Abstract
Background
Post-traumatic hydrocephalus (PTH) is one of the main complications of decompressive craniectomy (DC) after traumatic brain injury (TBI). Then, the recognition of risk factors and subsequent prompt diagnosis and treatment of PTH can improve the outcome of these patients. The purpose of this study was to identify factors associated with the development of PTH requiring surgical treatment in patients undergoing DC for TBI.
Methods
In this study, we collected the data of 190 patients (149 males and 41 females), who underwent DC for TBI in our Center. Then we analyzed the type of surgical treatment for all patients affected by PTH and the risk factors associated with the development of PTH.
Results
Post-traumatic hydrocephalus (PTH) developed in 37 patients out of 130 alive 30 days after DC (28.4%). The development of PTH required ventriculoperitoneal shunt (VPS) in 34 patients out of 37 (91.9%), while, in the remaining 3 patients, cerebrospinal fluid hydrodynamic (CSF) disturbances resolved after urgent cranioplasty and temporary external lumbar drain. Multivariate analysis showed that the presence of interhemispheric hygroma (p < 0.001) and delayed cranioplasty (3 months after DC) (p < 0.001) was significantly associated with the need for a VPS or other surgical procedure for PTH. Finally, among the 130 patients alive after 30 days from DC, PTH was associated with unfavorable outcome as measured by the 6-month Glasgow Outcome Scale score (p < 0.0001).
Conclusions
Our results showed that delayed cranial reconstruction was associated with an increasing rate of PTH after DC. The presence of an interhemispheric hygroma was an independent predictive radiological sign of PTH in decompressed patients for severe TBI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Post-traumatic hydrocephalus (PTH) is one of the main complications of decompressive craniectomy (DC) after traumatic brain injury (TBI) [3, 4, 11].
Post-traumatic hydrocephalus is also a complication of TBI in patients not having performed DC. However, in this study, the term PTH refers to the development of hydrocephalus in patients undergoing DC for severe head injury with uncontrollable intracranial hypertension.
In literature, the rate of PTH after DC ranges from 11.9 to 36% of adult patients and some authors have suggested that the presence of PTH is associated with unfavorable outcome after DC [3, 4, 11, 14, 15, 20]. For these reasons, the recognition of risk factors and subsequent prompt diagnosis of PTH can improve the outcome of these patients.
The development of hydrocephalus after DC is closely linked to the conversion of the cranial box from a closed system into an open one with the aim to increase brain compliance and reduce intracranial pressure in the presence of diffuse cerebral edema or a space occupying mass [18, 20]. On the other hand, the open box system can cause alterations in cerebral CBF and CSF dynamics and, usually 1 month or more after DC, the development of PTH or various forms of subdural collections may occur [3,4,5,6, 11, 17, 19, 21]. In this scenario, several studies suggested that early cranial reconstruction improves cerebral perfusion and CSF dynamics, usually altered by atmospheric pressure in patients with open skull, and can decrease the occurrence PTH requiring ventriculoperitoneal shunt (VPS) [16].
The purpose of this study was to identify factors associated with PTH requiring surgical treatment in patients undergoing DC for TBI.
Materials and methods
Data of 190 patients (149 males and 41 females), who underwent DC for sTBI in our Center between January 2003 and December 2011, were included in this study. Baseline clinical and radiological characteristics of patients and post-operative complications were summarized in Table 1.
Study outcomes focused specifically on the development of PTH after DC and the need for surgical treatment of this complication.
The indications for DC were based on current literature and the Brain Trauma Foundation guidelines for management of intracranial pressure (ICP) following traumatic brain injury, fourth edition [1].
Unilateral DC of at least 15-cm diameter was performed with the medial limit at least 2–2.5 cm lateral to the midline. Bifrontal DC was performed with the posterior limit at the coronal suture. All cases underwent expansive duraplasty with an allograft.
Demographic characteristics (age, gender), timing between injury and DC, admission Glasgow Coma Scale score (GCS), admission pupils’ reactivity and size, brain CT scan findings (subdural hematoma, brain contusion, etc.) and Rotterdam score, and surgical information including type and reason for DC were recorded and analyzed. Data concerning post-operative complications such as meningitis, hydrocephalus, subdural hygroma, incidence of new brain contusions, and expansion of contralateral post-traumatic mass lesion were collected and analyzed too. Then, all CT scans after the DC were reviewed and assessed for PTH or other CSF dynamic alterations (such as subdural or interhemispheric hygroma). Finally, we evaluated the number of patients that underwent cranioplasty within 3 months.
Definition and treatment of hydrocephalus
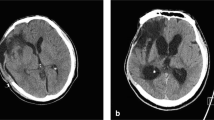
For the purposes of this study, PTH was defined as neuro-imaging evidence of progressive ventricular dilatation, with a modified Frontal Horn Index ≥ 33% associated with narrowed CSF spaces at the convexity, third ventricular enlargement, and periventricular lucencies on serial CT according to previous studies about PTH after DC [3, 4, 11, 14, 19].
For patients in vegetative status, clinical examination results were not included as a defining element in the determination of hydrocephalus. For patients showing an initial improvement of their clinical condition, impaired consciousness or a worsening neurologic status (not due to other causes) were included as a defining element in the determination of PTH. Moreover, all these patients required a surgical treatment due to neurological deterioration or considerable enlargement of their ventricles.
Finally, all patient included in this study presented the three criteria mentioned above: radiological, clinical (if not in vegetative status), and necessity of surgical treatment for hydrocephalus.
Primary end-point of the study
The primary end-point of this study focused specifically on the development of PTH after DC and the need for surgical treatment of this complication. After a careful review of literature, the variables associated with PTH were studied, including patient age, gender, timing between injury and DC, admission Glasgow Coma Scale score (GCS), admission pupils’ reactivity and size, pre-operative brain CT scan findings (subdural hematoma, brain contusion, midline shift, status of cisterns, etc.), Rotterdam score, type of DC, post-DC CT scan finding suggestive for CSF dynamic disturbances (subdural or interhemispheric hygromas), and the timing of cranioplasty.
We also analyzed the clinical outcome (secondary end-point of the study) according to the presence or not of PTH with the Glasgow Outcome Scale (5 point GOS) at 6-month follow-up: death, persistent vegetative state, and severe disability were assessed as unfavorable outcome (GOS 1–3) while GOS 4–5 (good recovery and moderate disability) as favorable outcome.
Statistical analyses
Data were analyzed with statistical package for social sciences (SPSS Inc., Chicago, Illinois, USA). Univariate analysis was performed by comparing patients who developed PTH and patients who did not develop PTH. Continuous variables were compared using Student’s t tests and Chi-square test for discrete variables. The multiple logistic regression was used to identify independent risk factors associated with the development of PTH. Statistical significance was set at p < 0.05.
Results
Post-traumatic hydrocephalus (PTH) developed in 37 patients out of 130 alive 30 days after DC (28.4%). The median interval from DC to the development of PTH requiring surgical treatment was 6.43 months (± 3.38) with a range from 1 to 15 months.
In 28 patients (75.7%), the diagnosis of PTH was formulated on the basis of a combination of radiological (modified Frontal Horn Index ≥ 33%, narrowed CSF spaces at the convexity, third ventricular enlargement, and periventricular lucencies) and clinical signs (worsened neurological examination or arrest of neurological improvement during rehabilitation). In the other 9 patients with comatose or minimal consciousness state, the diagnosis of PTH was based on considerable enlargement of ventricles associated with increasing size of pseudomeningocele on the side of the craniectomy flap.
The development of PTH required ventriculoperitoneal shunt (VPS) in 34 patients out of 37 (91.9%), while, in the remaining 3 patients, cerebrospinal fluid hydrodynamics (CSF) disturbances resolved after urgent cranioplasty and temporary external lumbar drain. Among the patients treated with VPS, in 20 cases, the shunt was implanted after cranioplasty. The remaining patients presented progressive enlargement of ventricular size at seriate CT scans associated with neurological worsening and progressive brain bulging outside of the borders of the craniectomy. For these reasons, we decided to perform in a single stage a cranioplasty and VPS placement in 7 cases, while only 4 patients underwent VPS few days before cranial reconstruction.
The patients undergoing a VPS placement after DC were significantly younger than those who did not undergo VPS insertion (p < 0.01). There was no significant difference in GCS at admission, pupils size and reactivity to light, and timing of DC between patients with and without PTH. Among radiological data (presence of SAH, state of cistern, midline shift, type of intracranial lesions, and Rotterdam Score), only the presence of SAH was significantly associated with PTH at univariate analysis (p = 0.027). Patients treated with bifrontal craniectomy presented higher rate of PTH rather than patients undergoing unilateral decompression (p = 0.03). The baseline characteristics (age, GCS at admission, pupils size and reactivity to the light, midline shift, state of cisterns, SAH, Rotterdam score, etc.) of the group of patients undergoing bifrontal DC compared with the group of unilateral DC were not statistically significantly different.
While meningitis and subdural hygroma were not statistically associated with PTH, interhemispheric hygroma was present in 35.1% of patients affected by PTH compared with the only 6% of the decompressed ones without PTH (p < 0.0001). The appearance of interhemispheric hygroma was observed in the early phase in the neuro-intensive care unit and preceded in all cases the ventricular enlargement. The median time between the DC and the appearance of hygroma was 12 days (range 6–21 days).
Finally, the 92% of decompressed patients (34/37) who developed PTH received cranioplasty after 3 months and delayed cranial reconstruction was significantly associated with PTH (p < 0.0001). Table 1 summarizes baseline demographic, clinical, and imaging data of the patients. The mean time of cranioplasty in our series was 5.97 months (± 3.39).
Multivariate analysis showed that the presence of interhemispheric hygroma (p < 0.001) and delayed cranioplasty (p ≤ 0.001) were significantly associated with the need for a VPS or others surgical procedure after DC (Table 2).
The baseline characteristics (age, GCS at admission, pupils size and reactivity to the light, midline shift, state of cisterns, SAH, Rotterdam score, etc.) of the group of patients who received cranioplasty within 3 months compared with the group of delayed cranioplasty (after 3 months) were similar (Table 3).
With regard to the clinical outcome of this series, overall mortality rate was 46.8% (89 out of 190 patients) while 60 patients (31.6%) died within 30 days after DC. Among the 130 patients alive after 30 days from DC, the outcome was poor (GOS severe disability, persistent vegetative state, and death) in 51.5% of patients (67 out of 130) while 63 patients (48.5%) presented a good outcome defined as GOS 4 (moderate disability; 41 patients) or GOS 5 (good recovery; 22 patients). In the group of 67 patients with poor outcome, 28 patients (41.8%) died (GOS 1), 19 (28.3%) presented persistent vegetative status (GOS 2), and 20 (29.9%) severe disability (GOS 3).
Finally, among the 130 patients alive after 30 days from DC, PTH was associated with unfavorable outcome as measured by 6-month Glasgow Outcome Scale score (p < 0.0001; Table 4).
Discussion
The DECRA and RESCUEicp trials found respectively that DC can decrease ICP value and mortality. However, in the same trials, DC was also associated with the increased rate of survival in either a vegetative state or severe disability [2, 13]. These results are partly due to the high rate of complications secondary to DC, including hemorrhagic complications, infectious complications, and disturbances of the CSF compartment [5,6,7,8,9,10,11,12,13,14,15].
Post-traumatic hydrocephalus (PTH) is one of the main complications of decompressive DC after TBI [3, 4, 11, 14, 15, 19,20,21]. So, a better understanding of the real incidence, pathophysiology, risk factors, and treatment of the PTH after DC is mandatory in order to optimize diagnostic and management strategies.
In a review published in 2015 about the complications associated with DC, PTH was reported in 14.8% (290/1966) of TBI patients undergoing DC (the diagnosis of PTH differed among the studies, defining this entity either radiographically as ventriculomegaly and/or clinically as symptoms of hydrocephalus) [15].
Honeybul et al. [11] reported that among the 159 patients who survived more than 6 months after DC, 26 (16.35%) developed clinical evidence of hydrocephalus and required a VP shunt. While more recently, Vedantam et al. [20] founded that the 25% of patients required a VP for PTH after DC for TBI. In our series, the rate of PTH after DC was slightly higher (28.4%). Indeed, PTH developed in 37 patients out of 130 alive 30 days after DC.
The pathophysiology of PTH after DC is closely linked to the conversion of the cranial box from a closed system into an open box and previous studies have suggested several possible mechanisms including disruption of CSF drainage due to arachnoid adhesions in the basal cisterns, loss of pulsatile intracranial CSF dynamics, and impaired venous drainage into the sagittal sinus [3, 4, 11, 14, 18,19,20,21].
Among these theories, Waziri et al. [21] postulated that, because the arachnoid granulations function as pressure dependent one-way valves from the subarachnoid space to the draining venous sinuses, the disruption of pulsatile ICP dynamics secondary to opening the cranial vault results in decreased CSF outflow. This could be a possible explanation why an early cranioplasty should restore normal intracranial pressure dynamics and spontaneous resolution of PTH and other CSF disturbances [16, 21]. On the contrary, delayed cranial reconstruction, by prolonging this disruption, may result in permanent dysfunction of the arachnoid granulations, such as the one seen in hydrocephalus induced by long-term CSF drainage [4, 5, 21]. Nonetheless, the previous hypothesis contrasted with the fact that a resolution of hydrocephalus after cranioplasty occurred only in some patients. However, the same authors (Waziri et al. [21]) suggest that time might play a role by prolonging the pathogenic mechanisms so that hydrocephalus becomes irreversible, even after cranioplasty.
This theory represents a possible explanation of our results: in our series, the 92% of decompressed patients (34/37) who developed PTH received cranioplasty after 3 months and delayed cranial reconstruction was independently associated with PTH (p < 00001).
This result contrasts with the study published by Honeybul et al. [11] where timing of cranioplasty was not associated with the development of PTH after DC. Indeed, in this series, the mean time of cranial reconstruction was near 3 months for both the group of PTH and no-PTH patients (86 days vs 96 days); and in these patients, for the authors, the mechanism of PTH may be related to the severity of the primary brain injury.
Previous studies reported young age, CSF infection, the presence of subarachnoid blood, and bilateral craniectomy as predisposing factors for post-traumatic hydrocephalus [3, 4, 11, 14, 19]. In our series these factors resulted associated with PTH only at the univariate analysis.
The second factor in our study, independently related to the development of hydrocephalus, was an interhemispheric hygroma. In more than 65% of patients, subdural interhemispheric CSF collections preceded the ventricular enlargement. This data confirmed the results of the studies of Kaen et al. [14] and De Bonis et al. [3]. In the first of these two studies, the presence of an interhemispheric hygroma was a predictive radiological sign of hydrocephalus development within the first 6 months after DC in patients with severe head injury with a sensitivity of 94% and a specificity of 96%.
De Bonis et al. [3, 4] reported that interhemispheric hygroma was present in 42% of patients with hydrocephalus and temporally preceded the occurrence of ventricular enlargement. These authors considered this entity as epiphenomenon of some alterations of CSF dynamics secondary to the damage of the arachnoid barrier cells and arachnoid–dura interface layer after TBI and secondary to the craniectomy close to the midline which may determine an increase in the venous outflow during the diastolic phase, with a subsequent increase of extracellular fluid absorption.
In our study, we do not evaluate the distance from the midline (cut-off 25 mm reported in previous studies) as risk factor for PTH because in every case we performed craniectomy near to the midline (about 2 cm) to maximize the decompression effect in case of refractory intracranial hypertension.
Finally, unfavorable outcome (GOS scores of 1–3) was observed in 27 patients (78.4%) with post-traumatic hydrocephalus. Patients with hydrocephalus after DC presented an increased risk for poor outcome (p < 0.0001).
The main limitations of this study included the following:
-
The association between PTH and unfavorable outcomes at 6-month follow-up may be most likely due to their association with more severe primary brain injury, and did not necessarily confirm a cause-and-effect relationship.
-
The result of cranioplasty on PTH reported in the present study could be partially affected by a bias related to the possible different severity of overall illness and health between the two analyzed groups (within and after 3 months).
Conclusions
Our results showed that delayed cranial reconstruction was associated with an increasing rate of PTH after DC. The presence of an interhemispheric hygroma was an independent predictive radiological sign of PTH in decompressed patients for severe TBI.
References
Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ et al. (2017) Guidelines for the management of severe traumatic brain injury, Fourth Edition. Neurosurgery 80(1):6–15.
Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P et al (2011) Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med 364(16):1493–1502
De Bonis P, Pompucci A, Mangiola A, Rigante L, Anile C (2010) Post-traumatic hydrocephalus after decompressive craniectomy: an underestimated risk factor. J Neurotrauma 27(11):1965–1970
De Bonis P, Sturiale CL, Anile C, Gaudino S, Mangiola A, Martucci M et al (2010) Decompressive craniectomy, interhemispheric hygroma and hydrocephalus: a timeline of events? Clin Neurol Neurosurg 115(8):1308–1312
Di Rienzo A, Iacoangeli M, Alvaro L, Colasanti R, Dobran M, Di Somma LG et al (2013) The sinking bone syndrome? Neurol Med Chir (Tokyo) 53(5):329–335
di Somma L, Iacoangeli M, Nasi D, Balercia P, Lupi E, Girotto R et al (2016) Combined supra-transorbital keyhole approach for treatment of delayed intraorbital encephalocele: a minimally invasive approach for an unusual complication of decompressive craniectomy. Surg Neurol Int 7(Suppl 1):S12–S16
Dobran M, Mancini F, Nasi D, Scerrati M (2017) A case of deep infection after instrumentation in dorsal spinal surgery: the management with antibiotics and negative wound pressure without removal of fixation. BMJ Case Rep 28:2017
Dobran M, Marini A, Gladi M, Nasi D, Colasanti R, Benigni R et al (2017) Deep spinal infection in instrumented spinal surgery: diagnostic factors and therapy. G Chir 38(3):124–129
Dobran M, Marini A, Nasi D, Gladi M, Liverotti V, Costanza MD et al (2017) Risk factors of surgical site infections in instrumented spine surgery. Surg Neurol Int 8:212
Dobran M, Nasi D, Esposito DP, Iacoangeli M (2016) Posterior fixation with C1 lateral mass screws and C2 pars screws for type ii odontoid fracture in the elderly: long-term follow-up. World Neurosurg 96:152–158
Honeybul S, Ho KM (2012) Incidence and risk factors for post-traumatic hydrocephalus following decompressive craniectomy for intractable intracranial hypertension and evacuation of mass lesions. J Neurotrauma 29(10):1872–1878
Huh PW, Yoo DS, Cho KS, Park CK, Kang SG, Park YS et al (2006) Diagnostic method for differentiating external hydrocephalus from simple subdural hygroma. J Neurosurg 105(1):65–70
Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J et al (2016) Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med 375(12):1119–1130
Kaen A, Jimenez-Roldan L, Alday R, Gomez PA, Lagares A, Alén JF, Lobato RD (2010) Interhemispheric hygroma after decompressive craniectomy: does it predict posttraumatic hydrocephalus? J Neurosurg 113(6):1287–1293
Kurland DB, Khaladj-Ghom A, Stokum JA, Carusillo B, Karimy JK, Gerzanich V et al (2015) Complications associated with decompressive craniectomy: a systematic review. Neurocrit Care 23(2):292–304
Malcolm JG, Rindler RS, Chu JK, Choksh F, Grossberg JA, Pradilla G et al. (2017) Early cranioplasty is associated with greater neurological improvement: a systematic review and meta-analysis. Neurosurgery 82:278–288.
Nasi D, Dobran M, Iacoangeli M, Di Somma L, Gladi M, Scerrati M (2016) Paradoxical brain herniation after decompressive craniectomy provoked by drainage of subdural hygroma. World Neurosurg 91:673.e1–4
Nasi D, Dobran M, Di Rienzo A, di Somma L, Gladi M, Moriconi E et al (2018) Decompressive craniectomy for traumatic brain injury: the role of cranioplasty and hydrocephalus on outcome. World Neurosurg. https://doi.org/10.1016/j.wneu.2018.05.028
Stiver SI (2009) Complications of decompressive craniectomy for traumatic brain injury. Neurosurg Focus 26(6):E7
Vedantam A, Yamal JM, Hwang H, Robertson CS, Gopinath SP (2018) Factors associated with shunt-dependent hydrocephalus after decompressive craniectomy for traumatic brain injury. J Neurosurg 128(5):1547–1552
Waziri A, Fusco D, Mayer SA, McKhann GM 2nd, Connolly ES Jr (2007) Postoperative hydrocephalus in patients undergoing decompressive hemicraniectomy for ischemic or hemorrhagic stroke. Neurosurgery 61:489–493
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Comments
In this retrospective observational study, Dr. Nasi et al. investigate the incidence rate of hydrocephalus following decompressive craniectomy (DC) for traumatic brain injury (TBI). In their patient cohort of 130 patients surviving the first month after DC, 37 patients (28%) were diagnosed with hydrocephalus defined by both progressive ventricular enlargement and clinical deterioration. The presence of an interhemispheric hygroma during the early phase after DC and cranioplasty later than 3 months after DC was independent risk factors for the development of symptomatic hydrocephalus requiring treatment. Interhemispheric hygroma may indeed be an indicator of disturbed cerebrospinal fluid circulation (caused by the trauma or the large skull defect resulting from the DC) and this radiological finding should be taken into account in evaluation of patients with clinical deterioration following DC, when hydrocephalus is suspected. The effect of cranioplasty timing is still debated and controversial. A recent (and larger) observational study by Morton et al. came to the opposite conclusion that cranioplasty later that 90 days after DC was associated with a lower risk of hydrocephalus. [1] This issue needs further exploration in future prospective (randomized?) studies.
Alexander Lilja-Cyron
Copenhagen, Denmark
1. Morton RP, Abecassis IJ, Hanson JF, et al (2017) Timing of cranioplasty: a 10.75-year single-center analysis of 754 patients. J Neurosurg 128(128):1-5
This article is part of the Topical Collection on Brain trauma
Rights and permissions
About this article
Cite this article
Nasi, D., Gladi, M., Di Rienzo, A. et al. Risk factors for post-traumatic hydrocephalus following decompressive craniectomy. Acta Neurochir 160, 1691–1698 (2018). https://doi.org/10.1007/s00701-018-3639-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-018-3639-0