Abstract
Background
The availability of minimal access instrumentation and endoscopic visualization has revolutionized the field of minimally invasive skull base surgery. The transorbital endoscopic approach using an eyelid incision has been proposed as a new minimally invasive technique for the treatment of skull base pathology, mostly extradural tumors. Our study aims to evaluate the anatomical aspects and potential role of the transorbital endoscopic approach for exposure of the sylvian fissure, middle cerebral artery and crural cistern.
Methods
An anatomical dissection was performed in four freshly injected cadaver heads (8 orbits) using 0- and 30-degree endoscopes. First, an endoscopic endonasal medial orbital decompression was done to facilitate medial retraction of the orbit. An endoscopic transorbital approach through an eyelid incision, with drilling of the posterior wall of the orbit and lesser sphenoidal wing, was then performed to expose the sylvian fissure and crural cisterns. A stepwise anatomical description of the approach and visualized anatomy is detailed.
Results
A superior eyelid incision followed by orbital retraction provided a surgical window of approximately 1.2 cm (range 1.0–1.5 cm) for endoscopic transorbital dissection. The superior (SOF) and inferior (IOF) orbital fissures represent the medial limits of the approach and are identified in the initial part of the procedure. Drilling of the orbital roof (lateral and superior to the SOF), greater sphenoidal wing (lateral to the SOF and IOF) and lesser sphenoidal wing exposed the anterior and middle fossa dura. A square-shaped dural opening provided visualization of the posterior orbital gyri, sylvian fissure and temporal pole. Intradural dissection allowed exposure of the sphenoidal portion of the sylvian fissure, M1, MCA bifurcation and M2 branches and lenticulostriate perforators. Dissection of the medial aspect of the sylvian and carotid cisterns with a 30-degree endoscope allowed exposure of the mesial temporal lobe and crural cistern.
Conclusions
The transorbital endoscopic approach allows successful exposure of the sphenoidal portion of the sylvian fissure and M1 and M2 segments of the middle cerebral artery. Angled endoscopes may provide visualization of the mesial temporal lobe and crural cistern. Although our anatomical study demonstrates the feasibility of intradural dissection and closure via an endoscopic transorbital approach, further studies are necessary to evaluate its role in the clinical scenario.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
“Minimally invasive” and “minimal access” surgical approaches are having an increasing impact on the current neurosurgical management of skull base and vascular pathology [5, 10, 12, 16, 23, 25, 29, 30, 39, 41, 47]. The basic principles of open skull base surgery traditionally involved removal of more bone to avoid brain retraction, while the basic principles of minimally invasive surgery involve well-placed small openings and endoscopes to avoid brain retraction. The Sylvian fissure and its contents, namely the middle cerebral artery, the surface of the insula as well as the uncus and crural cistern are frequent sites of neurosurgical pathology, namely aneurysms. Reaching these areas generally involves a pterional craniotomy, or some variation thereof, with dissection of the sylvian fissure and some degree of brain retraction, to achieve a view spanning from proximal to medial so the ICA and carotid bifurcation can be followed as they lead to M1 and more distal branches. However, the entrance of the vascularity into the Sylvian fissure lies just behind the orbit, and a potentially more sensible approach would be to use the opening provided by the orbit as a more direct approach to the proximal Sylvian fissure.
The evolution of endoscopic endonasal approaches (EEAs) has markedly improved the instrumentation and neurosurgical facility of endoscope assisted procedures [6, 10, 25, 37, 46, 49, 56, 57, 66]. Median and paramedian lesions, such as pituitary tumors and selected meningiomas and chordomas, are successfully resected through endoscopic approaches, with similar or better rates of gross total resection and complications than transcranial approaches [9, 11, 36]. The supraorbital eyelid incision has also gained popularity as a minimally invasive alternative to a pterional craniotomy for skull base pathology as well as for aneurysms with endoscopic assistance [1, 21, 33, 34, 52, 59, 61]. However, the supraorbital approach is limited inferiorly by the roof of the orbit, which limits access to the anatomic area directly behind the greater and lesser wings of the sphenoid, preventing ideal access to the proximal sylvian fissure and its contents. Using the orbit as an alternative option for surgical approaches led to the development of transorbital endoscopic surgery, also called TONES (transorbital neuroendoscopic surgery) [16, 44, 50].
Through superior or inferior eyelid approaches, lesions in the lateral portion of the skull base can be treated via a transorbital endoscopic approach, representing a minimally invasive alternative to transcranial surgery in selected cases [16, 44, 50]. While the supraorbital approach has been widely applied to a variety of skull base and vascular pathologies, the transorbital neuroendoscopic approach has had much more limited applications [3, 4, 24, 52, 61, 62, 65]. Indeed, transorbital endoscopic surgery has not been used for the management of intra-axial tumors or cerebral vascular lesions, and only limited anatomical studies have evaluated the feasibility of transorbital endoscopic surgery for such objectives [13, 16, 18, 22, 26].
In the current study, we aim to evaluate the feasibility, anatomical aspects and limitations of transorbital endoscopic surgery for exposure and dissection of the sylvian fissure, middle cerebral artery and surrounding cisterns, anatomic structures for which the approach appears ideally suited.
Methods
Anatomical dissection was performed in four freshly injected cadaver heads at the Weill Cornell Surgical Innovations Laboratory. The internal carotid arteries (ICAs) and jugular veins were cannulated and injected with silicone pigment compound (Dow Corning, Midland, MI, USA). The cadaveric heads were soaked in 70% ethyl alcohol for at least 24 h. Endoscopic dissections were done using rod lens endoscopes (4 mm, 18 cm, Hopkins II, 0° and 30°, Karl Storz, Tuttlingen, Germany) attached to a high-definition camera and a digital video recorder system (Full HD camera platform IMAGE 1, Karl Storz, Tuttlingen, Germany). A stepwise anatomical dissection of the transorbital endoscopic technique and measurements of the size of transorbital corridor, craniectomy extension and dural opening were performed.
Results
The cadaveric head was placed in a three-pin Mayfield head-holder in a neutral position. The initial part of the procedure consisted of the endoscopic endonasal approach. Via a uninarial approach, an ipsilateral uncinectomy, infundibulotomy and enlargement of the ostium of the right maxillary sinus were done. Posterior and anterior ethmoidectomies were performed using a tissue shaver, exposing the lamina papyracea, which was entered using a Cottle elevator. A Freer elevator was used to develop a plane in between the periorbital and lamina papyracea. This was adequately removed all the way up to the region of the orbital apex into the region of the annulus of Zinn, and a sphenoidotomy was carried out. The optic nerve was further decompressed using a Kerrison rongeur (Fig. 1). After completion of the medial orbital decompression, the transorbital endoscopic procedure was performed.
Endoscopic endonasal medial orbital decompression. A: Left-side uninarial approach depicting the uncinate process (UP), ethmoidal bulla (EB) and middle turbinate (MT); B: visualization of the ethmoidal bulla after resection of the uncinate process. The maxillary ostium (MO) is observed inferiorly to the EB; C: the sella (S), medial orbit (O) and optic nerve (ON) are observed after ipsilateral opening of the sphenoid sinus, ethmoidectomy and removal of the lamina papyracea
For didactic reasons, the transorbital endoscopic approach was divided into five steps: (1) skin incision and periorbita dissection; (2) craniectomy; (3) dural opening; (4) intradural dissection; (5) dural repair.
-
1.
Skin incision and periorbital dissection
Ttransorbital dissection is done via a superior eyelid incision (Fig. 2). The orbicularis layer is opened along the length of the incision to expose the periosteum of the lateral orbital rim, and dissection follows in a preseptal orbital plane. The orbital septum is a connective tissue structure that attaches peripherally at the periosteum of the orbital margin and merges centrally with the lid retractor structures. Careful dissection is recommended to avoid transgressing this plane to avoid injuries to the levator muscle and aponeurosis. The periorbita is gently elevated, from lateral to medial and superior to inferior, using a Freer elevator along the inner aspect of the lateral orbital rim toward the posterior wall of the orbit. An important landmark in this part of the approach, the meningolacrimal branch, usually runs anterior to the superior orbital fissure (mean distance: 1.57 cm; range: 1.0 cm–2.3 cm) (Fig. 3). Further dissection exposes the lateral aspect of the superior (SOF) and inferior orbital fissure (IOF) (Fig. 3), the main anatomical references for the transorbital craniectomy. Successful transorbital dissection followed by retraction of the orbit will create a surgical corridor of approximately 1.5 cm (range: 1.0–2.0 cm).
-
2.
Craniectomy
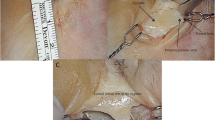
Superior eyelid approach. A: A skin incision is made over the superior eyelid. The supraorbital nerve represents the medial extent of the incision, which extends infero-laterally to the lateral canthus. B: After opening the orbital muscle layer, dissection is done following the preseptal plane to prevent injury to the levator muscle and aponeurosis. Retraction of the periorbital tissue will expose the orbital roof and lateral orbital wall
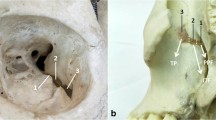
Orbital retraction and identification of the orbital roof and posterior-lateral walls of the orbit. Initial retraction exposes the meningolacrimal branch (1) anterior to the superior orbital fissure (SOF) (2), orbital roof (3) and lateral wall of the orbital (4). The meningolacrimal branch is located to guide the surgeon toward the SOF and is located approximately 1.57 cm anteriorly
The orbital roof, greater sphenoid wing and part of the orbital floor are exposed after adequate dissection. The extent of the transorbital craniectomy may vary according to the location and size of the lesion to be treated, but wide drilling of the orbital roof and greater sphenoidal wing is recommended to enlarge the surgical field and facilitate the use of instruments during the intradural stage. The superior and inferior orbital fissures are important anatomical references in the posterior wall of the orbit (Fig. 4). The orbital roof and basal frontal lobe are located superior to the SOF; the sphenoidal segment of the sylvian fissure, posterior orbital gyri and superior part of the temporal pole are lateral to the SOF and IOF, posterior to the greater and lesser sphenoidal wings; the temporal pole is fully exposed after extensive drilling of the greater sphenoidal wing, lateral to the IOF.
Landmarks for the endoscopic transorbital approach to the skull base and sylvian fissure. The superior and inferior orbital fissures are the main anatomical references to the approach through the posterior wall of the orbit. Lesions in the orbital roof and basal frontal lobe require removal of the orbital roof superior to the SOF; removal of the greater sphenoidal wing lateral to the SOF and IOF exposes the sphenoidal segment of the sylvian fissure, posterior orbital gyri and superior part of the temporal pole; extensive drilling lateral to the greater sphenoidal wing lateral to the IOF exposes the temporal lobe and bone removal inferior to the IOF leads to the infratemporal fossa. A: Left orbit showing the area of the craniectomy (marked area) used for the endoscopic transorbital approach to the sylvian region. Although the sylvian fissure might be exposed with smaller bone removal, extensive drilling is recommended to improve the surgical corridor during the intradural phase of the procedure. B: Craniectomy is only performed after identification of the SOF and IOF and exposure of the orbital roof and greater sphenoidal wing. C: Exposure of the optic canal and anterior clinoid process (ACP) is achieved after inferior retraction of the orbit. Removal of the ACP improves the exposure of the carotid cistern and allows exposure of the carotid bifurcation and middle cerebral artery origin. D: Opening of the SOF. The endoscopic transorbital approach allows direct access to the SOF and its contents. ACP: anterior clinoid process; *inferior orbital fissure (IOF); **superior orbital fissure (SOF)
Drilling is initiated with a cutting-bit high-speed drill (Midas Rex, Medtronic, Fort Worth, TX) to remove the orbital roof and lateral portion of the greater sphenoidal wing. As the drilling progresses laterally, the temporal muscle in the temporal fossa is exposed and represents the lateral limit of the craniectomy. The initial drilling in the lateral and superior parts of the posterior wall of the orbit gives more space for bimanual work and facilitates further removal of the medial part of greater sphenoidal wing. A diamond bit is used for drilling of the medial part of the greater sphenoidal wing because of the proximity to the SOF and IOF.
Once the orbital roof and greater sphenoid wing have been removed, the lesser sphenoid wing is exposed and removed (Figs. 5, 6 and 7). Penfield N.1 and angle endoscopic dissectors are used to detach the dura overlying the lesser sphenoidal wing prior to its removal using Kerrison rongeurs and drilling. The medial and lateral extension of its resection can be tailored according to the surgical goal. Lesions located in the lateral aspect of the sphenoid segment of the sylvian fissure will require more extensive lateral drilling, which can be extended until the inner aspect of the pterion cranial point (Fig. 5). The mesial temporal lobe, carotid cistern and proximal sylvian fissure lesions benefit from an extradural removal of the anterior clinoid process (Fig. 6). After a successful craniectomy (size: lateral, 1.8 cm; range, 1.2–2.5 cm; craniocaudal, 3.3 cm; range, 2.7–3.8 cm), exposure of the dura over the basal frontal lobe, temporal pole and sylvian fissure is achieved (Fig. 7)
Removal of the lateral portion of the lesser sphenoidal wing. Exposure of the sylvian fissure benefits from wide resection of the sphenoid ridge. Kerrison rongeurs (a) and high-speed drills (b) are used to remove the most lateral aspect of the lesser sphenoidal wing. *Intracranial projection of the pterion point
Anterior clinoid process and approaches to the sylvian fissure and carotid cistern. A: Exposure of the medial portion of the sylvian and carotid cisterns is improved after extradural removal of the anterior clinoid process. This bone projection is attached to the anterior fossa in three points: planum sphenoidale medially; lesser sphenoid wing laterally; optic strut (OS) inferiorly. It composes the anterior part of the roof of the cavernous sinus and is superior to the SOF. B: The meningo-orbital band (MOB) contains the meningo-orbital artery and is located medial to the SOF (**). It should be dissected and cut prior to drilling of the ACP. Complete anatomical detachment of the ACP may not be performed because of the projection of the optic strut inferiorly to the optic canal (*). ACP, anterior clinoid process; MOB, meningo-orbital band; OS, optic strut; *optic canal; **superior orbital fissure (SOF)
Dural exposure. A: dural exposure prior to resection of the medial (ACP) and lateral limits of the lesser sphenoidal wing. B: Wide drilling of the orbital roof and sphenoidal wings allows exposure of the dura over the basal frontal lobe, temporal pole and sylvian fissure. ACP: anterior clinoid process; MOB: meningo-orbital band; TM: temporal muscle; **superior orbital fissure (SOF)
The anterior clinoid process (ACP) is a posterior projection of the medial portion of the lesser sphenoidal wing that composes the anterior part of the roof of the cavernous sinus (Fig. 6). It is attached to the anterior fossa in three points: planum sphenoidale medially; lesser sphenoid wing laterally; optic strut inferiorly. Safe complete anatomical detachment of the ACP is difficult to achieve through an endoscopic transorbital approach since the optic strut is inferior to the optic canal and medial to the SOF. However, removal of the infero-lateral aspects of the ACP superior to the SOF is easily performed using a high-speed drill.
-
3.
Dural opening
Like the craniectomy, durotomy can be tailored according to the location of the lesion. However, a large dural opening is recommended to achieve an optimal surgical window for intradural dissection. Although a single linear incision over the sylvian fissure can be attempted, this might be insufficient for adequate exposure and mobilization of the frontal and temporal lobes. Therefore, we recommend performing three incisions over the dura, carried out in an “H” format, which will lead to a final square-shaped dural opening, exposing the basal frontal lobe, sylvian fissure and temporal lobe. The initial incision is performed over the sylvian fissure, running from medial to lateral. Two perpendicular incisions are then performed at the medial and lateral limits of the sylvian incision, running from inferior to superior over the frontal lobe and from superior to inferior over the temporal lobe. Kerrison rongeurs are then used to remove the dura and make the final square-shaped opening (Fig. 8), measuring approximately 2.0 × 2.0 cm (Fig. 9).
-
4.
Intradural dissection
-
(a)
The sylvian fissure
The sylvian fissure is composed of a superficial and a deep part. The superficial part presents a stem and three rami, the anterior horizontal, anterior ascending and posterior rami. The deep (cisternal) part is divided into an anterior part, the sphenoidal compartment, and a posterior part, the operculoinsular compartment (Figs. 10, 11, 12, 13 and 14). The sphenoidal compartment arises in the region of the limen insulae, at the lateral margin of the anterior perforated substance. The sphenoidal compartment is a narrow space posterior to the sphenoid ridge, between the frontal and the temporal lobes, that communicates medially with the carotid cistern. The operculoinsular compartment is formed by two narrow clefts, the opercular cleft, between the opposing lips of the frontoparietal and the temporal opercula and the insular cleft, between the insula cortex and the opercula.
Initial exposure of the frontal and temporal lobes and sylvian fissure. Exposure of the posterior orbital gyri and superior part of the temporal pole is required for successful exposure and dissection of the sylvian fissure. Initial intradural dissection is performed with a 0-degree endoscope supported by an endoscope holder. This allows application of bimanual microsurgical techniques using aspirators and microscissors. Dissection starts with identification of the superficial MCA branches, such as the anterior temporal arteries (**), which guide the surgeon toward the main trunk of the M1 segment. M1: sphenoidal segment of the middle cerebral artery (MCA); **anterior temporal branch
Sylvian fissure and sylvian veins. Large sylvian veins draining into the sphenoparietal sinus may obstruct the surgical corridor and block visualization of the sylvian fissure. Small veins may be ligated; however, large draining vessels should be preserved if possible through careful dissection and mobilization toward the temporal lobe
Middle cerebral artery. a Dissection of the sylvian fissure leads to exposure of the distal portion of the M1 segment, MCA bifurcation and superior and inferior trunks of the MCA. The limen insula is shown at the site of the genu of the MCA (**), and the uncus is observed inferior to M1. b Further dissection with a 30-degree endoscope demonstrates the proximal segment of M1 exiting the carotid cistern and early temporal branches (*). The lateral wall of the cavernous sinus is exposed after retraction of the temporal lobe. *Early temporal branch; **genu of the middle cerebral artery
Sphenoid segment of the MCA (M1 segment). A Exposure of the medial portion of the sylvian cistern and its contents, with a 30-degree endoscope. The medial (*) and lateral (**) lenticulostriate branches exit the superior and posterior walls of the MCA and run toward the anterior perforated substance to supply the internal capsule and deep nuclei. B The uncus is observed at the medial aspect of the temporal lobe, inferior to the M1 segment and closely related to the lateral wall of the cavernous sinus. Through an endoscopic transorbital transuncus approach, the amygdala and hippocampus may be resected. *Medial lenticulostriate branches; **lateral lenticulostriate branches
Insular segment of the MCA (M2 segment). a Lateral dissection of sylvian fissure leads to exposure of its operculoinsular segment. The MCA bifurcates at the distal part of the M1 and turns around the limen insula, originating the genu of the MCA. b The superior and inferior trunks of the MCA run close to the inferior circular sulcus toward the posterior part of the operculoinsular segment of the sylvian fissure. In their trajectory, they develop perforating branches that cross perpendicularly to irrigate the insula
Although the lateral superficial part of the sylvian fissure cannot be adequately exposed via an endoscopic transorbital approach, the sphenoidal and operculoinsular segments are suitable regions for this technique. A successful dural opening exposes the posterior orbital gyri, the temporal pole and the superficial part of the sphenoidal segment of the sylvian fissure (Fig. 10). Dissection proceeds with opening of the arachnoid membranes over the anterior aspect of the sylvian fissure with endoscopic knifes and microscissors. A ball dissector is carefully used to separate the arachnoid from the temporal branches of the MCA and deep sylvian fissure veins. This venous complex is connected medially to the sphenoparietal sinus and laterally to the superficial sylvian veins, and its extension may limit the approach. A large deep sylvian vein complex may limit the mobilization of the temporal lobe and the opening of the sylvian fissure, while a small venous complex (Fig. 11) allows direct exposure of the sphenoid segment. Small veins crossing the sylvian fissure between the frontal and temporal lobe may be ligated and cut, as performed in transcranial approaches.
-
(b)
Middle cerebral artery
The MCA originates in the carotid cistern, at the medial end of the deep portion of the sylvian fissure, lateral to the optic chiasm and posterior to the olfactory tract division into medial and lateral olfactory striae. It can be divided into four segments: sphenoidal (M1), insular (M2), opercular (M3) and cortical (M4). The M1 segment runs laterally from its origin, parallel to the lesser sphenoid wing and approximately 1 cm posterior to the sphenoidal ridge. Anterior to the limen insula, the MCA turns posteriorly (genu of the MCA), originating the M2 segment. It runs over the lateral insular surface, usually as two main trunks (superior and inferior), which will originate other branches and cross the circular sulcus of the insula (M3 segment) and reach the cortical surface of the frontal, temporal and parietal lobes (M4 branches). The proximity of the M1 segment to the sphenoidal ridge makes it a good candidate for transorbital endoscopic approaches. Careful wide opening of the sphenoidal segment of the sylvian fissure allows exposure of the limen insula and identification of the M2 branches at the lateral insular surface.
Endoscopic transorbital identification of the MCA is possible after opening of the sylvian fissure and identification of the temporopolar and anterior temporal branches of the MCA, which run over the anterior part of the temporal lobe (Figs. 9 and 10). These branches will lead the surgeon to the main trunk of the MCA in the sylvian cistern. This cistern extends medially along the sylvian fissure and over the insula to the carotid cistern. It contains the middle cerebral artery, its bifurcation and major branches, anterior perforating arteries, temporopolar and anterior temporal arteries (Figs. 12 and 13). Once the main trunk of the M1 segment has been found, dissection can be carried medially toward the carotid cistern, with exposure of the carotid bifurcation as well as the site of origin of M1 and A1. The medial lenticulostriate branches originate from the superior-posterior wall of the M1 and are observed during its course toward the anterior perforated substance (Fig. 12). Lateral dissection exposes the MCA bifurcation, limen insula and genu of the MCA, M2 segment and its superior and inferior trunks and lateral lenticulostriate branches (Figs. 12 and 14).
-
(c)
Lateral dissection—anterolateral surface of the insula
Opening of the sphenoidal and operculoinsular segments of the sylvian fissure allows exposure of the limen insula and anterior and lateral insular surface (Figs. 12 and 14). Mobilization of the frontal and temporal operculum allows visualization of the inferior circular sulcus and M2 branches in the anterior-inferior aspect of the insula. Perforating branches, originating from M2, enter the lateral insula surface perpendicularly and may be visualized after lateral mobilization of the M2 trunks. The superior-posterior part of the lateral insular surface may not be adequately exposed through this approach since it is hidden deeply below the posterior part of the frontotempoparietal operculum.
-
(d)
Medial dissection—carotid, crural and ambiens cistern
Dissection toward the medial aspect of the sylvian cistern will lead to the carotid cistern (Fig. 15). It is located between the sylvian and chiasmatic cisterns and contains the supraclinoid portion of the internal carotid artery, the origins of the ophthalmic, posterior communicating (PcomA) and anterior choroidal arteries (AChA), the carotid bifurcation, initial portions of the A1 and M1 segments, and the perforating branches from the carotid artery. Posteriorly, this cistern communicates with the crural and ambiens cistern, which can also be visualized using 30-degree endoscopes and lateral retraction of the temporal lobe or after an anterior temporal lobectomy.
Carotid cistern. Exposure of the carotid cistern with a 30-degree endoscope. a The carotid bifurcation and origin of A1 and M1 are shown. It is possible to visualize the trajectory of A1 above the left optic nerve. b The uncus is observed in its typical location medial to the supraclinoidal ICA and inferior to the M1 segment. After exiting the interpeduncular fossa, the oculomotor nerve runs medial to the uncus on its way to the roof of the cavernous sinus
The crural cistern is located between the cerebral peduncle and the posterior part of the uncus (Fig. 16) and contains the P2a segment of the posterior cerebral artery, perforating branches to the cerebral peduncles, AChA and medial posterior choroidal artery. The ambient cistern is situated posterior-superior to the crural cistern, between the midbrain and the temporal lobe, and extends from the posterior edge of the uncus anteriorly to the lateral edge of the collicular plate posteriorly. It contains the posterior cerebral artery and lateral posterior choroidal arteries, thalamogeniculate branches, inferior temporal and parieto-occipital arteries, and the trochlear nerve.
A Crural cistern and uncus. After retraction of the temporal lobe, it is possible to observe the relation of the uncus and crural cistern. The AChA originates from the distal part of the supraclinoidal and runs posterolaterally to supply the mesial temporal lobe, internal capsule and optic tract. Branches from the Pcom and AchA cross the crural cistern to supply the uncus, as demonstrated in the figure (*). Medial to the tentorial edge, the origin of the oculomotor nerve, between the P2a and SCA, and its course toward the roof of the cavernous sinus are observed. The basal vein of Rosenthal, distal part of the AChA and optic tract are observed in the upper part of the crural cistern. B Exposure of the crural cistern after resection of the uncus. After resection of the uncus, the inferior choroidal point (**) and cerebral peduncles are fully exposed. C Inferior choroidal point, crural and ambiens cistern. Close-up view of Fig. 15b. The ACha and inferior ventricular vein run through the inferior choroidal point (*). From cranial to caudal, the optic tract, basal vein and ACha are observed at the roof of the crural cistern. The lateral mesencephalic sulcus (**) is exposed in the lateral aspect of the midbrain at the level of the inferior choroidal point. AChA anterior choroidal artery; ICA internal carotid artery; P2a anterior part of the perimesencephalic segment of the posterior cerebral artery; SCA superior cerebellar artery
Adequate exposure of this region requires dissection of the uncus with careful mobilization of its feeding branches originating from the PComA and AChA as well as identification of the oculomotor nerve at its medial surface (Fig. 16). Complete exposure of the cerebral peduncles may require significant medial retraction of the temporal lobe. In such cases, preservation of feeding branches to the uncus may not be possible, and ligation of those vessels associated or not with uncus resection improves the surgical exposure (Fig. 16b). It is paramount to avoid damage to the AChA branches that supply the optic tract and internal capsule. Use of a 30 degree endoscope pointed upward allows visualization of the roof of the crural cistern, with exposure of the optic tract and geniculate bodies (Fig. 16c), while an inferiorly pointed 30 degree endoscope demonstrate the oculomotor nerve and oculomotor triangle in the roof of the cavernous sinus, tentorial edge, supraclinoid internal carotid artery and origin of the PcomA and AChA (Fig. 17).
-
5.
Dura repair
Oculomotor nerve and interpeduncular cistern. Exposure of the carotid and interpeduncular cisterns with a 30-degree endoscope pointing downwards. a The oculomotor exits the interpeduncular fossa between the posterior cerebral artery and SCA, and it runs into the oculomotor triangle in the posterior part of the roof of the cavernous sinus. b Close-up view of Fig. 16a. AChA anterior choroidal artery; ICA internal carotid artery; PcomA posterior communicating artery; P2a anterior part of the perimesencephalic segment of the posterior cerebral artery; SCA superior cerebellar artery
Closure of the dural defect was done using fascia temporalis grafts (Fig. 18) inserted in two layers as inlay and outlay grafts. These can be sutured together in the middle as a “button” graft [43]. As in endoscopic endonasal surgery, the grafts must be larger than the dural defect and cover its borders entirely. The pressure of the orbit over the dura reconstruction keeps the grafts in place.
Discussion
Transorbital endoscopic surgery
Surgical approaches through and around the orbit have been implemented since the early twentieth century. In 1913, Frazier described the orbital roof craniotomy technique [28], which was later modified and performed via an eyebrow incision [1, 20, 34, 53, 61]. More recently, eyelid approaches to the anterior cranial base have also been proposed but generally in an “upward” trajectory toward the roof of the orbit and basal frontal lobe [3, 51].
However, the concept of pure transorbital endoscopic approaches to a variety of locations around the skull base was only proposed in 2010 by Moe et al. [44]. Without the need to remove the orbital rim, this might be considered the first description of a “true” transorbital approach [58]. As proposed by the authors, the orbit can be divided into four different quadrants, each with its specific approach: medial (precaruncular approach), superior (superior eyelid approach), lateral (lateral retrocanthal approach) and inferior (preseptal lower eyelid). Superior and lateral approaches, as the one performed in our study, allow exposure of the anterior and middle cranial fossae and superior orbital fissure [8, 44].
The transorbital endoscopic approach was initially developed as an alternative route for endoscopic treatment of skull base lesions [19, 42, 44]. As a natural corridor, the orbits have been used as an additional route to different skull base regions. Previous anatomical studies have demonstrated the utility of these approaches to reach multiple skull base structures, including the lateral wall of the cavernous sinus [2, 8, 18], Meckel’s cave [26], the petrous carotid [26] and optic nerve [22]. In this study, we expand the previously described targets to include the Sylvian fissure, middle cerebral artery and the crural cistern and its contents.
Intradural dissection via the transorbital endoscopic approach
The potential role of transorbital endoscopic surgery to the approach to intradural pathologies has been scarcely evaluated, with a single anatomical study published about it so far [14]. Chen et al. reported the feasibility of selective amygdalohippocampectomy via a transorbital endoscopic approach using a superior eyelid approach [14]. The authors exposed the temporal pole and performed a limited corticetomy directed to the temporal horn. With neuronavigation guidance, the amygdala and hippocampus were identified and resected, without major restrictions.
In this study, we demonstrate that the sylvian fissure is also readily accessible via a transorbital endoscopic approach. Guided by anatomical landmarks, such as the SOF and IOF, tailored bone resection and dural opening can be performed. Using an endoscope holder, bimanual dissection and careful dissection of the arachnoid membranes allow opening of the sylvian cistern. Unlike transcortical approaches, transcisternal dissection requires manipulation of veins and arterial branches that may cross from one side to the other as well as eventual coagulation and ligation of those vessels. Dissection should be performed following standard microsurgical principles using knives, microscissors and aspirators; classic endoscopic endonasal maneuvers, such as the two-aspirator technique and retraction using curettes may be implemented for resection of intra-axial lesions, but should be avoided during transcisternal dissection to reduce trauma to the normal surrounding parenchyma.
Potential benefits and clinical applications
As demonstrated in our study, the sylvian fissure and its surroundings, including the basal frontal lobe and temporal lobe, are adequately exposed via a transorbital endoscopic approach. Benefits of this approach include improved cosmetic results, lack of temporalis muscle dissection and the resulting atrophy, reduced brain retraction and excellent visualization. Using an oculoplastic surgeon for the opening and closing may further improve the cosmetic results.
Unlike classic lateral craniotomies, the transorbital endoscopic approach does not require scalp incisions or manipulations of the temporalis muscle. Those approaches to the sylvian region require temporalis muscle dissection, which may lead to muscle atrophy and cosmetic deformities [63]. Additionally, frontalis muscle palsy due to injury of the frontotemporal branches of the facial nerve may follow after lateral craniotomies [64]. Minimally invasive microsurgical approaches, such as eyelid [3] and supraorbital eyebrow [35] craniotomies, share the cosmetic benefits of the transorbital endoscopic approach and also result in superior cosmetic outcomes when compared to classic approaches [3, 16, 17].
The anterior view of the sphenoid segment of the sylvian fissure and frontal and temporal lobes achieved via the transorbital approach allows adequate exposure of this region with reduced brain retraction. If the surgical target is located in the frontal lobe or temporal pole, surgery may proceed with almost no brain manipulation; however, successful dissection of the sylvian fissure will require careful retraction of the surrounding parenchyma. The frontoorbitozygomatic approach allows similar visualization of the anterior and lateral aspects of the frontal and temporal lobes, but requires a large craniotomy followed by extensive drilling of the skull base, which may lead to postoperative deformities if not adequately performed.
Potential candidates for transorbital endoscopic surgery include not only extradural tumors with intradural extension and intradural lesions, but also vascular lesions in the sylvian region such as MCA aneurysms. Aneurysms of the P2 segment in the crural cistern are also visible. Brainstem cavernous malformations that come to the pial surface in the crural cistern may also be approached in this manner. Resection of spheno-orbital meningiomas via this approach has already been reported with successful results and no major complications [16, 44, 50]. Intradural lesions, such as frontobasal, temporal and insular gliomas and mesial temporal sclerosis, may be approached through this technique. To this point, a single study reported the use of endoscopic-guided transorbital surgery for amydalahyppocampectomy in two patients [13]. Although the authors report the use of microscopes in the initial part of the procedure, this report demonstrates the clinical feasibility of resection of intra-axial lesions through transorbital endoscopic-guided approaches.
Vascular lesions are special challenges for endoscopic approaches. Despite this, previous reports demonstrated the effectiveness of different endoscopic approaches in selected cases. Transcranial endoscopic-assisted microneurosurgery, through supraorbital craniotomies [27, 33, 52], has been reported as an effective option for surgical clipping of anterior circulation aneurysms. Additionally, an endoscopic endonasal approach for clipping of aneurysms [30, 32, 48, 60] and resection of cavernomas [15, 31, 38, 45] has been advocated by groups with large experience in endoscopic skull base surgery, with successful results in selected cases. The experience of those groups reinforces the potential of transorbital endoscopic surgery. As shown in our anatomical study, the transorbital endoscopic approach is a feasible option for exposure of the sylvian fissure, supraclinoidal ICA, ICA bifurcation and middle cerebral artery and its branches. Therefore, through careful dissection, exposure and clipping of MCA bifurcation aneurysms may be feasible via this technique, and the proximal to distal exposure provides excellent proximal control. Aneurysms originating in other locations such as the ICA bifurcation and the origin of the AChA and PcomA may be reached, but may demand larger craniectomies and more complex intradural dissection. Moreover, distal MCA aneurysms may be more easily exposed through a lateral craniotomy. Cavernomas located in the basal frontal lobe, anterior part of the insula, superior anterolateral brainstem or temporal pole are potential candidates for transorbital resection due to their superficial location and adequate exposure after a successful approach.
Limitations of the technique
Common concerns related with the transorbital endoscopic approach are postoperative CSF leaks and orbital damage. Clinical results are limited, and it is difficult to draw definitive conclusions regarding these complications. So far, however, CSF leaks have not been reported as a major complication [7, 16, 40, 55]. To this point, the fascia lata has been effectively used to repair transorbital dural defects in most reports. The presence of the orbit and its contents keeps the skull base reconstruction in place and may justify the lower CSF rates of transorbital compared to extended endoscopic approaches. Data regarding the results of transorbital surgery for intra-axial pathology is even scarcer. A recent study by Chen et al. [13] reports the results of two patients submitted to amygdalohippocampectomy via an endoscopic transorbital approach. In those cases, external ventricular drains were inserted before the procedure, the dura was loosely reapproximated, and a free local tissue graft was inserted. Although no major CSF leak was reported, one of the patients developed an orbital pseudomeningocele, which was treated with insertion of a ventriculoperitoneal shunt.
To our knowledge, significant orbital damage and visual loss have not been reported to date [7, 16, 55]. However, it is a real risk and needs to be addressed. The technique used in our article included an endoscopic endonasal medial orbit decompression prior to the transorbital dissection. It is important to state that the decision to perform the current anatomical study in such way was based on our initial clinical experience with two spheno-orbital meningiomas operated on via the transorbital endoscopic approach (not published but accepted for publication). At this stage of our surgical experience with the transorbital endoscopic approach, the addition of endoscopic endonasal decompression of the optic canal and lamina papyracea seems useful to facilitate medial retraction of the orbit and mobilization of the optic nerve to enlarge the surgical corridor and to avoid significant increases in intraorbital pressure. However, we believe that as expertise grows, a pure transorbital endoscopic approach probably will be sufficient. Additionally to the dissections described in the study, we performed two transorbital approaches without the medial decompression. In those cases, there was no significant difference in the size of the surgical corridors (approximately 1.5 cm). Although it has been effectively done in the laboratory, this may be more complex in the clinical scenario, since less bone removal may imply more orbital mobilization. Certainly, more gentle and careful manipulation of the orbit will be required to avoid visual injuries, especially in long cases.
Intradural transorbital endoscopic dissection adds a significant challenge to the surgeon, who must be aware of the limitations of this technique. More common “minimally invasive” transcranial approaches, such as the lateral supraorbital (LSO), may be used to reach similar anatomical targets, and a critical comparison needs to be done. First, the transorbital endoscopic approach requires comprehensive knowledge of skull base anatomy through a new surgical perspective. Second, the cisternal dissection and mobilization of arteries and veins are performed in a limited corridor, which demands previous experience with endoscopic skull base techniques and training in an anatomical laboratory. Lastly, intradural vascular complications may be troublesome. Although easily performed in an anatomical study, manipulation of intracranial vessels during surgery may lead to intraoperative hemorrhages secondary to arterial or venous damage that may be hard to control. As in endoscopic endonasal surgery, coordination with an assistant surgeon to keep the endoscope lens clean during hemostasis is paramount. The surgical corridor obtained with the approach should be adequate for application of temporary clipping, compression with cotton balls and coagulation with bipolar electrocautery whenever necessary. When an LSO approach is selected, wider exposure and 3D vision provided by the surgical microscope are available; besides, the anatomy is observed through a more common anterior-lateral perspective. However, this approach may not adequately expose the inferomedial temporal lobe region and retrosellar area [54] and may lead to paralysis of the frontotemporal branch of the facial nerve and cosmetic deformities secondary to the frontal craniotomy.
Study limitations
Results from our study represent the findings obtained during surgical dissections performed in a fully equipped skull base anatomical laboratory. Therefore, our surgical anatomy results do not necessarily represent the exact characteristics observed in real-life surgery. Continuous drainage of the CSF, eventual intradural hemorrhages and brain herniation into the surgical corridor may limit the benefits of this approach. Additionally, we tried our best to analyze the anatomical characteristics of the sylvian fissure and its surroundings as well as the potential pros and cons of the technique for treatment of pathologies in this region. Since clinical experience with this route is still in its early phase, only future clinical studies may prove whether our results have an application in the clinical scenario.
Conclusions
The transorbital endoscopic approach allows successful exposure of the sphenoidal portion of the sylvian fissure and M1 and M2 segments of the middle cerebral artery. After careful dissection, the carotid cistern and its components including the ICA bifurcation, A1 and M1 origin and perforating branches, can be exposed. Angled endoscopes may provide visualization of mesial temporal lobe structures and interpeduncular and crural cisterns. Besides the usual concerns related with the transorbital endoscopic approach, a limited surgical window for intradural bimanual dissection, potentially hard to control hemorrhages and lack of adequate surgical tools are some of the limitations of the technique. Although our anatomical study demonstrates the feasibility of intradural dissection via a transorbital endoscopic approach, further studies are necessary to evaluate its role in clinical situations.
References
Al-Mefty O (1987) Supraorbital-pterional approach to skull base lesions. Neurosurgery 21:474–477
Alqahtani A, Padoan G, Segnini G, Lepera D, Fortunato S, Dallan I, Pistochini A, Abdulrahman S, Abbate V, Hirt B, Castelnuovo P (2015) Transorbital transnasal endoscopic combined approach to the anterior and middle skull base: a laboratory investigation. Acta Otorhinolaryngol Ital 35:173–179
Andaluz N, Romano A, Reddy LV, Zuccarello M (2008) Eyelid approach to the anterior cranial base. J Neurosurg 109:341–346
Andaluz N, Zuccarello M (2014) Supraorbital and transorbital minicraniotomies. Response J Neurosurg 121:1291–1293
Banu MA, Guerrero-Maldonado A, McCrea HJ, Garcia-Navarro V, Souweidane MM, Anand VK, Heier L, Schwartz TH, Greenfield JP (2014) Impact of skull base development on endonasal endoscopic surgical corridors. J Neurosurg Pediatr 13:155–169
Banu MA, Kim JH, Shin BJ, Woodworth GF, Anand VK, Schwartz TH (2014) Low-dose intrathecal fluorescein and etiology-based graft choice in endoscopic endonasal closure of CSF leaks. Clin Neurol Neurosurg 116:28–34
Bly RA, Morton RP, Kim LJ, Moe KS (2014) Tension pneumocephalus after endoscopic sinus surgery: a technical report of multiportal endoscopic skull base repair. Otolaryngology—head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery 151:1081–1083
Bly RA, Ramakrishna R, Ferreira M, Moe KS (2014) Lateral transorbital neuroendoscopic approach to the lateral cavernous sinus. Journal of Neurological Surgery. Part B, Skull base 75:11–17
Cappabianca P, Cavallo LM, Esposito F, De Divitiis O, Messina A, De Divitiis E (2008) Extended endoscopic endonasal approach to the midline skull base: the evolving role of transsphenoidal surgery. Adv Tech Stand Neurosurg 33:151–199
Cappabianca P, Schwartz TH, Jane JA, Jr., M D, Zada G (2014) Introduction: Endoscopic endonasal skull base surgery. Neurosurgl Focus 37.Introduction
Castelnuovo P, Dallan I, Battaglia P, Bignami M (2010) Endoscopic endonasal skull base surgery: past, present and future. Eur Arch Otorhinolaryngol 267:649–663
Cavallo LM, Messina A, Gardner P, Esposito F, Kassam AB, Cappabianca P, de Divitiis E, Tschabitscher M (2005) Extended endoscopic endonasal approach to the pterygopalatine fossa: anatomical study and clinical considerations. Neurosurg Focus 19:E5
Chen HI, Bohman LE, Emery L, Martinez-Lage M, Richardson AG, Davis KA, Pollard JR, Litt B, Gausas RE, Lucas TH (2015) Lateral transorbital endoscopic access to the hippocampus, amygdala, and entorhinal cortex: initial clinical experience. ORL J Otorhinolaryngol Relat Spec 77:321–332
Chen HI, Bohman LE, Loevner LA, Lucas TH (2014) Transorbital endoscopic amygdalohippocampectomy: a feasibility investigation. J Neurosurg 120:1428–1436
Dallan I, Battaglia P, de Notaris M, Caniglia M, Turri-Zanoni M (2015) Endoscopic endonasal transclival approach to a pontine cavernous malformation: case report. Int J Pediatr Otorhinolaryngol 79:1584–1588
Dallan I, Castelnuovo P, Locatelli D, Turri-Zanoni M, AlQahtani A, Battaglia P, Hirt B, Sellari-Franceschini S (2015) Multiportal combined transorbital transnasal endoscopic approach for the management of selected skull base lesions: preliminary experience. World Neurosurg 84:97–107
Dallan I, Castelnuovo P, Turri-Zanoni M, Fiacchini G, Locatelli D, Battaglia P, Sellari-Franceschini S (2016) Transorbital endoscopic assisted management of intraorbital lesions: lessons learned from our first 9 cases. Rhinology 54:247–253
Dallan I, Di Somma A, Prats-Galino A, Solari D, Alobid I, Turri-Zanoni M, Fiacchini G, Castelnuovo P, Catapano G, de Notaris M (2016) Endoscopic transorbital route to the cavernous sinus through the meningo-orbital band: a descriptive anatomical study. J Neurosurg:1–8
Dallan I, Locatelli D, Turri-Zanoni M, Battaglia P, Lepera D, Galante N, Sellari-Franceschini S, Castelnuovo P (2015) Transorbital endoscopic assisted resection of a superior orbital fissure cavernous haemangioma: a technical case report. Eur Arch Otorhinolaryngol 272:3851–3856
Delashaw JB Jr, Jane JA, Kassell NF, Luce C (1993) Supraorbital craniotomy by fracture of the anterior orbital roof. Tech Note J Neurosurg 79:615–618
Delashaw JB Jr, Tedeschi H, Rhoton AL (1992) Modified supraorbital craniotomy: technical note. Neurosurgery 30:954–956
Di Somma A, Cavallo LM, de Notaris M, Solari D, Topczewski TE, Bernal-Sprekelsen M, Ensenat J, Prats-Galino A, Cappabianca P (2016) Endoscopic endonasal medial-to-lateral and transorbital lateral-to-medial optic nerve decompression: an anatomical study with surgical implications. J Neurosurg:1–10
Ditzel Filho LF, Prevedello DM, Jamshidi AO, Dolci RL, Kerr EE, Campbell R, Otto BA, Carrau RL, Kassam A (2015) Endoscopic endonasal approach for removal of tuberculum sellae meningiomas. Neurosurg Clin N Am 26:349–361
Dlouhy BJ, Chae MP, Teo C (2015) The supraorbital eyebrow approach in children: clinical outcomes, cosmetic results, and complications. J Neurosurg Pediatr 15:12–19
Fernandez-Miranda JC, Prevedello DM, Gardner P, Carrau R, Snyderman CH, Kassam AB (2010) Endonasal endoscopic pituitary surgery: is it a matter of fashion? Acta Neurochir 152:1281–1282 author reply 1282
Ferrari M, Schreiber A, Mattavelli D, Belotti F, Rampinelli V, Lancini D, Doglietto F, Fontanella MM, Tschabitscher M, Rodella LF, Nicolai P (2016) The inferolateral Transorbital endoscopic approach: a preclinical anatomic study. World Neurosurg 90:403–413
Fischer G, Stadie A, Reisch R, Hopf NJ, Fries G, Bocher-Schwarz H, van Lindert E, Ungersbock K, Knosp E, Oertel J, Perneczky A (2011) The keyhole concept in aneurysm surgery: results of the past 20 years. Neurosurgery 68:45–51 discussion 51
Frazier CH (1913) I. An approach to the hypophysis through the anterior cranial fossa. Ann Surg 57:145–150
Gardner PA, Prevedello DM, Kassam AB, Snyderman CH, Carrau RL, Mintz AH (2008) The evolution of the endonasal approach for craniopharyngiomas. J Neurosurg 108:1043–1047
Gardner PA, Vaz-Guimaraes F, Jankowitz B, Koutourousiou M, Fernandez-Miranda JC, Wang EW, Snyderman CH (2015) Endoscopic endonasal clipping of intracranial aneurysms: surgical technique and results. World Neurosurg 84:1380–1393
He SM, Wang Y, Zhao TZ, Zheng T, Lv WH, Zhao LF, Chen L, Sterling C, Qu Y, Gao GD (2016) Endoscopic endonasal approach to mesencephalic cavernous malformations. World Neurosurg 90:701 e707–701 e710
Heiferman DM, Somasundaram A, Alvarado AJ, Zanation AM, Pittman AL, Germanwala AV (2015) The endonasal approach for treatment of cerebral aneurysms: a critical review of the literature. Clin Neurol Neurosurg 134:91–97
Ho CL, Hwang PY (2015) Endoscope-assisted transorbital keyhole surgical approach to ruptured supratentorial aneurysms. Journal of Neurological Surgery. Part A, Central European Neurosurgery 76:376–383
Jane JA, Park TS, Pobereskin LH, Winn HR, Butler AB (1982) The supraorbital approach: technical note. Neurosurgery 11:537–542
Jho HD (1997) Orbital roof craniotomy via an eyebrow incision: a simplified anterior skull base approach. Minimally invasive neurosurgery: MIN 40:91–97
Kasemsiri P, Carrau RL, Ditzel Filho LF, Prevedello DM, Otto BA, Old M, de Lara D, Kassam AB (2014) Advantages and limitations of endoscopic endonasal approaches to the skull base. World Neurosurg 82:S12–S21
Khan OH, Anand VK, Schwartz TH (2014) Endoscopic endonasal resection of skull base meningiomas: the significance of a “cortical cuff” and brain edema compared with careful case selection and surgical experience in predicting morbidity and extent of resection. Neurosurg Focus 37:E7
Kimball MM, Lewis SB, Werning JW, Mocco JD (2012) Resection of a pontine cavernous malformation via an endoscopic endonasal approach: a case report. Neurosurgery 71:186–193 discussion 193-184
Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH (2013) Endoscopic endonasal compared with anterior craniofacial and combined cranionasal resection of esthesioneuroblastomas. World Neurosurg 80:148–159
Koutourousiou M, Gardner PA, Stefko ST, Paluzzi A, Fernandez-Miranda JC, Snyderman CH, Maroon JC (2012) Combined endoscopic endonasal transorbital approach with transconjunctival-medial orbitotomy for excisional biopsy of the optic nerve: technical note. Journal of Neurological Surgery Reports 73:52–56
Lee DL, McCoul ED, Anand VK, Schwartz TH (2012) Endoscopic endonasal access to the jugular foramen: defining the surgical approach. Journal of Neurological Surgery. Part B, Skull base 73:342–351
Locatelli D, Pozzi F, Turri-Zanoni M, Battaglia P, Santi L, Dallan I, Castelnuovo P (2016) Transorbital endoscopic approaches to the skull base: current concepts and future perspectives. J Neurosurg Sci 60:514–525
Luginbuhl AJ, Campbell PG, Evans J, Rosen M (2010) Endoscopic repair of high-flow cranial base defects using a bilayer button. Laryngoscope 120:876–880
Moe KS, Bergeron CM, Ellenbogen RG (2010) Transorbital neuroendoscopic surgery. Neurosurgery 67:ons16–ons28
Nayak NR, Thawani JP, Sanborn MR, Storm PB, Lee JY (2015) Endoscopic approaches to brainstem cavernous malformations: case series and review of the literature. Surg Neurol Int 6:68
Ottenhausen M, Banu MA, Placantonakis DG, Tsiouris AJ, Khan OH, Anand VK, Schwartz TH (2014) Endoscopic endonasal resection of suprasellar meningiomas: the importance of case selection and experience in determining extent of resection, visual improvement, and complications. World Neurosurg 82:442–449
Patel KS, Komotar RJ, Szentirmai O, Moussazadeh N, Raper DM, Starke RM, Anand VK, Schwartz TH (2013) Case-specific protocol to reduce cerebrospinal fluid leakage after endonasal endoscopic surgery. J Neurosurg 119:661–668
Peris-Celda M, Da Roz L, Monroy-Sosa A, Morishita T, Rhoton AL Jr (2014) Surgical anatomy of endoscope-assisted approaches to common aneurysm sites. Neurosurgery 10(Suppl 1):121–144 discussion 144
Prevedello DM, Ditzel Filho LF, Solari D, Carrau RL, Kassam AB (2010) Expanded endonasal approaches to middle cranial fossa and posterior fossa tumors. Neurosurg Clin N Am 21:621–635 vi
Ramakrishna R, Kim LJ, Bly RA, Moe K, Ferreira M Jr (2016) Transorbital neuroendoscopic surgery for the treatment of skull base lesions. J Clin Neurosci 24:99–104
Raza SM, Quinones-Hinojosa A, Lim M, Boahene KD (2013) The transconjunctival transorbital approach: a keyhole approach to the midline anterior skull base. World Neurosurg 80:864–871
Reisch R, Fischer G, Stadie A, Kockro R, Cesnulis E, Hopf N (2014) The supraorbital endoscopic approach for aneurysms. World Neurosurg 82:S130–S137
Reisch R, Perneczky A, Filippi R (2003) Surgical technique of the supraorbital key-hole craniotomy. Surg Neurol 59:223–227
Salma A, Alkandari A, Sammet S, Ammirati M (2011) Lateral supraorbital approach vs pterional approach: an anatomic qualitative and quantitative evaluation. Neurosurgery 68:364–372 discussion 371-362
Schaberg M, Murchison AP, Rosen MR, Evans JJ, Bilyk JR (2011) Transorbital and transnasal endoscopic repair of a meningoencephalocele. Orbit 30:221–225
Snyderman CH, Carrau RL, Kassam AB, Zanation A, Prevedello D, Gardner P, Mintz A (2008) Endoscopic skull base surgery: principles of endonasal oncological surgery. J Surg Oncol 97:658–664
Snyderman CH, Pant H, Carrau RL, Prevedello D, Gardner P, Kassam AB (2009) What are the limits of endoscopic sinus surgery?: the expanded endonasal approach to the skull base. The Keio Journal of Medicine 58:152–160
Sonig A, Nanda A (2013) Transorbital approach to the anterior cranial skull base. World Neurosurg 80:810–812
Srinivasan VM, Kan P, Germanwala AV, Pelargos P, Bohnen A, Choy W, Yang I, Smith ZA (2016) Key perspectives on woven EndoBridge device for wide-necked bifurcation aneurysms, endoscopic endonasal clipping of intracranial aneurysms, retrosigmoid versus translabyrinthine approaches for acoustic neuromas, and impact of local intraoperative steroid administration on postoperative dysphagia following anterior cervical discectomy and fusion. Surg Neurol Int 7:S720–S724
Szentirmai O, Hong Y, Mascarenhas L, Salek AA, Stieg PE, Anand VK, Cohen-Gadol AA, Schwartz TH (2016) Endoscopic endonasal clip ligation of cerebral aneurysms: an anatomical feasibility study and future directions. J Neurosurg 124:463–468
van Lindert E, Perneczky A, Fries G, Pierangeli E (1998) The supraorbital keyhole approach to supratentorial aneurysms: concept and technique. Surg Neurol 49:481–489 discussion 489-490
Wilson DA, Duong H, Teo C, Kelly DF (2014) The supraorbital endoscopic approach for tumors. World Neurosurg 82:S72–S80
Yasuda CL, Costa AL, Franca M Jr, Pereira FR, Tedeschi H, de Oliveira E, Cendes F (2010) Postcraniotomy temporalis muscle atrophy: a clinical, magnetic resonance imaging volumetry and electromyographic investigation. J Orofac Pain 24:391–397
Youssef AS, Ahmadian A, Ramos E, Vale F, van Loveren HR (2012) Combined subgaleal/myocutaneous technique for temporalis muscle dissection. Journal of Neurological Surgery. Part B, Skull base 73:387–393
Zada G (2014) Editorial: the endoscopic keyhole supraorbital approach. Neurosurg Focus 37:E21
Zhang M, Singh H, Almodovar-Mercado GJ, Anand VK, Schwartz TH (2016) Required reading: the most impactful articles in endoscopic endonasal skull base surgery. World Neurosurg 92(499–512):e492
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Almeida, J.P., Ruiz-Treviño, A.S., Shetty, S.R. et al. Transorbital endoscopic approach for exposure of the sylvian fissure, middle cerebral artery and crural cistern: an anatomical study. Acta Neurochir 159, 1893–1907 (2017). https://doi.org/10.1007/s00701-017-3296-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-017-3296-8