Abstract
Background
Tumors of the middle fossa or cavernous sinus (CS), or intraorbital tumors, can penetrate each other through the superior orbital fissure (SOF) or neighboring tissue. These complicated pathologies are often treated with highly invasive surgical procedures. In this article, we demonstrate surgical anatomic dissections of the CS, SOF, orbital apex (OA), and dura mater extending to the periorbita from the middle fossa, by performing an epidural dissection via a lateral orbitotomy approach, and discuss findings that may provide guidance during surgery in these regions.
Methods
Lateral orbitotomy was performed on latex-injected cadaver heads by making a 2-cm skin incision lateral to the lateral canthus, drilling the lesser and greater sphenoid wings that form the SOF borders, and removing the bone section between the middle fossa and orbit. Dura mater from the middle fossa to the periorbita was exposed to perform anterior clinoidectomy. Meningeal dura was dissected from the endosteal dura, which forms the lateral wall of the CS, to expose the CS, SOF, and OA for dissections.
Results
Changing the orientation of the microscope from posterior to anterior enabled regional control for dissection from the Gasserian ganglion to the OA. Cranial nerves that pass through the CS, SOF, and OA were dissected and exposed. The annular tendon was opened, revealing the oculomotor nerves and its branches, as well as the abducens and nasociliary nerves, which pass through the oculomotor foramen and course within the OA and orbit.
Conclusions
This approach causes less tissue damage; provides control of the surgical area in spheno-orbital tumors invading the fissure and foramen by changing the orientation of the microscope toward the orbit, OA, SOF, CS, and middle fossa; and expands the indication criteria for lateral orbitotomy surgery. This approach, therefore, represents an alternative surgical method for excising complicated tumors in these regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cranial surgical approaches should be planned according to the location, size, and anatomical relation of the intracranial pathologies, and the intervention should be the least invasive technique possible that provides the greatest anatomical control [1, 2, 11, 13, 19]. Modern neurosurgery targets the best possible surgical outcome with minimal tissue damage and maximum patient satisfaction [1, 21]. Advances in technology and microsurgical techniques, and a combination of intervention routes or their modifications, have increased the number of possible alternatives to access skull base pathologies.
Middle fossa tumors can invade the orbit directly through the neighboring tissue or cavernous sinus (CS), optic canal, or superior orbital fissure (SOF) [24]. A transcranial approach is preferred for middle fossa tumors, whereas transcranial or orbital approaches are preferred for tumors located in the orbit and orbital apex (OA) [3, 13–16, 18, 20, 24, 25]. Minimally invasive cranial approaches with keyhole craniotomy were introduced to neurosurgical practice in the 1990s for aneurysm surgery [9, 19, 26, 27]. Pernezcky and Van Lindert [19, 27] introduced supraorbital keyhole craniotomy, and Jho [11] introduced orbital roof craniotomy, which are common minimally invasive interventions used for anterior cranial fossa surgery. However, minimally invasive interventions for the middle fossa have recently been reported [2, 13].
Lateral orbitotomy, which is a more straightforward and less-invasive method than the transcranial approach, is primarily used for intraorbital and retrobulbar lesion surgeries [14, 15]. Maroon and Kennerdel [14] reported in 1976 that it is possible to expose the temporal dura by drilling the greater sphenoid wing to achieve better control over the orbit in lateral orbitotomies performed for intraorbital tumors. It has been shown in recent years that the CS can be reached by removing the lateral orbital wall and drilling in the greater sphenoid wing; using this approach, sphenoid wing tumors penetrating to the orbit can be operated on without invading the SOF or anterior clinoid (AC) [2, 13]. However, to the best of our knowledge, no previous anatomic study has demonstrated whether minimally invasive approaches can be used for tumors invading both the middle fossa and the SOF or OA.
We hypothesized that by using a lateral orbitotomy as a minimal invasive approach, both the middle fossa and the CS, SOF, OA, and orbital regions can become accessible by changing the orientation of the microscope. Using surgical anatomical dissections, we demonstrated that the region from the Gasserian ganglion to the OA, and the intraorbital structures can be accessed surgically by performing a step-by-step extradural dissection, which have already been displayed by Dolenc [4–8] with lateral orbitotomy.
Materials and methods
Materials
This study was conducted using five adult cadaver heads that were injected with latex and fixed in formalin solution in the Albert L. Rhoton anatomy laboratory of Bahçeşehir University (Istanbul, Turkey). Dissections were made by microsurgical tools and surgical microscope (Leica M530 OH6, Leica Microsystems Wetzlar, Germany).
Surgical procedure
Skin incision, lateral orbitotomy, and drilling
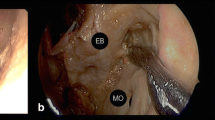
The heads were fixed at a 45-degree angle. We used the lateral orbital wall approach procedure described by Altay et al. to access the CS [2]. A 2-cm linear incision was made laterally from the level of the lateral epicanthus (Fig. 1a). First, the lateral rim of the periosteum of the orbit was dissected to expose the junction of the zygomatic arch inferior to the lateral orbital rim and superior to the frontozygomatic suture (Figs. 1b and 2a). Next, traction was exerted to the temporal muscle until the pterion area was exposed by lateral dissection (Fig. 1c). The intraorbital part of the lateral orbital wall was dissected toward the SOF without harming the periorbital tissue. However, to avoid exerting traction to the orbit, we did not try to expose the SOF (Fig. 2b and c). The 2-cm lateral orbital rim section between the frontozygomatic suture and the region close to the site where the zygomatic arch joins with the lateral orbital rim was cut using a high-speed drill (Fig. 3a and b). After the obstacle created by the concavity of the lateral orbital rim was removed, the periorbital dissection progressed toward the lateral border of the SOF without exerting traction on the periorbita. The frontal dura was exposed by drilling the lateral wall of the posterior orbit. The temporal dura and sphenoid ridge, which is located between the temporal and frontal dura, were exposed by drilling the greater sphenoid wing starting from the orbitotemporal junction (Fig. 3c and d). To reach the floor of the middle fossa and the Gasserian ganglion, it was necessary to drill the greater sphenoid wing 0.5–1 cm further toward the underside of the zygomatic arch. Thus, we created a space that enabled dissection from the lateral part of the orbit to the floor of the middle fossa.
For the left lateral orbitotomy, the zygomaticofacial foramina, from which the zygomaticofacial and zygomaticotemporal nerves exit, was exposed following periosteum dissection. The periorbita was gently dissected and slight traction was exerted to reveal the entrance of the zygomaticofacial and zygomaticotemporal nerves into the zygomaticofacial foramina [23]. These nerves, which are sensory branches of the zygomatic nerve, needed to be cut to perform the lateral orbitotomy (a and b). Specimen c that have zygomatico-orbital foramina but not zygomaticofacial foramina
After completing adequate dissection of the periosteum and the periorbita, left lateral orbitotomy was performed by cutting a 2-cm section between the frontozygomatic suture and the orbitozygomatic junction while preserving the periorbita (a, b). The great sphenoid wing and lateral orbital wall were drilled until the frontal and temporal dura and the sphenoid ridge in between were exposed (c). The periorbita was dissected toward the lateral wall of the SOF, and slight traction was exerted during drilling of the lateral orbital wall to prevent injury. As the obstacle created by the orbital rim was eliminated, less traction was exerted, and a safe dissection area was created toward the SOF (d)
Lateral orbitotomy margins and bony landmarks
Resected section; From the frontozygomatic suture at the superior to the junction of the zygomatic arch to the lateral orbital rim at the inferior. Areas requiring drilling; between the inferior margin of SOF on the lateral orbital wall and the superior margin of the inferior orbital fissure, orbito-temporal junction of the greater sphenoid wing to access middle fossa (Figs. 3 and 4).
Left lateral orbitotomy and the drilled lateral wall of the posterior orbit with an illustrative drawing. As the periorbita and dura were dissected, the exposed lateral wall of the posterior orbit was removed. Following epidural dissection of the sphenoid ridge, the dural fold is initially exposed. This dura emerges from the temporal dura and blends into the periorbita. Passage of the dura was readily visualized with the nerve hook, which was passed from the lateral edge of the SOF (a). Temporal dural extension coursing from the most lateral border of the SOF and the accompanying meningo-orbital artery and vein were reached without exerting significant traction (a, and arrows in b). In addition to the neurovascular structures passing through the SOF, which is an oblique fissure connecting the middle fossa to the orbit, the extension of the temporal dura passes through the SOF and blends into the periorbita (star in a–c). Thus, a connection between the orbital and middle fossa was provided by the SOF (a–c). The temporal dura, which passes from the lateral edge of SOF, can hide the AC and the proximal part of the sphenoid ridge while forming the fold (star in c). After cutting this dura, the epidural dissection around the sphenoid ridge was continued. Then, we exposed very thin endosteal dura, which forms the lateral wall of the CS, passes through medial border of the SOF along with the nerves, and blends into the periorbita at the level of the OA and AC (d); arrows: meningo-orbital artery and vein; SOF superior orbital fissure; OA orbital apex; CS cavernous sinus; AC anterior clinoid
Epidural dissection
In the epidural area, the temporal and frontal dura were stripped from the bone as described before [4–8, 10, 12]. When the sphenoid ridge and the lateral and inferior wall of the posterior orbit were removed, we were able to access the first dural extension originating from the temporal dura and extending toward the orbit (Fig. 4a–c). To reach the CS, AC, and medial margin of the SOF, this dural extension, which creates the last lateral point of the SOF and includes the meningo-orbital artery, was cut (Fig. 4d). After eliminating the dura connection between the temporal dura and periorbita, epidural dissection was performed toward the temporal floor to expose the CS (Fig. 5a). The temporal dura was carefully dissected from the lateral wall of the CS to the superior border, and the CS was exposed (Fig. 5a–d).
The temporal dura was freed after cutting the dura that blends into the left periorbita. The temporal dura was dissected from the endosteal layer that forms the lateral wall of the CS, starting from the middle fossa floor to the lower part of the CS. The dissection plane between the meningeal and endosteal dura is shown by arrows in a. The AC was completely exposed after dissecting the dura and removing the lateral and inferior section of the SOF. The trochlear and ophthalmic nerves, which course along the lateral wall of the CS, as well as the endosteal dura, became visible (b). When the microscope was oriented superior to the CS, dissection of the meningeal dura was advanced, revealing entry of the oculomotor nerve superior to the CS and its close relation with the AC (c). When the microscope was oriented to the SOF, the anterior parts of the CS, SOF, and AC were completely exposed. When epidural dissection was continued over the frontal side of the AC, the extradural optic nerve was revealed. Advancing the dissection medially, the oculomotor nerve medial to the SOF, and trochlear and ophthalmic nerves that course along the lateral wall of the CS, can be seen better (d); CN II optic nerve; CN III oculomotor nerve; CN IV trochlear nerve; V 1 ophthalmic nerve; AC anterior clinoid; ICA internal carotid artery; SOF superior orbital fissure
Anterior clinoidectomy, orbital apex, and middle fossa
The lesser sphenoid wing, which forms the superior border of the SOF, was drilled toward the AC. The proximal part of the lesser sphenoid wing was dissected from the epidural area, and the AC was exposed. Next, the sphenoid ridge was thinned toward the optic canal by drilling, and the connection with the optic strut was freed prior to extradural anterior clinoidectomy. Thus, all of the CS as well as the clinoid triangle, clinoid segment of the internal carotid artery (ICA), and distal and proximal rings surrounding the ICA were exposed (Fig. 6a–c). When we changed the orientation of the microscope from posterior to anterior, the SOF and OA appeared in the field-of-view for dissection. It was necessary to cut the annular tendon to enable dissection of the OA and oculomotor foramen. Thus, by performing lateral orbitotomy with epidural dissection of the middle fossa, we obtained a surgical dissection area that enabled access to the Gasserian ganglion, CS, and SOF from the epidural area, and to the OA and lateral, inferior, and superior (partially) part of the orbit from the periorbital area.
Optic strut, constituting the lateral wall of the optic canal and optic nerve running beneath AC, was exposed after drilling of AC (a). Clinoidal triangle was exposed after drilling optic strut and AC. The clinoidal triangle corresponds to the carotid collar between the distal and proximal rings formed by the dura separating the AC from the oculomotor nerve, and to the clinoidal segment of the ICA (b–d). Optic nerve attached to ICA in intradural area was dissected off (c). At this stage, when the dura was opened at the level of the distal dural ring, the intradural supraclinoid ICA, anterior cerebral artery, posterior communicating artery, anterior choroidal artery, optic nerve, and oculomotor nerve are readily exposed. AC Anterior clinoid; CN II optic nerve; CN III oculomotor nerve; CN IV trochlear nerve; V 1 ophthalmic nerve; ICA internal carotid artery; SOF superior orbital fissure
After completion of the dissection, the lateral orbital rim was fixed using a mini screw-plaque. When necessary, the lateral and inferior wall of the posterior orbit wall and the drilled greater sphenoid wing can be supported with low-profile mesh or synthetic materials for easy and effective reconstruction.
Results
Anatomical orientations (anterior, posterior, inferior, superior, lateral, and medial) are given relative to the upright positioning of the head.
Anatomic monitoring
With the same epidural dissection steps in the pterional approach [4–6, 10, 12] where direction of approach goes along lesser sphenoidal wing and AC were accessed to the CS, SOF, and OA using lateral orbitotomy by more horizontal direction as a minimal invasive method.
Superior orbital fissure and Dural fold
Following skin incision, we exposed the zygomaticofacial and zygomaticotemporal foramina and superficial sensory nerves which zygomaticofacial and zygomaticotemporal nerves originating from the zygomatic nerve by subcutaneous and periosteal dissection [23] (Fig. 2). These nerves needed to be cut to enable lateral orbitotomy. After performing lateral orbitotomy, the lateral wall of the posterior orbit was drilled, which allowed for easy access to the frontal and temporal dura (Fig. 3c and d). Similarly, we drilled in between the sphenoid ridge to permit access to the lateral border of the SOF (Fig. 4a and b). When the lesser and greater sphenoid bone sections that form the lateral border of the SOF were removed, the lateral part of the dural extension, which blends into the periorbita from the temporal dura and contains the meningo-orbital artery and vein, was exposed (Fig. 4a–c). The inferior wall of the SOF was removed, and the bone curtain between the middle fossa and the orbit was partially removed to completely reveal the temporal dura, which extends to the SOF below the lesser sphenoid wing and blends into the periorbita; this is also known as the dural fold [17] (Fig. 4a–d). Using lateral orbitotomy for dissecting the dural fold, there was no significant traction requirement for the temporal dura or periorbita.
The meningeal and endosteal dura
The dura of the middle fossa divides into two layers toward the CS. One of these layers, the endosteal dura, forms the lateral wall of the CS, whereas the other layer, the meningeal dura, covers the temporal lobe [4, 5, 8, 10, 12, 28]. After cutting the dura that traverses the lateral part of the SOF and blends into the periorbita, the temporal dura was freed and could be dissected easily toward the proximal part of the sphenoid ridge. Dissection of the AC was possible only after this dural connection was cut [5, 8]. The endosteal dura does not create an obstacle at this stage of the dissection because the endosteal layer covers the nerves that pass through the medial part of the SOF and blends into the periorbita at the level of the OA (Figs. 4c and 5a). After removing the inferior section of the SOF, which is created by the greater sphenoid wing up to the medial border of the SOF, we continued the dissection and completely exposed the anterior part of the CS, AC, SOF, and endosteal dura (Fig. 5a–d). Then, the thicker meningeal layer was dissected by stripping the endosteal layer, starting from the inferior border of the CS toward the superior border (Fig. 5a and b). The trochlear and ophthalmic nerves that course along the lateral wall of the CS were observable at this stage.
The lateral wall of the cavernous sinus
We continued dissection of the dura with the microscopic view oriented toward the superior CS, during which the entry point of the oculomotor nerve to the CS roof and its close relation with the AC were revealed (Fig. 5b and c). After dissecting the meningeal layer up to the superior border of the CS, the CS became entirely visible starting from the posterior SOF. It was observed that the oculomotor nerve enters into the roof of the CS. It was also observed that the trochlear nerve, which is inferior to the oculomotor nerve and superior to the ophthalmic nerve, courses toward the SOF along the lateral wall of the CS together with the ophthalmic nerve (Fig. 5c and d).
The anterior clinoid, clinoidal triangle, and carotid collar
The epidural dissection was continued toward the frontal side over the AC, and the extradural optic nerve was exposed (Fig. 5d). Advancing the dissection medially, the oculomotor nerve medial to the SOF, and trochlear and ophthalmic nerves that course along the lateral wall of the CS, can be seen better [5, 8, 22, 28]. After dissecting the dura, which engulfed the AC, the AC was freed from the superomedial margin of the optic canal as well as the optic strut (Fig. 6a). After the anterior clinoidectomy was performed, the clinoidal triangle that forms the anterior-superior margin of the CS was revealed (Fig. 6b and c) [5, 8, 28]. The clinoidal triangle corresponds to the carotid collar, which is located between the distal and proximal rings formed by the dura, and which separates the AC from the oculomotor nerve medial to the AC and to the clinoidal segment of the ICA (Fig. 6b–d) [4, 5, 7, 8, 28]. During dissection of the distal dural ring, it was necessary to open the dura. At this stage, the intradural supraclinoid ICA, anterior cerebral artery, posterior communicating artery, anterior choroidal artery, optic nerve, and oculomotor nerve were readily exposed. Intradural dissection was not continued, but it was assumed that continuation would create an exposure that could reach the anterior communicating artery and even the basilar apex (Fig. 6e).
Dissection of the cavernous sinus
Dissection of the CS was performed after anterior clinoidectomy (Fig. 7a–d). To visualize branching of the ophthalmic nerve, which courses via the lower part of the lateral wall of the CS and is formed by coalescing loose nerve fibers, the ophthalmic nerve was dissected. The ophthalmic nerve reveals the nasociliary branch medially, which extends into the annular tendon and oculomotor foramen (Fig. 7b–d). The dissection revealed that the ophthalmic nerve bifurcates to the frontal and lacrimal nerves that enter the SOF along with the trochlear nerve outside the annular tendon (Fig. 7c and d). It was necessary to dissect and pull aside the frontal nerve at the lateral wall of the CS to expose the nasociliary nerve (Fig. 7b–d). The dissection also revealed that, after entering the CS roof, the oculomotor nerve traverses below the trochlear and frontal nerves and extends into the oculomotor foramen inside the annular tendon along with the abducens and nasociliary nerves. To visualize the abducens nerve, which courses lateral to the ICA and is the most medially located cranial nerve inside the CS, it was necessary to dissect the ophthalmic nerve and its branches and to move the nasociliary nerve away from its anatomical position (Fig. 7c and d).
During dissection of the CS from the posterior of the SOF, the trochlear nerve at the lateral wall is observed to extend to the SOF, following a course outside the annular tendon and forming a sickle-like curve below the oculomotor nerve and above the ophthalmic nerve (a). The ophthalmic nerve, which courses along the most inferior part of the lateral wall of the CS, first yields the nasociliary branch medially, which extends into the annular tendon and oculomotor foramen, and then gives rise to the frontal and lacrimal branches that enter the SOF along with the trochlear nerve outside the annular tendon (b and c). The frontal nerve had to be dissected and moved away from the lateral wall to reveal the nasociliary nerve (b). After entry from the roof of the CS, the oculomotor nerve is shown to pass under the trochlear and frontal nerves and extend toward the oculomotor foramen inside the annular tendon along with the abducens and nasociliary nerves. The nasociliary nerve had to be dissected to reveal the abducens nerve that courses lateral to the ICA inside the CS (c and d). CN III oculomotor nerve; CN IV trochlear nerve; V 1 ophthalmic nerve; CN VI abducens nerve; star internal carotid artery
The Gasserian ganglion
After drilling the greater sphenoid wing from the orbitotemporal junction to the underside of the zygomatic arch, the angle of the microscope was oriented posterior to the CS. The dissection was continued posterior to the CS to reveal the Gasserian ganglion and maxillary, mandibular, and proximal ophthalmic nerves (Fig. 8).
An illustrative drawing shows the microscope orientation to the posterior part of the CS (a). After drilling the greater sphenoid wing from the orbitotemporal junction toward the underside of the zygomatic arch, the microscope was directed posteriorly. Epidural dissection was continued from the posterior of the CS, revealing the Gasserian ganglion and ophthalmic, maxillary, and mandibular nerves (b). CN III oculomotor nerve; CN IV trochlear nerve; V 1 ophthalmic nerve; V 2 maxillary nerve; V 3 mandibular nerve
Superior orbital fissure, oculomotor foramen, and orbital apex
The CS and OA were unified by removing the lesser and greater sphenoid wing sections that form the lateral and inferior part of the posterior orbital wall, which are separated from the CS and OA by a bone curtain but are interconnected via the SOF. Thus, the annular tendon, which is located inside the OA and to which the ocular muscle tendons are attached [23], was recognized and cut easily between the lateral and superior recti muscles (Fig. 9b). The oculomotor foramen through which the cranial nerves pass, was dissected. The superior and inferior divisions of the oculomotor nerve, nasociliary, and abducens nerves were dissected through the oculomotor foramen located inside the annular tendon (Fig. 10b). The abducens nerve was observed to be extended inside the internal surface of the lateral rectus muscle after passing superior to the muscle (Figs. 10b, 11b and 12a). The trochlear, frontal, and lacrimal nerves were observed to be entering the orbit outside and lateral to the annular tendon (Figs. 10b and 11b).
Left annular tendon cutting with an illustrative drawing showing the microscope orientation (a). The OA and CS are connected through the SOF. After removing the lesser sphenoid wing and the lateral and inferior parts of posterior orbital wall, the orbit and middle fossa became unified. For dissection of the oculomotor foramen, which is located inside the annular tendon at the OA and through which the cranial nerves pass, the annular tendon was cut between the lateral rectus and superior rectus muscles (b); OA orbital apex; CS cavernous sinus; SOF superior orbital fissure
Left oculomotor foramen and OA dissection with an illustrative drawing showing the microscope orientation (a). The abducens nerve enters the lateral rectus muscle superior to the muscle in the oculomotor foramen located inside the annular tendon. Nasociliary nerve courses above the abducens nerve. Dissection reveals the appearance of superior and inferior divisions of the oculomotor nerve at the OA and entry of the trochlear, frontal, and lacrimal nerves to the orbit outside and lateral to the annular tendon (b); OA orbital apex; CN III oculomotor nerve; CN IV trochlear nerve; CN VI abducens nerve
Left oculomotor foramen and OA dissection with an illustrative drawing showing the microscope orientation (a). When the abducens and nasociliary nerves are pulled inferiorly, and the trochlear, frontal, and lacrimal nerves outside the annular tendon are pulled superiorly, branching of the oculomotor nerve inside the annular tendon to the superior and inferior divisions is completely revealed (b); OA orbital apex; CN III oculomotor nerve; CN IV trochlear nerve; CN VI abducens nerve
When dissection inside the annular tendon is continued toward the intraorbital section, the ophthalmic artery extending from the optic foramen, optic nerve, and distal parts of oculomotor nerve divisions, were exposed. After clearing the intraorbital fat tissues and the nasociliary nerve was moved away from the abducens nerve, the inferior division of the oculomotor nerve at the OA was observed to branch distally to the inferior rectus, inferior oblique, and medial rectus muscles (a, b). When the superior edge of the lateral rectus muscle is pulled downwards, the most proximal parts of these distal branches, the ophthalmic artery, as well as the optic nerve can be reached. The inferior division of the oculomotor nerve initially yields a branch that courses just under the optic nerve before entering the medial rectus muscle, then branches to innervate the inferior oblique and medial rectus muscles. The nerve that projects to the inferior oblique muscle can be easily identified, as it yields a motor branch to the ciliary ganglion. The nerve that leads to the medial rectus muscle is located more medially (a). When the inferior edge of the lateral rectus muscle is pulled upwards, more distal branches of the inferior division of the oculomotor nerve can be reached (b). CN II optic nerve; CN III oculomotor nerve;
CN VI abducens nerve; io branch of the inferior division of the CN III innervating the inferior oblique muscle; mr branch of the inferior division of the CN III innervating the medial rectus muscle; ir branch of the inferior division of the CN III innervating the inferior rectus muscle
Fatty tissue in front of the OA was cleaned, and the inferior division of the oculomotor nerve was observed to yield distal branches that innervate the inferior rectus, inferior oblique, and medial rectus muscles (Fig. 12a and b). When the superior border of the lateral rectus muscle was pulled inferiorly, the most proximal parts of these distal branches, i.e., the ophthalmic artery and optic nerve, were exposed. First, the inferior division of the oculomotor nerve branches to the medial rectus muscle, passing just below the optic nerve. Then, branching of the nerve innervating the inferior oblique rectus muscle, which can be differentiated from other branches by locating the motor branch to the ciliary ganglion, as well as more medially branching nerve that innervate the inferior rectus muscle was observed (Fig. 12a). More distal parts of the inferior division of the oculomotor nerve were exposed by pulling the inferior border of the lateral rectus upwards (Fig. 12b). These distal branches were observed to course the medial rectus muscle, inferior rectus muscle, and inferior oblique muscle. Cranial nerves and its branches when viewing the inside of the left orbit anteriorly were illustrated in Fig. 13.
Thus, an area enabling dissection of the lateral and inferior parts of the orbit, OA, SOF, and CS complex was created by lateral orbitotomy. Because lateral orbitotomy is a well-known and widely used surgical technique for intraorbital pathologies, we did not continue with dissection of the intraorbital tissues.
Discussion
Principles of the epidural approach and dissection steps to the CS and OA were first described and performed by Dolenc [4–6, 8, 10, 12]. Minimal invasive interventions using epidural dissection were developed on the grounds of these approaches. In our study, we demonstrated that the indications and criteria for lateral orbitotomy which performed for CS tumor by Altay et al. [2] may be expanded. Using this technique, we were able to perform epidural dissection of the CS and neurovascular tissues passing through the SOF to reach the OA. Using lateral orbitotomy, which has previously been used for intraorbital pathologies only, we created a dissection area starting from the Gasserian ganglion and continuing to the OA, which could be expanded into the intraorbital areas when required. Cranial nerves located within this space extend distally and yield branches; therefore, it was not difficult to recognize cranial nerves passing through the CS, SOF and oculomotor foramen during cadaveric dissection. In tumor surgery, it may be difficult to identify cranial nerves when they are displaced or surrounded by tumor tissue. This approach provides control over the oculomotor, trochlear, ophthalmic, and abducens nerves and their branches at their proximal or distal sections. It is possible to dissect these nerves up to their normal anatomical locations during surgery for tumors that affect these nerves, particularly tumors invading the SOF and OA. For tumors that have both middle fossa and intraorbital components passing between the different parts of the cranium through the fissure, foramen or adjacent invasion, transcranial approaches such as pterional and orbitozygomatic frontotemporal craniotomy (OZC) are often used to achieve surgical control over these areas [5, 6, 16, 20, 23–25]. However, larger bone resections are required during OZCs to minimize brain traction [20, 23]. Thus, although this approach can provide complete control over the CS, optic canal, SOF, and inside of the orbit [23], performing craniotomy and closing the defect extends the surgery time and necessitates important bone reconstruction procedures for cosmetic purposes. The anatomical dissection that we performed with lateral orbitotomy can provide access to almost all anatomical areas that are accessible via a classical pterional and OZC approaches but with less tissue damage. Drilling the bone located between the middle fossa and the orbit with lateral orbitotomy unifies these two separate compartments and provides control over the cranial nerves which along the lateral wall of the CS and entry of the oculomotor nerve to the roof of the CS can be easily observed. At the same time, the superior and inferior divisions of the oculomotor nerve, nasociliary, and abducens nerves can be dissected through the oculomotor foramen located inside the annular tendon. When required, drilling of the greater sphenoid wing makes it easier to visualize the Gasserian ganglion, as well as the maxillary and mandibular nerves extending toward the foramen rotundum and ovale.
Intraorbital tumors can also penetrate the intracranial area via invasion of the OA and SOF. A conventional OZC approach is unquestionably superior to other methods for lesions predominantly within the orbit and intracranial extensions via infiltration of the OA or SOF. However, whereas lateral orbitotomy is used in the initial approach to complex intraorbital lesions, it is also possible to extend the surgical area by drilling the sphenoid wing to access intracranial content. This possibility makes this approach a minimally invasive surgical intervention with tandem properties. Thus, we can say that this approach facilitates identification of the cranial nerves, reduces the risk of injury, and aids orientation. Therefore, this approach can be considered an alternative minimally invasive intervention for surgery on spheno-orbital or intraorbital tumors which invaded OA and oculomotor foramen.
Dissection of tumors extending from the middle fossa or CS through SOF to OA, or vice versa, by lateral orbitotomy need surgical experience and anatomic knowledge. Morbidity in this area, where so many nerves are bundled at SOF, with ICA and optic nerves, is extremely high and mostly not accepted in practice today. Radiosurgery generates acceptable outcomes for these tumors. Nonetheless, for tumors that can be surgically removed or require volume reduction or biopsy, surgical intervention via lateral orbitotomy can be employed. We consider that the present anatomical study, which demonstrates the stages of lateral orbitotomy and epidural dissection step by step, and in detail, would shed light for future clinical studies.
As with anterior fossa approaches, there is a need to develop minimally invasive alternatives for operating within the middle fossa [2, 13]. One recent report demonstrated the ability to reach the middle fossa with lateral orbitotomy [13]. However, in that clinical study, lateral orbitotomy was performed for uncomplicated meningiomas that had invaded the orbit without invading the SOF, AC, or CS. In a cadaveric study, Altay et al. [2] reported that the CS can be reached with lateral orbitotomy using drilling the greater-lesser sphenoid wing, and performing an anterior clinoidectomy. This report was the first to apply this alternative approach for CS surgery. Thus, emerging evidence suggests a trend toward minimally invasive approaches for these complex anatomical areas. However, no previous anatomic study has demonstrated that minimally invasive approaches can be used for tumors invading both the middle fossa and the SOF or OA. In our study using lateral orbitotomy, which is regarded as a minimally invasive method, we demonstrated that it was possible to achieve control over critical neurovascular structures during surgical dissection of complex tumors invading the not only CS but also SOF and OA. Our findings can provide guidance to surgeons operating on tumors located in these areas.
The zygomatico-facial and zygomatico-temporal nerves, which carry only sensory nerve fibers, are the first neural tissue and obstacle which encountered during lateral orbitotomy. These nerves provide sensory innervation to the temple and cheek [23]. It is not possible to preserve these nerves during lateral orbitotomy. However, previous clinical studies using lateral orbitotomy have not reported clinical outcomes related to injury of these sensory nerves [2, 13]. The dural anatomy and relationships are very important for lateral orbitotomy approach because the dural fold is the second obstacle to achieving anterior clinoidectomy. The endosteal layer, which forms the lateral wall of the CS and blends into the periorbita at the level of the OA, covers the trochlear, frontal, and lacrimal nerves that pass outside the annular tendon and inside the SOF. Whereas the other layer, which faces the temporal lobe and proceed as temporal dura, is the meningeal layer. Also known as the dural fold, it originates from the temporal dura and blends into the periorbita. To perform anterior clinoidectomy with either lateral orbitotomy or another transcranial intervention, dural fold that is first obstacle between middle fossa and orbit, should be cut to continue the dissection [4–8].
The most significant disadvantage lies in attempting to gain control over well-known anatomic structures through a small area created by lateral orbitotomy using pterional and OZC approaches and different orientations. The surgeons may experience difficulties due to anatomical confusion caused by tumors occupying more than one compartment, particularly in conditions that require tackling both bleeding in CS pathologies and gaining anatomical orientation. Hemostatic agents can help stop bleeding; however, it is obvious that it is not as simple as a cadaveric dissection. Another disadvantage of this approach lies in the proximal control of ICA. It was reported that ICA could be exposed through Glasscock’s triangle instead of using neck dissection [4, 5, 7, 8]. However, access to this area can be difficult and risky for gaining proximal control in lesions encircling or infiltrating ICA. We believe that dissection practices should be performed in the anatomy laboratory to best familiarize with normal anatomic contiguity of neurovascular structures. Mariniello et al. [13] used lateral orbitotomy in uncomplicated spheno-orbital meningioma, and subsequently, Altay et al. [2] used lateral orbitotomy in a study of cadaveric dissection to CS and then in an isolated case of meningioma located in the CS. The incision may be concern cosmetically but both studies reported that incision was well tolerated. However, there are no reports on the use of this method in complicated multi-compartmental tumors. Although there is a lack of clinical implementation, our anatomical study suggests that this method is not impossible using different anatomical orientations.
In conclusion, we demonstrated that the indications and criteria for this surgical technique may be expanded with dissections performed at the CS, SOF, and OA corridor. An accurate determination of the lesion borders with ever-expanding neuroradiological imaging techniques will play an important role in the surgical planning of lateral orbitotomy interventions. Because this technique provides control over the orbit, OA, SOF, and middle fossa by changing the angle of the microscope, it can be used as a new and alternative surgical method for tumors of these areas. Nonetheless, although this technique provides control over important neurovascular structures within a small area, sufficient experience with adequate anatomical studies and dissection should be achieved prior to clinical implementation. Although it is not possible to completely remove tumors with this technique, growth of the residual tumor tissue can be controlled using Gamma Knife radiosurgery, which results in less tissue damage and enables better reconstruction. Advancing technology will provide further techniques in the field of microsurgery, which we believe will increase the importance of the lateral orbitotomy approach.
Limitations
The most significant limitation of our anatomical study is the lack of clinical implementation.
References
Abdel Aziz KM, Bhatia S, Tantawy MH, Sekula R, Keller JT, Froelich S, Happ E (2011) Minimally invasive transpalpebral “eyelid” approach to the anterior cranial base. Neurosurgery 69:ons195–206; discussion 206-197
Altay T, Patel BC, Couldwell WT (2012) Lateral orbital wall approach to the cavernous sinus. J Neurosurg 116:755–763
Arai H, Sato K, Katsuta T, Rhoton AL Jr (1996) Lateral approach to intraorbital lesions: anatomic and surgical considerations. Neurosurgery 39:1157–1162, discussion 1162-1153
Dolenc V (1999) Extradural approach to intracavernous ICA aneurysms. In: Reulen H-J (ed) Neurosurgical management of aneurysmal subarachnoid haemorrhage. Acta neurochirgica supplements, vol 72. Springer, Verlag Wien, Vienna, pp 99–106
Dolenc VV (1989) Anatomy and surgery of the cavernous sinus. Springer-Verlag Wien, New York
Dolenc VV (1997) Transcranial epidural approach to pituitary tumors extending beyond the sella. Neurosurgery 41:542–552
Dolenc VV (1999) A combined transorbital-transclinoid and transsylvian approach to carotid-ophthalmic aneurysms without retraction of the brain. In: Reulen H-J (ed) Neurosurgical management of aneurysmal subarachnoid haemorrhage. Acta neurochirurgica supplement, vol 72. Springer, Verlag Wien, Vienna, pp 89–97
Dolenc VV (2003) Microsurgical anatomy and surgery of the central skull base. Springer-Verlag Wien, New York
Fries G, Perneczky A (1998) Endoscope-assisted brain surgery: part 2–analysis of 380 procedures. Neurosurgery 42:226–231, discussion 231-222
Hakuba A, Matsuoka Y, Suzuki T, Komiyama M, Jin T, Inoue Y (1987) Direct approaches to vascular lesions in the cavernous sinus via the medial triangle. In: Dolenc VV (ed) The cavernous sinus. Springer, Verlag Wien, Vienna, pp 272–284
Jho HD (1997) Orbital roof craniotomy via an eyebrow incision: a simplified anterior skull base approach. Minim Invasive Neurosurg 40:91–97
Lesoin F, Pellerin P, Autrique A, Clarisse J, Jomin M (1987) The direct microsurgical approach to intracavernous tumors. In: Dolenc VV (ed) The cavernous sinus. Springer, Verlag Wien, Vienna, pp 323–331
Mariniello G, Maiuri F, de Divitiis E, Bonavolonta G, Tranfa F, Iuliano A, Strianese D (2010) Lateral orbitotomy for removal of sphenoid wing meningiomas invading the orbit. Neurosurgery 66:287–292, discussion 292
Maroon JC, Kennerdell JS (1976) Lateral microsurgical approach to intraorbital tumors. J Neurosurg 44:556–561
Maroon JC, Kennerdell JS (1984) Surgical approaches to the orbit. Indications and techniques. J Neurosurg 60:1226–1235
Natori Y, Rhoton AL Jr (1994) Transcranial approach to the orbit: microsurgical anatomy. J Neurosurg 81:78–86
Natori Y, Rhoton AL Jr (1995) Microsurgical anatomy of the superior orbital fissure. Neurosurgery 36:762–775
Ouyang T, Zhang N, Wang L, Li Z, Chen J (2015) Sphenoid wing meningiomas: surgical strategies and evaluation of prognostic factors influencing clinical outcomes. Clin Neurol Neurosurg 134:85–90
Perneczky A, Fries G (1998) Endoscope-assisted brain surgery: part 1–evolution, basic concept, and current technique. Neurosurgery 42:219–224, discussion 224-215
Pieper DR, A-MO (1999) Cranio-orbito-zygomatic approach. Operat Tech Neurosurg 2:2–9
Reisch R, Marcus HJ, Hugelshofer M, Koechlin NO, Stadie A, Kockro RA (2014) Patients’ cosmetic satisfaction, pain, and functional outcomes after supraorbital craniotomy through an eyebrow incision. J Neurosurg 121:730–734
Rhoton AL Jr (2002) The cavernous sinus, the cavernous venous plexus, and the carotid collar. Neurosurgery 51:S375–S410
Rhoton AL Jr (2002) The orbit. Neurosurgery 51:S303–S334
Ringel F, Cedzich C, Schramm J (2007) Microsurgical technique and results of a series of 63 spheno-orbital meningiomas. Neurosurgery 60:214–221, discussion 221-212
Roth J, Fraser JF, Singh A, Bernardo A, Anand VK, Schwartz TH (2011) Surgical approaches to the orbital apex: comparison of endoscopic endonasal and transcranial approaches using a novel 3D endoscope. Orbit 30:43–48
Steiger HJ, Schmid-Elsaesser R, Stummer W, Uhl E (2001) Transorbital keyhole approach to anterior communicating artery aneurysms. Neurosurgery 48:347–351, discussion 351-342
van Lindert E, Perneczky A, Fries G, Pierangeli E (1998) The supraorbital keyhole approach to supratentorial aneurysms: concept and technique. Surg Neurol 49:481–489, discussion 489–490
Yasuda A, Campero A, Martins C, Rhoton AL Jr, de Oliveira E, Ribas GC (2008) Microsurgical anatomy and approaches to the cavernous sinus. Neurosurgery 62:1240–1263
Acknowledgments
Thanks to Nurdan Ulutas for drawings.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
Ethical approval was not required for this type of study at our institute.
Additional information
Comments
This article applies in full extent to the principles of pterional epidural approach to cavernous sinus and orbital apex pioneered by Vinko Dolenc 25 years ago (1). These principles are applied here in cadaver study by minimally invasive route using lateral orbitotomy approach and by turning microscope from anterior (toward orbital apex) to posterior directions (toward CS and middle fossa). As surgery in this complex anatomical part of the central skull base is extremely demanding and includes serious morbidities (2), mostly not accepted anymore nowadays and thus treated in a different way, such cadaver study is not only an excellent repetition of parasellar microanatomy but also stresses out the key questions on very narrow indications and very serious limitations of some minimally invasive approaches versus more traditional ones.
Roman Bosnjak
Ljubljana, Slovenia
1. Dolenc VV. Anatomy and surgery of the cavernous sinus New York, Dordrecht, Heidelberg, London: Springer-Verlag; 1989
2. Dolenc V V. Microsurgical Anatomy and Surgery of the Central Skull Base. Wien, Austria: Springer-Verlag; 2003.
The authors present a nice anatomical description of the lateral orbitotomy with extended variations exposing the clinoidal region, cavernous sinus, SOF, and the Gasserian ganglion. There is a natural access to the sphenoid bone and fronto-temporal region when the lateral orbital wall is removed between frontozygomatic process and orbitozygomatic junction, and as it is shown, a relative wide exposure can be achieved. The lack of clinical experience makes this paper less attractive, however this is a nice anatomical effort to expose this region in a rather minimally access way. Yet, although the proximal control of carotid can be achieved in the clinoidal segment, this approach is not optimal for tackling ophthalmic artery aneurysms while having a temporary clip positioned in the clinoid segment. The visualization might be limited. For other lesions, and specifically if the goal is a biopsy or subtotal resection, the approach could be very appropriate. Skull base surgeons should get familiar with this minimally access exposure, and get appropriate exposure and knowledge about this alternative with cadaveric dissection.
Amir Dehdashti
NY, USA
Rights and permissions
About this article
Cite this article
Ulutas, M., Boyacı, S., Akakın, A. et al. Surgical anatomy of the cavernous sinus, superior orbital fissure, and orbital apex via a lateral orbitotomy approach: a cadaveric anatomical study. Acta Neurochir 158, 2135–2148 (2016). https://doi.org/10.1007/s00701-016-2940-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-016-2940-z