Abstract
Background
Electromagnetic (EM)-guided neuronavigation is an innovative technique and a viable alternative to opto-electric navigation. We have performed a safety and feasibility study using EM-guided neuronavigation for posterior fossa surgery in the semi-sitting position in a selected subset of patients.
Methods
Out of 284 patients with posterior fossa tumours operated upon over a period of 40 months, a subset of 15 patients was thought to possibly benefit from EM navigational guidance and was included in this study. There were six children and nine adults (aged between 8 and 84 years; mean age, 34.6 years) with different neoplasms in the brainstem or close to the midline. All patients had contrast-enhanced three-dimensional (3D) magnetic resonance imaging (MRI) of the head preoperatively. EM-guided navigation was used to identify and preserve the venous sinuses during craniotomy and to determine the trajectory to the lesion using various approaches. Neuronavigation accuracy was repeatedly checked for deviations measured in millimetres on screen shots during surgery before and after dural opening in the coronal (z = vertical), axial (x = mediolateral) and sagittal (y = anteroposterior) plane.
Results
Referencing of the patient in the supine position was fast and easy. There was no loss of navigation accuracy after repositioning of the patient in the semi-sitting position (mean, 2.5 mm ± 0.92 mm). Identification of the pathological structure using EM navigation was achieved in all instances. Optimal angulation of the neck was selected individually to permit a comfortable position for the surgeon with full access to the lesion avoiding over-flexion. Deviation of accuracy at the surface of the target lesion ranged between 2.5 and 5.8 mm (mean, 3.9 mm ± 1.1 mm).
Conclusions
EM-guided neuronavigation in the semi-sitting position was safe and technically feasible. It enabled fast and accurate referencing without loss of navigation accuracy despite repositioning of the patient. In contrast to conventional opto-electric neuronavigation there were no line of sight problems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Posterior fossa lesions may be challenging for surgery, especially when they are small and deep-seated involving midline structures like the brainstem, the cerebellar peduncles or the tegmental mesencephalic region. To obtain optimal results it is pivotal to choose the best surgical approach, to secure appropriate angulation of the patient’s head and to plan the optimal trajectory to reach the pathological lesion minimising tissue dissection especially in recurrent tumours surrounded by scar tissue [4, 5, 13, 6, 21]. Opto-electric neuronavigation has been shown to be a useful adjunct for neurosurgeons to localise supratentorial pathologies, and it has become a standard technology used worldwide [17, 18, 20]. Neuronavigation techniques, however, are not commonly used for surgery in the posterior fossa, and little is known about their possible applications. Especially in the semi-sitting position, its usefulness has been doubted because of concerns in navigation accuracy secondary to brainshift caused by gravity and loss of cerebrospinal fluid (CSF). Nevertheless, opto-electric navigation was shown to have certain advantages also in the semi-sitting position in planning of a lateral suboccipital approach, in brainstem cavernoma surgery, and in the treatment of trigeminal neuralgia [5, 8, 9, 21, 29].
Electromagnetic (EM) navigation was introduced more than a decade ago in neurosurgery, but in contrast to opto-electric navigation it has gained more widespread recognition only recently [7, 10, 11, 15, 19, 24, 27]. It has certain advantages over opto-electric navigation; in particular, there are no line-of-sight problems.
Thus far there are no data about its use and its possible advantages in surgery for difficult-to-approach tumours in the posterior fossa in the semi-sitting position. After having explored the application of EM navigation for other neurosurgical procedures [11, 12], we decided to perform a safety and feasibility study.
Methods and materials
Over a period of 40 months, we operated 284 patients with tumours in the posterior fossa. Navigational guidance was thought to be useful in a subset of 15 patients after thorough strategical preoperative discussion. Informed patient consent was obtained.
Inclusion criteria were primarily small or deep-seated lesions involving midline or paramedian structures thought to be difficult for intraoperative identification, and also recurrent tumours, supposed not to be markedly affected by brainshift and gravitation after opening of the dura and releasing CSF.
Exclusion criteria were big and straightforward-to-reach lesions, and lesions in the cerebellar hemispheres or in the cerebellopontine angle. Also, patients with a patent foramen ovale (PFO) were not considered because of the increased risk for air embolism in the semi-sitting position.
Technical specifications of the EM-system
The AxiEM neuronavigation system (Medtronic, Minneapolis, MN, USA) was used for EM navigation. This system has a field strength of 100 A/m at 50 mm off face of the transmitter coil array (TCA) and a decrease by 1/r3 (NORM IEC 61000-4-8). The field created by the emitter varies between 0 and 3.57 Gauss over the working volume (compared to the earth’s magnetic field of ~1 Gauss). The “usable field” corresponds to a roughly cubic shaped area of 650 × 525 mm. While we relied on fiducials fixed to the osseous skull in our initial study [22], we subsequently used surface-rendering of the face as outlined in detail elsewhere [11, 12].
Imaging data acquisition
All patients underwent three-dimensional (3D) T1-weighted magnetic resonance imaging (MRI) after single-dose gadolinium injection for visualisation of the targeted pathological lesion preoperatively (MPRAGE sequence, TR 1,980 ms, TE 3.98 ms, TI 1,100 ms, voxel size 1 × 1 × 1 mm3, FOV 256, 176 slices; single-dose contrast media per kilogram bodyweight). This dataset was also used for identification of the cerebral venous sinus system of the posterior fossa to confirm its precise location before and after craniotomy. In non-enhancing tumours additionally a T2-weighted 3D MR dataset was acquired (SPACE sequence, TR 3,200 ms, TE 379 ms, voxel size 1 × 1 × 1mm3, FOV 256, 176 slices). A work station was used for preoperative 3D reconstructions (Stealth Station; Medtronic Navigation, Minneapolis, MN, USA).
Intraoperative registration of the patient in the supine position
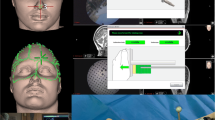
The patient was intubated and ventilated according to a standard protocol [14]. After attaching the dynamic reference frame (DRF) to the forehead just above the eyebrow to maintain adequate distance to the Mayfield clamp fixed later on, the DRF was secured by adhesive tape. The transmitter coil was fixed to the table at the side where the DRF was attached. Finally, registration was achieved by surface matching over the patient’s face and head and additionally over the occipital and suboccipital area bilaterally in the supine position without the Mayfield clamp (Fig. 1). After automatical computing of a correlation matrix, accuracy was controlled for deviations in the coronal (z = vertical), axial (x = mediolateral) and sagittal (y = anteroposterior) planes on screen shots (measured in millimetres) of the position of tragus, bregma and nasion (Fig. 2). In addition, accuracy was confirmed by checking surface markers of the skull in frontal and parietal regions (sagittal and coronal sutures), and especially in the occipital and suboccipital region after careful elevation and anteflexion of the head.
Intraoperative registration of the 3D T1-weighted MR dataset by surface matching of the face and head performed in the supine position without sharp head fixation in the Mayfield clamp. After elevation of the head, surface matching in the occipital and suboccipital region was also achieved. In this instance the transmitter coil is placed left to the patient’s head, close to the patient’s forehead with the dynamic reference frame (DRF) attached to the left forehead. The navigation pointer for surface matching is shown close to the right coronal suture
Accuracy control after surface matching in the supine position by checking for deviations of anatomical landmarks on screen shots (distance between the red and orange points measured in millimetres). Here, deviations are determined for the nasion in coronal (z = vertical), axial (x = mediolateral) and sagittal (y = anteroposterior) planes. Other landmarks which are checked routinely are bregma, tragus, mastoid and inion after careful head elevation
To better account for total spatial inaccuracy also a resulting vector was calculated from the Cartesian coordinates x, y and z in millimetres (see Fig. 3).
After removing the transmitter coil the electrodes for intraoperative neurophysiological monitoring (IOM) of somatosensory evoked potentials (SSEP) of the median and tibial nerves and for acoustic evoked potentials (AEP) were fixed to the scalp of the patient and recorded for a short period to obtain baseline values.
Positioning of the patient in the semi-sitting position for surgery
Thereafter, the patient’s head was fixed in the Mayfield clamp taking particular care with the pins in order not to displace the DRF. The patient’s position was changed then from supine to the semi-sitting position for surgery with the head in anteflexion to gain access to the posterior fossa. The transmitter coil was fixed again to the operating table in a manner to allow free detection of the DRF. After checking the virtual trajectory to the pathological structure on the navigation screen by virtual tip extension, accounting also for cisternal anatomy, the optimal degree of head flexion was chosen before locking the Mayfield clamp (Fig. 4). In each patient the optimal angulation was selected individually both with regard to gain full view of the pathological process and to permit a comfortable position for the surgeon without over-flexion of the patient’s neck.
After locking the Mayfield clamp in anteflexion, another accuracy check was performed by checking for deviations of the position of tragus, bregma, nasion and for osseous markers in the occipital and suboccipital region (mastoid process and inion). Then, the venous sinuses were identified by EM navigation and marked on the patient’s skin, allowing them to be protected easily during craniotomy. Subsequently, SSEPs and AEPs were recorded again after removing the transmitter coil to check for interferences by the EM field of the navigation system.
Surgery aided by EM navigation
All procedures were performed by neurosurgeons with longstanding experience in posterior fossa surgery. First, a customised craniotomy was performed guided by EM navigation as needed to approach the pathological process taking the location of the dural sinuses into account. After removal of the bone flap and before opening the dura, the EM navigation stylet with the virtual tip extension was used to assess precisely the direction and the distance to the target lesion, and also to confirm the location of the venous sinuses. Then, after microsurgical opening of the dura and CSF release, the EM stylet was used again for orientation, taking into account the trajectory, before opening the dura (screen shot). This helped to assess and correct for the amount of brainshift.
Thereafter, the operation was continued by standard microneurosurgical techniques, following the trajectory to the target point either via a supracerebellar, transcerebellar or telovelar route, depending on the site of the lesion [13]. The surgeon alternated several times between microsurgical preparation under microscopical view and on-line tracking navigation of the tip of the EM stylet to check the distance and to control the direction to the target lesion. Once the target lesion was reached by microscopical view, the tip of the EM stylet was positioned on it, and the actual position was compared with the position displayed on the navigation screen. This was documented by photos via the microscope and screen shots on the EM navigation system. Deviations in all three axes were measured.
Both sets of information, based on the microscopical view and the position as outlined on the navigation system were synthesised taking the amount of brainshift into account. During surgery, localisation of the tumour, the tumour borders and distinct anatomical landmarks of the aqueduct, the tentorium cerebelli, the floor of the fourth ventricle, the surface of the brainstem or prominent vessels visualised on the 3D MRI dataset were marked by the EM stylet. Measurements in all three axes were repeatedly performed on screen shots to check for deviation of navigation accuracy during surgery.
Postoperative imaging
Postoperative computed tomography (CT) scans were obtained routinely 6 h after surgery to rule out postoperative complications like bleeding, infarction, brain swelling, hydrocephalus or pneumocephalus. In children, a contrast-enhanced MRI scan was performed additionally during a period of 72 h after operation to rule out tumour remnants. In adults, a postoperative MRI scan was obtained routinely at the 3-month follow-up examination.
Results
Patient characteristics and clinical data including histopathological diagnoses are summarised in Table 1. There were four women and 11 men. The study included six children and nine adults. Mean age at surgery was 34.6 years (range, 8–84 years).
Feasibility
EM navigation for surgery in the semi-sitting position was feasible in all 15 selected cases. Easy and fast referencing of the patient by surface matching in the supine position without sharp head fixation including the occipital and suboccipital region was possible without difficulty in all instances. Referencing took about 5 min in trained hands. The time for installation of the system including registration in the supine position and repositioning of the patient for surgery in the semi-sitting position with refixation of the transmitter coil and rechecking of navigation accuracy added up to about 30 min. The location of the venous sinuses was reliably confirmed. Identification of the target lesion using the EM navigation was achieved in all instances.
Accuracy
EM navigation accuracy for anatomical landmarks after surface matching is shown in Table 2. The overall mean deviation of EM navigation accuracy (resulting vector) for anatomical landmarks was 2.48 mm (± 0.75 mm) in the supine position and 2.5 mm (± 0.92 mm) after repositioning of the patient in the semi-sitting position for surgery, which was statistically not significant (p = 0.44).
EM navigation accuracy for different anatomical landmarks after dural opening and CSF release is shown in Table 3.
The deviation of navigation accuracy for the surface of the target lesion ranged between 2.5 and 5.8 mm (mean, 3.88 ± 1.15). Brain shift during surgery was remarkable for the cerebellar hemispheres due to gravity and loss of CSF.
Safety
There were no complications related to the application of the EM navigation system. Also, there were no line-of-sight problems.
The use of the EM navigation system interfered with monitoring of evoked potentials. Reliable monitoring was only possible after temporary removal of the transmitter coil from the desk-holder since it produced artefacts. Therefore, during navigation monitoring was stopped. Whenever EM navigation was requested, easy repositioning of the transmitter coil in the desk-holder was feasible.
The metallic wound retractor impaired accurate identification of the tip of the EM stylet at the beginning of the operation before performing the craniotomy. EM navigation with the tip of the EM stylet in that phase therefore was only possible after temporary removal of the retractor. With the operation proceeding more to the depth, however, after removal of the bone flap no more interferences occurred, so that there was no need to remove the wound retractor.
Usefulness
In all 15 patients EM navigation was considered very useful by the neurosurgeon, in particular when guiding localisation to small midline lesions.
In all cases a slight deflexion of the patient’s initial head position in the semi-sitting position, which had been fixed without navigation guidance, was possible after determining the angulation of the trajectory with the EM system, resulting in more relaxed jugular veins obviating venous congestion.
Discussion
Neuronavigation has become a standard technology for various neurosurgical procedures within less than two decades [5, 8, 9, 17, 18, 21]. Especially, it is well established for surgery of gliomas and other tumours in the cerebral hemispheres and the diencephalon [4, 20]. Navigation can also be helpful to tailor petrosectomies to reach lesions at the jugular foramen [6]. Although neuronavigation is being used routinely nowadays, it is important not to forget its limitations, in particular with regard to factors influencing its accuracy.
Navigational guidance has only rarely been studied for posterior fossa surgery in the semi-sitting position [8, 9] and often it has been thought not to be particularly helpful for optimising surgical approaches in this setting. Nevertheless, the mean target registration error amounted to 1.4 mm in a study on neuronavigation used in conjunction with a suboccipital lateral approach which is comparable to registration errors in supratentorial surgery [8, 9].
There are only a few reports on intradural navigation in the posterior fossa providing very limited information on navigation accuracy.
Brainshift is the most vexing problem in neuronavigation, and despite various attempts to minimise its effect [26], it may result in considerable inaccuracy. In the semi-sitting position, brainshift due to gravity and loss of CSF may affect certain structures markedly, especially with sagging of the cerebellar hemispheres. On the other hand, it is apparent that downward displacement of anterior and midline structures would be much less pronounced. Therefore, while neuronavigation might not be useful for the majority of posterior fossa lesions, it might have a place to guide the approach to more anteriorly located lesions which are difficult to identify primarily.
Opto-electric navigation
Opto-electric navigation systems are widely used [5, 6, 17, 18], but they have some disadvantages like line-of-sight problems and the need of rigid head fixation without the possibility of changing the position of the patient’s head during surgery without loss of navigation accuracy. Opto-electric navigation in the prone position or in a semi-sitting position requires meticulous and time-consuming registration procedures, and interference with the Mayfield clamp is common because of line-of-sight problems. This particular issue may result in prolonged and repeated registration procedures to reach an acceptable accuracy for surgery. Stieglitz et al. [26] showed that loss of neuronavigation accuracy with opto-electric systems can occur at several points during surgery and even before skin incision.
EM navigation
EM navigation is a relatively new technique and its use is still limited in comparison to the common opto-electric navigation systems. It has become a well-accepted technical aid for placement of ventricular catheters in children, newborns and even in preterms [1, 2, 7, 10, 11, 15, 22] because it obviates the need for sharp head fixation. Another advantage in comparison to the opto-electric navigation systems is that there are no line-of-sight problems when tracking the instruments during surgery. Instruments and staff can come in and out of the EM field with no disruption to the surgical navigation information. The widening applications of EM surgery include now neuroendoscopy [24], aneurysm surgery [12], placement of Ommaya reservoirs for chemotherapy [28] and of external ventricular drains in the intensive care unit [16].
Accuracy of EM navigation has been shown to be comparable with that of opto-electric navigation ranging between 0.71 to 3.51 mm [23] and 0.7 to 4.4 mm [3].
The present study extends the limited experience with this technique demonstrating its feasibility, safety and usefulness in selected instances for posterior fossa surgery in the semi-sitting position.
Navigation in posterior fossa surgery
Previous studies reported only about experience with opto-electric navigation systems in the semi-sitting position. Navigation was thought to be helpful in lateral suboccipital craniotomy in order to identify sinus anatomy and increasing the safety of this approach [8, 9]. Another study investigated its usefulness for thermocoagulation of the Gasseri ganglion in the treatment of trigeminal neuralgia in a sitting position [29]. Navigation was also applied in brainstem cavernoma surgery, especially to guide decision where to make the pial incision [21]. Reliable accuracy of neuronavigation to the brainstem was shown recently in a series of 38 patients with brainstem cavernomas [4]. In addition, the use of navigation was supposed to decrease surgical complications and morbidity in haemangioblastoma removal in the posterior fossa [5].
Since midline anatomical structures like the brainstem are tethered to the cranial base, brainshift appears to be less pronounced. Despite these encouraging preliminary data, however, only limited statements about the true value of navigation in posterior fossa surgery are possible because of the general lack of quantification of deviations in navigation accuracy in previous studies.
Pitfalls using navigation in the semi-sitting position
Navigation in the semi-sitting position is especially challenging because of the additional impact of gravity and the downward displacement of the cerebellar hemispheres after opening of the dura and CSF release. Accuracy of navigation was shown to differ between the frontal region and the occipital region. Surface-based registration accuracy was shown to be better in the face and in the frontal region, and error increased as the target location was further away from the face. In one study, mean surface registration error in the face zone was 0.9 ± 0.35 mm, while for targets located at 60, 105, and 150 mm from the facial surface it was 2.0, 3.2, and 4.5 mm, respectively [25]. Such an increase of inaccuracy with increased distance from the face when using surface-matching was also seen in our study and it has to be considered when using navigation in the posterior fossa.
EM navigation in the semi-sitting position
Overall, the present study demonstrated that EM navigation in the semi-sitting position was a useful adjunct to localise small or deep seated tumours in the brainstem, the cerebellar peduncles and the midbrain. Brainshift after CSF loss was not a major problem, as demonstrated also by measurements at the obex and the median sulcus on the floor of the fourth ventricle. EM navigation allowed a comfortable registration procedure for the patient by surface matching of the face in a supine position. Sufficient accuracy was demonstrated when surface landmarks were rechecked after definitive positioning of the patient for surgery in the semi-sitting position. Referencing of the system was easy without any line-of-sight problems.
The Mayfield clamp can be safely used with EM navigation. In contrast to the findings of Stieglitz et al. [26] on opto-electric navigation, minor movements in the Mayfield clamp do not influence EM navigation accuracy because the DRF is directly fixed on the patient’s forehead. IOM was easily performed with EM navigation by removing the transmitter coil temporarily and placing it in its holder again when needed.
The EM navigation stylet can be advanced easily to deep areas such as the pineal region or the midbrain because of its thinness and its length. Because of its pliability, it can practically be introduced into any narrow and deep anatomical space, enabling the use of surgical navigation also in procedures that were previously limited by the rigid optical-based tracking technology.
Navigation accuracy at the target lesion in the depth was acceptable and sufficient in the semi-sitting position.
Conclusions
EM-guided neuronavigation in the semi-sitting position for posterior fossa tumours was both safe and technically feasible. It enabled fast and accurate referencing of the patient for navigation without loss of accuracy despite sharp head fixation and repositioning of the patient from supine to semi-sitting position. It helped in localising the target in the depth when approaching the brainstem and it was considered a useful adjunct.
References
Aufdenblatten CA, Altermatt S (2008) Intraventricular catheter placement by electromagnetic navigation safely applied in a paediatric major head injury patient. Childs Nerv Syst 24(9):1047–1050
Azeem SS, Origitano TC (2007) Ventricular catheter placement with a frameless neuronavigational system: a 1-year experience. Neurosurgery 60(4 Suppl 2):243–247, discussion 247–248
Barszcz S, Roszkowski M, Daszkiewicz P, Jurkiewicz E, Maryniak A (2007) Accuracy of intraoperative registration during electromagnetic neuronavigation in intracranial procedures performed in children. Neurol Neurochir Pol 41(2):122–127
Chen LH, Zhang HT, Chen L, Liu LX, Xu RX (2014) Minimally invasive resection of brainstem cavernous malformations: surgical approaches and clinical experiences with 38 patients. Clin Neurol Neurosurg 116:72–79
Chen W, Zhang G, Lin C, Yang Y, Cai D, Huang M, Xu Y, Cai C, Li W, Lin C (2012) Clinical use of a neuronavigation system in hemangioblastoma resection of posterior cranial fossa. Minim Invasive Ther Allied Technol 21(3):234–240
Cinibulak Z, Krauss JK, Nakamura M (2013) Navigated minimally invasive presigmoidal suprabulbar infralabyrinthine approach to the jugular foramen without rerouting of the facial nerve. Neurosurgery 73(1 Suppl Operative):ons3–ons15
Clark S, Sangra M, Hayhurst C, Kandasamy J, Jenkinson M, Lee M, Mallucci C (2008) The use of noninvasive electromagnetic neuronavigation for slit ventricle syndrome and complex hydrocephalus in a pediatric population. J Neurosurg Pediatr 2(6):430–434
Gharabaghi A, Rosahl SK, Feigl GC, Liebig T, Mirzayan JM, Heckl S, Shahidi R, Tatagiba M, Samii M (2008) Image-guided lateral suboccipital approach: part 1-individualized landmarks for surgical planning. Neurosurgery 62(3 Suppl 1):18–22, discussion 22–23
Gharabaghi A, Rosahl SK, Feigl GC, Safavi-Abbasi S, Mirzayan JM, Heckl S, Shahidi R, Tatagiba M, Samii M (2008) Image-guided lateral suboccipital approach: part 2-impact on complication rates and operation times. Neurosurgery 62(3 Suppl 1):24–29, discussion 29
Hayhurst C, Beems T, Jenkinson MD, Byrne P, Clark S, Kandasamy J, Goodden J, Nandoe Tewarie RD, Mallucci CL (2010) Effect of electromagnetic-navigated shunt placement on failure rates: a prospective multicenter study. J Neurosurg 113(6):1273–1278
Hermann EJ, Capelle HH, Tschan CA, Krauss JK (2012) Electromagnetic-guided neuronavigation for safe placement of intraventricular catheters in pediatric neurosurgery. J Neurosurg Pediatr 10(4):327–333
Hermann EJ, Petrakakis I, Götz F, Lütjens G, Lang J, Nakamura M, Krauss JK (2015) Surgical treatment of distal anterior cerebral artery aneurysms aided by electromagnetic navigation CT angiography. Neurosurg Rev. doi:10.1007/s10143-015-0611-9
Hermann EJ, Rittierodt M, Krauss JK (2008) Combined transventricular and supracerebellar infratentorial approach preserving the vermis in giant pediatric posterior fossa midline tumors. Neurosurgery 63(1 Suppl 1):ONS30–ONS35, discussion ONS35-37
Hong B, Biertz F, Raab P, Scheinichen D, Ertl P, Grosshennig A, Nakamura M, Hermann EJ, Lang JM, Lanfermann H, Krauss JK (2015) Normobaric hyperoxia for treatment of pneumocephalus after posterior fossa surgery in the semisitting position: a prospective randomized controlled trial. PLoS One (in press)
Kandasamy J, Hayhurst C, Clark S, Jenkinson MD, Byrne P, Karabatsou K, Mallucci CL (2011) Electromagnetic stereotactic ventriculoperitoneal csf shunting for idiopathic intracranial hypertension: a successful step forward? World Neurosurg 75(1):155–160, discussion 32–33
Mahan M, Spetzler RF, Nakaji P (2013) Electromagnetic stereotactic navigation for external ventricular drain placement in the intensive care unit. J Clin Neurosci 20(12):1718–1722
Nakamura M, Krauss JK (2010) Image-guided resection of small lesions in the cavernous sinus and Meckel’s cave. Eur J Surg Oncol 36(2):208–213
Nakamura M, Stöver T, Rodt T, Majdani O, Lorenz M, Lenarz T, Krauss JK (2009) Neuronavigational guidance in craniofacial approaches for large (para)nasal tumors involving the anterior skull base and upper clival lesions. Eur J Surg Oncol 35(6):666–672
Omara AI, Wang M, Fan Y, Song Z (2014) Anatomical landmarks for point-matching registration in image-guided neurosurgery. Int J Med Rob 10(1):55–64
Orringer DA, Golby A, Jolesz F (2012) Neuronavigation in the surgical management of brain tumors: current and future trends. Expert Rev Med Devices 9(5):491–500
Ramina R, Mattei TA, de Aguiar PH, Meneses MS, Ferraz VR, Aires R, Kirchhoff DF, de Carvalho Kirchhoff D (2011) Surgical management of brainstem cavernous malformations. Neurol Sci 32(6):1013–1028
Rodt T, Köppen G, Lorenz M, Majdani O, Leinung M, Bartling S, Kaminsky J, Krauss JK (2007) Placement of intraventricular catheters using flexible electromagnetic navigation and a dynamic reference frame: a new technique. Stereotact Funct Neurosurg 85(5):243–248
Rosenow JM, Sootsman WK (2007) Application accuracy of an electromagnetic field-based image-guided navigation system. Stereotact Funct Neurosurg 85(2–3):75–81
Sangra M, Clark S, Hayhurst C, Mallucci C (2009) Electromagnetic-guided neuroendoscopy in the pediatric population. J Neurosurg Pediatr 3(4):325–330
Shamir RR, Freiman M, Joskowicz L, Spektor S, Shoshan Y (2009) Surface-based facial scan registration in neuronavigation procedures: a clinical study. J Neurosurg 111(6):1201–1206
Stieglitz LH, Fichtner J, Andres R, Schucht P, Krähenbühl AK, Raabe A, Beck J (2013) The silent loss of neuronavigation accuracy: a systematic retrospective analysis of factors influencing the mismatch of frameless stereotactic systems in cranial neurosurgery. Neurosurgery 72(5):796–807
Suess O, Kombos T, Kurth R, Suess S, Mularski S, Hammersen S, Brock M (2001) Intracranial image-guided neurosurgery: experience with a new electromagnetic navigation system. Acta Neurochir (Wien) 143(9):927–934
Weiner GM, Chivukula S, Chen CJ, Ding D, Engh JA, Amankulor N (2015) Ommaya reservoir with ventricular catheter placement for chemotherapy with frameless and pinless electromagnetic surgical neuronavigation. Clin Neurol Neurosurg 5(130C):61–66
Zhang WC, Zhong WX, Li ST, Zheng XS, Yang M, Shi J (2012) Neuronavigator-guided percutaneous radiofrequency thermocoagulation in the treatment of trigeminal neuralgia. Ir J Med Sci 181(1):7–13
Acknowledgments
We thank Hans Heissler for his technical support in the production of Fig. 3.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hermann, E.J., Petrakakis, I., Polemikos, M. et al. Electromagnetic navigation-guided surgery in the semi-sitting position for posterior fossa tumours: a safety and feasibility study. Acta Neurochir 157, 1229–1237 (2015). https://doi.org/10.1007/s00701-015-2452-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-015-2452-2