Abstract
Bleeding from brainstem cavernomas may cause severe deficits due to the absence of non-eloquent nervous tissue and the presence of several ascending and descending white matter tracts and nerve nuclei. Surgical removal of these lesions presents a challenge to the most surgeons. The authors present their experience with the surgical treatment of 43 patients with brainstem cavernomas. Important aspects of microsurgical anatomy are reviewed. The surgical management, with special focus on new intraoperative technologies as well as controversies on indications and timing of surgery are presented. According to several published studies the outcome of brainstem cavernomas treated conservatively is poor. In our experience, surgical resection remains the treatment of choice if there was previous hemorrhage and the lesion reaches the surface of brainstem. These procedures should be performed by experienced neurosurgeons in referral centers employing all the currently available technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cavernous malformations (also called cavernomas or cavernous angiomas) are well-circumscribed lesions formed by sinusoidal vascular channels. When located in the brainstem, however, the occurrence of hemorrhages (even small ones) may lead to devastating neurological deficits. Cavernous malformations are currently classified in the group of vascular malformations of the central nervous system (CNS), which also comprises the venous angiomas (currently best designated as developmental venous anomalies, DVAs), the arteriovenous malformations and the capillary telangiectasias [1]. Recently, it has been proposed that these three entities may in fact represent the same spectrum of a unique disease, which presents itself in different stages along the course of its natural history [2].
Cavernous malformations tend to expand slowly in size and carry a relative small annual risk of hemorrhage. Their expansive and mass effect potentials vary mainly in dependence of recurrent intra-sinusoidal hemorrhages [3, 4]. Because of the low pressure inside these lesions, the hemorrhages of CNS’ cavernous malformations rarely extend into the ventricles or to the subarachnoid space [5].
Most cavernous malformations occur sporadically and lonely [6]. However, multiple cavernous malformations may be found in up to 24% of patients and, in general, about 14% of patients have a familial history [7], with a dominant pattern of inheritance with incomplete penetration.
Only 10–30% of intracranial cavernous malformations are located in the posterior fossa. These lesions expand slowly with a relatively low annual risk of bleeding. Hemorrhage from brainstem cavernomas may cause devastating neurological deficits [8]. In fact, unlike cavernous malformations from other locations, the absence of non-eloquent nerve tissue in the brainstem, the presence of white matter pathways composed of ascending and descending fascicles as well as the several nerves nuclei make even small hemorrhages a frightening event [3].
In this paper the retrospective analysis of a multicenter casuistic of brainstem cavernous malformations treated surgically is reported. Important aspects of microsurgical anatomy of brainstem considered essential for proper selection of surgical approach are reviewed. A literature review on the surgical management of such lesions, with special focus on new intraoperative technologies (such as navigation and electrophysiological monitoring) as well as controversies regarding indication and timing of surgery is presented.
Casuistics and results
The surgical and outpatient follow-up charts from three neurosurgical institutions (Neurological Institute of Curitiba, Brazil; Santa Paula Hospital, São Paulo, Brazil and Assistência Neurológica, São Bernardo do Campo, Brazil) were retrospectively reviewed to evaluate the outcome of 43 patients harboring brainstem cavernous malformations treated surgically between 1999 and 2009. These lesions were classified according to their anatomical location as follows: ventral midbrain (1 case), lateral midbrain-thalamus (1 case), dorsal midbrain-thalamus (1 case), ventral midbrain-pons (3 cases), dorsal midbrain (8 cases), lateral pons (15 cases), dorsal pons (7 cases), lateral pontomedullary (1 case), dorsal pontomedullary (3 cases), lateral medulla (2 cases), dorsal medulla (1 case).
In this series 31 patients (72%) presented preoperatively only one episode of bleeding, while 10 patients (23%) had two episodes and 2 patients (4.65%) presented three or more previous hemorrhages. The surgical approaches used were fronto-temporal, transylvian (6 cases), infratentorial-supracerebellar (8 cases), suboccipital-telovelar (10 cases), retrosigmoid (15 cases), transtentorial-suboccipital (2 cases) and far-lateral (2 cases) (Fig. 1).
Total surgical removal at the first operation was possible in 42 patients (97%). One patient with a pontomedullary cavernoma associated with a large venous angioma, who presented four prior bleedings and was previously irradiated elsewhere remained with a residual lesion. This lesion could be totally removed in a second procedure.
New transient postoperative deficits were observed in six patients (20%): three presented diplopia due to a transient oculomotor nerve paresis (with complete resolution after 6 months), two presented Parinaud’s sign (both with complete remission after 4 months) and one patient presented vertical nystagmus, vertigo, and ataxia (which also resolved after 6 months). Two patients presented postoperatively hydrocephalus requiring shunt insertion. There was no mortality in this series.
In the 6-month follow-up 14 patients (32%) showed an improvement of preoperative neurological deficits. The patients with the pontomedullary cavernomas with multiple previous hemorrhages and irradiation remained with new neurological deficits.
Discussion
Natural history
The bleeding rates of brainstem cavernous malformation vary among the literature series between 0.6 and 6% per patient/year [6, 9, 10]. After the first bleeding, however, the rates of re-bleeding can reach 60% per patient/year [7]. Age over 35 years and lesions larger than 10 mm are associated with higher risk of bleeding [11]. Female patients have higher risk of recurrent hemorrhages suggesting that hormonal factors may be related with the pathophysiology of progression and bleeding [1, 7].
Bleeding episodes from brainstem cavernous malformations are rarely asymptomatic. However, in the majority of cases, even large hemorrhages in the brainstem cause deficits that are surprisingly limited to the ocular facial motility; rarely do severe motor deficits occur [10].
The most common neurological deficits are related to VI and VII cranial nerves. Symptoms such as headaches, nauseas and impairment of consciousness (usually related to increased intracranial pressure) are most common with bleedings from cavernous malformations located in the midbrain, and are usually related to obstructive hydrocephalus secondary to aqueduct’s compression [10].
Symptoms related to V, VII and VIII cranial nerves are typical of bleedings from cavernous malformations of the pons, while cardiovascular and respiratory instability, refractory hiccups and gastrointestinal bleedings are commonly observed in lesions located in the medullary region [7].
Diagnostic imaging
Computed tomography (CT) is usually the initial diagnostic procedure performed in most of the patients and is very useful in demonstrating the presence of bleeding (Fig. 2). The differentiation between hematomas of hypertensive origin from those caused by bleeding of a cavernous malformations may be difficult even with high-resolution CT and thin slices scans [12]. Magnetic resonance imaging (MRI) is the “gold-standard” method for diagnosing CNS’ cavernous malformations (Fig. 3). These lesions present usually at the MRI a typical appearance described as “blackberry” or “popcorn” which is related to the multiple previous hemorrhages. A hypersignal in both T1 and T2 surrounded by an irregular halo of hyposignal of variable length is observed. The T2-weighted Gradient Echo images or, more recently, the susceptibility weighted images (SWI) are extremely useful due to their high sensitivity to blood. It enables the diagnosis of micro-bleedings which could go unnoticed with other sequences. In fluid-attenuated inversion recovery (FLAIR) sequences, these lesions present a typical central hypersignal related to the presence of metahaemoglobin surrounded by a perilesional rim with hyposignal due to hemosiderin deposit. MRI is also useful to demonstrate the relationship between the cavernous malformation and the adjacent neurological structures. Modern techniques, such as diffusion tensor imaging (DTI) have proved to be extremely useful in demonstrating the involvement or displacement of the surrounding white matter tracts. It is an essential tool in planning the surgical approach for brainstem cavernous malformations [13, 14]. All preoperative images should be incorporated into navigation systems enabling a precise surgical procedure with very low morbidity rates [1].
Cavernous malformations are grouped in the class of CNS vascular malformations, but due to the absence of arterial feeders or active arteriovenous shunts they are usually not demonstrated by digital angiography [1, 4, 15, 16]. The association between cavernous malformations and the presence of concomitant satellite developmental venous anomaly (DVAs or venous angiomas) is well-documented. Therefore, preoperative evaluation through angiographic sequences of CT or MRI may be helpful, since the presence of such lesions, depending on their location, may change radically the planned surgical trajectory.
Microsurgical anatomy and surgical approaches
The precise knowledge of the brainstem anatomy, both of its superficial and deep structures, including white matter tracts and cranial nerves nuclei is essential for surgical planning of brainstem cavernous malformations.
Usually, brainstem cavernous malformations, which bleed, have a prominent point to the brainstem pial surface or to the walls of the fourth ventricle that can be identified by MRI. This point usually indicated the best route to access the lesion. In deep-seated lesions there may be a layer of parenchyma of 3–5 mm between the hematoma or lesion cavity and the outer surface of the brainstem. In such cases, coagulation and incision on the brainstem’s surface may be necessary.
Several studies detailing the functional brainstem anatomy led to the characterization of the so-called “safe entry-zones”, through which deep lesions in the brainstem can the accessed without damaging fascicles or brainstem nuclei [17, 18].
For a detailed analysis of the microsurgical anatomy of the brainstem and its implications in the selection of the best surgical approach to brainstem cavernous malformations, we divide such lesions in three basic groups with basis in a previous clinically validated classification [10]. It is already known that these groups represent entities with different functional prognosis due to their particular location:
-
Group I cavernomas localized exclusively within the pons;
-
Group II pontomesencephalic lesions;
-
Group III medullary lesions.
Taking into account such classification, we present a general overview about what we consider the most suitable microsurgical approaches to each particular location of the brainstem, as well as valuable anatomical landmarks in order to avoid injury to important underlying nervous structures. Each approach is illustrated with cadaveric specimens obtained from microsurgical dissections performed in our laboratory, as well as pre-, intra- and postoperative images of the author’s series of operated brainstem cavernous malformations [19]. We also demonstrate nuances of the surgical techniques with illustrative videos.
In our opinion, it is possible to divide microsurgical approaches to brainstem cavernous malformations in six great groups, corresponding to either ventral or dorsal approach to each location.
Microsurgical anatomy of the ventral midbrain
The midbrain can be divided in two morphological and structural different regions: a ventral portion (the midbrain tegmentum) and a dorsal portion (the midbrain tectum) (Fig. 4).
The midbrain can be divided into two morphologically and structurally different regions: a ventral portion, the mesencephalic tegmentum and a dorsal portion, the mesencephalic tectum. The red nucleus is an important structure of the midbrain. A “safe entry zone” has been proposed since the fibers of the corticospinal tract occupy only the intermediate 3/5 of the peduncle. This narrow window is delimited: above by the posterior cerebral artery, below by the superior cerebellar artery, medially by the emergence of the III cranial nerve and basilar artery and laterally by the pyramidal tract
Important microscopic structures of the midbrain tegmentum are the substantia nigra, the red nucleus, the reticular formation, the cerebral aqueduct and the periaqueductal gray substance. Ventral to the midbrain tegmentum, the two cerebral peduncles, separated by the interpeduncular fossa, appears as two great bundles of fibers originated from the superior border of the pons and diverging cranially to deeply penetrate the cerebral hemispheres.
Surgical approaches to ventral midbrain
Cavernous malformations located in the ventral midbrain can be divided into two topographic areas in relation to the best-indicated surgical approach:
-
Lesions situated anteromedially to the cerebral peduncles, in which the fronto-temporal transylvian approach is the best option (Fig. 5). In these mesencephalic cavernous malformations, the IV cranial nerve in the cisterna ambiens is the only clearly identifiable structure on the midbrain’s surface. The lateral geniculate body, the most posterior and superior structure, is scarcely visible, and the most superficial fasciculus which should be avoided is the medial lemniscus.
Fig. 5 a Magnetic resonance imaging used for preoperative navigation planning in a patient with a cavernous malformation located in the ventral midbrain, which was resected through a fronto-temporal transylvian approach. b Postoperative control demonstrating complete lesion resection and the absence of ischemia related to the procedure. c Intraoperative photos: left fronto-temporal transylvian approach. The cavernoma (CAV) is located medial to the oculomotor nerve (III)
-
Lesions located anterolaterally to the cerebral peduncles (at the level of the medial lemniscus and lateral geniculate bodies) in which the optimal approach is the subtemporal transtentorial approach (either isolated or combined with a fronto-temporal transylvian approach).
A “safe entry-zone” exists to avoid damage to the fibers of the corticospinal tract passing through the cerebral peduncle. This narrow window is delimited above by the posterior cerebral artery, below by the superior cerebellar artery, medially by the emergence of the III cranial nerve and basilar artery and laterally by the pyramidal tract.
Microsurgical anatomy of the ventral pons
At ventral surface, the pons is separated from the medulla by the pontomedullary sulcus. Three cranial nerves have their apparent origin at this sulcus on each side: the abducens nerve (which emerge between the bulbar pyramid and the pons), the facial nerve (which emerges between the olive and the pons) and the vestibulocochlear nerve (which maintains a close relation with the ipsilateral VII nerve situated medially). Between the facial and vestibulocochlear nerves emerge the nervus intermedius, which corresponds to the sensitive root of the VII nerve (Fig. 6).
Anterior view of the brainstem. The authors divide the surgical approaches to brainstem cavernous malformations with basis on the affected regions (midbrain, pons and medulla) and in dependence on their location (ventral or dorsal). At the ventral surface, the medulla oblongata is separated superiorly from the pons by the inferior pontine (also called pontomedullary) sulcus. The trigeminal nerve—which has two components: a larger sensitive root, and a smaller motor root—is the only nerve which has its apparent origin in the pons, emerging in its antero-lateral surface, medial to the middle cerebellar peduncle. In fact, the point of the emergence of this nerve constitutes the limit between the pons itself and the arms of the pons. The interpeduncular fossa, through which the oculomotor nerves pass, is limited anteriorly by two diencephalic structures: the mammillary bodies. The deep superior part of the interpeduncular fossa presents small orifices for the passage of small vessels, the posterior perforated substance
The pontine nuclei present as small group of gray matter in the middle of a dense net of crossing transversal white fibers. These nuclei are the final destiny of the cortico-pontine fibers and the origin of the ponto-cerebellar fibers, which reach the cerebellum through the middle cerebellar peduncle, also called arms of the pons.
Although many cranial nerves nuclei are localized in the dorsal part of the pons, the trigeminal nerve (which has two components: a larger sensitive root, and a smaller motor root) is the only cranial nerve which has its apparent origin in the pons. It emerges in its antero-lateral surface, medial to the middle cerebellar peduncle. In fact, the point of the emergence of this nerve constitutes the limit between the pons itself and the arms of the pons. At the ventral surface of the pons, the crossing white matter tracts of the middle cerebellar peduncle form a longitudinal depression, which is coincidentally the site of location of the basilar artery and, for such reason had been classically denominated basilar sulcus.
It is also important to emphasize that the space between the basilar artery and the ventral surface of the pons is occupied by a large number of small perforating vessels, which are essential to vascular supply of the deep neurological structures.
Surgical approaches to ventral pons
The ventral pons is one of the less frequent anatomical areas for brainstem cavernous malformations. The surgical route most employed for the lesions which partially extends into the anterolateral site of cerebellopontine angle is the retrosigmoid approach (Fig. 7) [12, 14].
The combined petrosal approach has also been suggested as an alternative to cavernous malformations of the ventral pons situated in front of the exit point of the V or VII–VIII cranial nerves [11]. Strictly, median lesions are usually attacked from the side of non-dominant sigmoid sinus. In cavernous malformations of the ventral pons with a significant hemorrhagic component the reticular formation is generally displaced laterally or posteriorly and the access through the anterolateral surface is usually preferred.
Although surgical access through the ventral side of the pons carries the risk of motor deficits due to lesions of cortico-pontine fibers (once these fibers are not closely packed but intercalated with transversal fasciculi of the cortico-ponto-cerebellar pathway) there is a reasonable chance of avoiding neurological deficits if the bulging and discoloration produced on the surface of the pons by the bleeding of the cavernous malformations is used as a surgical window.
Some authors have shown that at the exit point of the V cranial nerve at the pons an area with a low-density of motor fibers can be identified (about 1 cm wide and 1 cm lateral from the midline), providing a safe “entry-zone” to reach the deep structures of pons without incurring in additional neurological deficits. Although medial extension toward the root of the V cranial nerve for about 0.5 cm is possible, it must be reminded that the route of access must not direct too medial in order to avoid getting close to the midline where motor fibers are abundant [3].
Microsurgical anatomy of the ventral medulla
At the ventral surface, the medulla oblongata is separated superiorly from the pons by the pontomedullary sulcus. The medullary surface presents one median deep fissure, the anterior median fissure, and two longitudinal sulcus on each side (which are roughly parallel and continues with those of the spinal cord): the anterior lateral sulcus and the posterior lateral sulcus. These sulci divide the medullae into three portions, which as seen from their surface continue with the spinal cord funiculli—anterior, lateral and posterior.
On each side of the anterior median fissure, there is a longitudinal prominent column, the medullary pyramids, which are limited laterally by the anterior lateral sulcus. These structures are composed by the descending corticospinal fibers originated in the ipsilateral motor cortex. In the lower part of the ventral medulla, 75–90% of these fibers cross obliquely the midline forming an interdigiting bundle, the so-called pyramidal decussation. These crossing fibers will form the lateral corticospinal tracts of the spinal cord while the uncrossing fibers will constitute the anterior corticospinal tract.
Laterally to the pyramids and extending up to 2 cm below the pons, two ovoid prominences, the medullary ollivae (also called ollivary eminences) bulges into the ventral surface of the upper medulla. Underlying these structures, there is a dense grouping of gray matter, the inferior ollivary nuclei, which form important brainstem relay for the extrapyramidal connections between the cerebral cortex and the cerebellar hemispheres. Ventral to the olivae, the roots of the hypoglossal nerve emerge from anterior lateral sulcus. Posterior to the olivae, at a region known as the paraollivary fossa, the roots of the IX and X cranial nerves emerge from the posterior lateral sulcus. Caudally, also emerging from the posterior lateral sulcus, emerge the roots of the cranial or medullary part of the XI cranial nerve.
At the deep ventral part of the medulla, posterior to the pyramidal tracts and anterior to the central canal, is situated the spinal lemniscus (composed by fibers of the ascending spino-thalamo-cortical sensory pathway), which crosses the midline immediately below the olives (Fig. 8).
a Laterally to the pyramids and extending up to 2 cm below the pons, two ovoid prominences, the medullary olivae or ollivary eminences, bulges in the ventral surface of the upper medulla. b Ventrally to the olivae, the radiculae of the hypoglossal nerve emerges from the anterior lateral sulcus. At the deep ventral part of the medulla, posterior to the pyramidal tracts and anterior to the central canal, is situated the spinal lemniscus (composed by fibers of the ascending spinothalamocortical sensory pathway), which decussates immediately below the olives
Other valuable anatomical landmarks located at the ventral medulla are the two vertebral arteries, which join each other most of the times at the level of pontomedullary sulcus to form the basilar artery. The anterior spinal artery, which may emerge either from basilar or from the vertebral arteries, occupies the median ventral fissure in the lower part of the medulla and descends at the anterior spinal sulcus, to supply the ventral part of the spinal cord (Fig. 9).
The two vertebral arteries, which join each other at the level of the pontomedullary sulcus, to form the basilar artery, are also valuable anatomical landmarks in the ventral medullary region. On each side of anterior median fissure there are two prominent longitudinal columns, the bulbar pyramids, which are bordered laterally by the anterolateral sulcus. These structures are composed of descending corticospinal fibers. At the inferior ventral medulla, 75–90% of these fibers cross obliquely the median plane at the decussation of pyramids
Surgical approaches to the ventral medulla
Cavernous malformations located in the ventral portion of the medullae are very rare. In such cases the best surgical approach for this region seems to be the far-lateral with its possible variants (supra, para or transcondylar approaches). Because of the superficial position and close relation of the motor fibers on each side, it is possible to gain strictly median surgical access to the ventral medulla (the ideal route in order to access to the deep ventral part of the medulla avoiding the pyramidal fibers) in those cases in which the cavernous malformation has an exophytic component. On the other hand, a paramedian oblique route, at the level of the anterior lateral sulcus (between the roots of the XII cranial nerve and C1) is recommended.
Microsurgical anatomy of the dorsal midbrain
The dorsal midbrain, also called tectum of midbrain, corresponds, in a surgical view, to the region of the quadrigeminal cistern and is limited inferiorly by the superior surface of the cerebellum and superior medullary velum, superiorly by the pineal body and superolaterally by both thalamus. Important macroscopic structures of this region are the trochlear nerves and the quadrigeminal body (Fig. 10).
The dorsal midbrain, also called midbrain tectum, is macroscopically limited: below by the superior surface of the cerebellum, superior cerebellar peduncle and superior medullary velum and superolaterally by the pineal body above and both thalamus. Above the emergence of the trochlear nerves, four round masses, the superior and inferior colliculi form the quadrigeminal plate. The region above the quadrigeminal plate and posterior to pineal body pursues a rich channel of venous structures: the internal cerebral veins, proceding from the roof of the third ventricle, the basal veins of Rosenthal, to which the veins from inferior horn of the lateral ventricles drain, and the great cerebral vein (also called Vein of Galen). Both internal cerebral veins, together with the inferior sagittal sinus join posteriorly to form the straight sinus
The trochlear nerve is the only cranial nerve with its apparent origin in the dorsal surface of the brainstem. It is a very tiny nerve, which emerges a few millimeters from the midline on each side, continues laterally around the midbrain and further turns in a ventral direction.
Above the emergence of the IV nerves, four round masses, the superior and inferior colliculi form the so-called quadrigeminal plate. It is important to remember that the region superior to the quadrigeminal plate and posterior to pineal body pursues a rich channel of venous structures which make the surgical approach to these region challenging: among them we highlight the internal cerebral veins, which proceed from the roof of the third ventricle, the basal veins of Rosenthal, which drains the veins from the inferior horn of the lateral ventricles, and the great vein of Galen, formed by the confluence of the these structures (Fig. 10).
Surgical approaches of dorsal midbrain
The dorsal midbrain is the region that presents the highest density of auditory and oculomotor fibers. Two surgical approaches to the dorsal midbrain are possible: the supracerebellar-infratentorial and the suboccipital-transtentorial approaches (Fig. 11) [11].
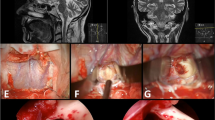
a Magnetic resonance imaging used for preoperative navigation planning in a patient with a brainstem cavernous malformation located in the dorsal midbrain. b Surgical view of a supracerebellar-infratentorial approach. c Surgical exposure of the cavernous malformation (Cav) and tentorium (T). d, e Removal of the cavernous malformation (arrows)
The trochlear nerves nuclei are situated in the ventral part of the cerebral aqueduct (also called Sylvian aqueduct) at the level of the inferior colliculi. Injury to this nerve caused by traction or pressure on the surface of the superior medullary velum has already been reported [5].
On the midline, also ventral to the cerebral aqueduct, are located the three somatic nuclei of the III cranial nerve. Posterior to these somatic nuclei lies the visceral nucleus of the III cranial nerve (the nucleus of Edinger-Westphal), responsible for the pupillary light reflex (constriction). The medial longitudinal fasciculus can be found anterior and lateral to these nuclei. Also in this region, the trigeminal lemniscus lies laterally to the cerebral aqueduct.
In order to access the deep portion of the dorsal midbrain a median intercollicular approach (between the superior and inferior colliculus) is possible, even though small quantities of intercollicular fibers are usually present. If necessary, it is possible to pass across the cerebral aqueduct as far as the central mesencephalic region, but not without compromising ocular motility, visual reflex or conjugate eye movements. If the cavernous malformation partially enters into the third ventricle, it is advisable to enter it through the suprapineal recess.
Microsurgical anatomy of dorsal pons
The floor of the fourth ventricle has a rhomboid shape and can be further divided into two triangles of different size: a greater superior and a smaller inferior triangle. The superior triangle corresponds to the dorsal segment of the pons, while the inferior corresponds to the dorsal segment of the medulla. Boththese triangles are separated by thin transversal fibers which cross transversally from the vestibular area until the median sulcus: the medullary striae of the fourth ventricle (Fig. 12).
The fourth ventricle floor has a rhomboid shape and can be subdivided into two triangles of different sizes: a major superior (corresponding to the dorsal portion of the pons dorsal) and a smaller inferior (corresponding to the dorsal portion of the medulla). There are two “safe entry zones” in this region: inferior lesions, located in the dorsal portion of the medulla, are preferably accessed through the infracollicular approach, while pontine lesions are preferably accessed through the supracollicular approach
In all its extension the floor of the fourth ventricle presents a midline depression, the median sulcus, which disappears cranially at the cerebral aqueduct and caudally at the central canal of the medullae. On each side of the median sulcus, there is an ovoid structure, the median eminence, which is limited laterally by the sulcus limitans. On each side of this structure, the sulcus limitans becomes deeper, forming the so-called fovea: one superior to the medullary stria (the superior fovea) and other below (the inferior fovea).
Medially to the superior fovea the medial eminence becomes larger, forming an elevated ovoid structure, the facial colliculus, which is constituted microscopically by the fibers of the facial nerve which surround the nucleus of the VI nerve. Laterally to the sulcus limitans and extending laterally on each side in direction to the lateral recesses, there is a great triangular space, the vestibular area (which corresponds microscopically to the vestibular and cochlear nuclei).
In the superior portion of floor of the fourth ventricle, which corresponds to the dorsal surface of the pons, two “safe entry zones” have been described, one above and other below the facial nerve’s colliculus: corresponding to the described supra and infracollicular approach [20, 21].
Surgical approaches of dorsal pons
The surgical access to the dorsal pons is performed through the floor of the fourth ventricle. In order to reach the area superior to the stria medullaris, a suboccipital craniotomy is usually performed, with further elevation of both tonsillae and opening of the roof of the fourth ventricle in the region between the inferior medullary velum and the tela choroidea (the so-called telovelar approach (Fig. 13).
a Magnetic resonance imaging used for preoperative navigation planning in a patient with a cavernous malformation located in the dorsal pons. b Surgical exposure through a suboccipital telovelar approach. c, d Cavernous malformation located at the floor of the fourth ventricle floor (arrows). e Surgical cavity after radical removal of the lesion (arrow)
This approach seems to be more anatomical than the transvermian approach (performed through the splitting of the inferior portion of the vermis) and, according to some authors, presents lower rates of complications such as cerebellar mutism, although this is still a debate in the literature [22].
After reaching the structures of the floor of the fourth ventricle, in order to reach a lesion in the dorsal portion of the pons, a supracollicular approach is recommended. This approach is performed between the traversing fibers of the facial nerve crossing above the upper pole of the IV nerve nucleus and the fibers of the trochlear nerve crossing within the superior medullary velum. The lateral boundary of the approach is formed by the superior cerebellar peduncle and the trigeminal motor nuclei, which are located at the very lateral edge of the rhomboid fossa, close to the superior cerebellar peduncle. Medially, the medial longitudinal fasciculus restricts the surgical access and a strict midline approach will almost surely damage both medial longitudinal fasciculus with subsequent bilateral ophthalmoplegia [23]. Even from a paramedian approach, the access to the deep portion of the dorsal pons presents risks of damage to the nuclei and to the ascending and descending reticular formation, such as the caudal pontine reticular and the pontine raphe nucleus, which are located lateral and medial to the fasciculus longitudinalis medialis. Fortunately, unilateral lesions of these structures do not seem to produce permanent neurological deficits in terms of consciousness, regulation of sleep-wake cycle and vigilance [21].
At the level of the facial colliculus the lateral lemniscus (formed by the ascending acoustic fasciculus) and the spinal lemniscus (formed by the ascending spino-thalamo-cortical sensory pathway) lie anterolaterally and anteromedially, respectively, to the nucleus of the VI cranial nerve, at a depth of about 1 cm. Using a transventricular approach the possibility of damaging the lateral lemniscus and the spinal lemniscus is rare once the abducens’ nucleus is easily identifiable. As the position of the trigeminal lemniscus and motor and sensorial trigeminal nuclei are even more lateral to the lateral lemniscus, at the junction of the pons with the middle cerebellar pedicle, they are also rarely affected when a transventricular approach is performed.
Microsurgical anatomy of dorsal medulla
The lower part of the fourth ventricle, below the stria medullaris, represents the dorsal part of the medulla and is limited inferolaterally by the inferior cerebellar peduncles and by the gracile and cuneiform tubercles.
Separated at the midline by the median sulcus, two small triangles with the vertex downward can be observed: the hypoglossal trigone (which corresponds microscopically to the XII nerve nuclei) and lateral to the hypoglossal trigone, another triangular area, with a slight grayer color (in latin: area cinerea), the vagal trigone (which corresponds microscopically to the dorsal X nerve nuclei). These two pairs of triangles appear to resemble a leather pen; hence, this region is also known as “calamus scriptorius”.
Lateral to the inferior fovea, immediately under the ependymal surface, is located the solitary tract nucleus, which receives visceral sensation and taste from the facial, glossopharyngeal and vagal nerves. At the relative depth of about 0.5 cm and in front of the solitary tract nucleus is situated the ambiguous nucleus, the origin of the somatomotor fibers of the IX, X and XI cranial nerves which supplies the striated muscles of the pharynx and larynx (Fig. 14).
The lower part of the fourth ventricle, below the stria medullaris, represents the dorsal part of the medulla and is limited inferolaterally by the inferior cerebellar peduncles and by the gracile and cuneiform tubercles. Separated at the midline by the median sulcus, two small triangles with the vertex downward can be observed: the hypoglossal trigone (which corresponds microscopically to the XII nerve nuclei), and lateral to the hypoglossal trigone, another triangular area, with a slight grayer color (in latin: area cinerea), the vagal trigone (which corresponds microscopically to the dorsal X nerve nuclei). At the relative depth of about 0.5 cm and in front of the solitary tract nucleus is situated the ambiguous nucleus, the origin of the somatomotor fibers of the IX, X and XI cranial nerves which supplies the striated muscles of the pharynx and larynx
Surgical approaches of dorsal medulla
The surgical approach recommended to access cavernous malformations located in the posterior portion of the medulla is also the median suboccipital approach. In order to avoid potentially irreversible deficits of deglutition, phonation and taste, morphometrical studies have shown that the infracollicular paramedian approach should be performed in an area with a maximum extension of 0.9 cm between the facial colliculus and hypoglossal and vagal trigone [23]. In our experience direct electrophysiological stimulation can be regarded as safe, reliable and fast adjuvant technique for intraoperative localization of such motor nuclei).
Surgical timing
Some authors recommend performing surgery of brainstem cavernous malformations in the sub-acute stage, several days or weeks after the initial hemorrhage [24]. This time delay would provide more time for neurological stabilization and allow better differentiation between the hematoma and the cavernous malformation itself on MRI. According to these authors, the knowledge of the exact location of the vascular portion of the lesion within the cavity of the hematoma, especially in those cases of extensive bleeding, may be essential during selection of the best surgical approach. Recent series suggest that surgery performed in the sub-acute phase (in a period of 10–30 days after ictus) is associated with a better prognosis when compared with delayed surgery [25, 26].
There are also those who advocate performing surgery as soon as possible [17, 27]. According to these authors, this strategy would prevent the occurrence of reactive gliosis, hyaline degeneration, and the presence of extra-lesional calcifications, which may appear months after the original bleeding and lead to significant increase in surgical difficulty.
Surgical indications
Some studies suggest that only patients with multiple bleedings or progressive neurological deterioration would benefit from surgical treatment. According to these reports in the long-term follow-up surgical removal of incidental cavernous malformations does not present any functional benefit in relation to the natural history of the disease.
Most of the surgical literature, however, demonstrates that unlike cavernous malformations in other locations of the CNS, brainstem lesions have a higher risk of recurrent bleeding and progressive neurological deficits. In some series, 75% of the lesions (especially those located in the ponto-mesencephalic transition) presented at the time of diagnosis radiological evidence of multiple previous hemorrhages [11]. Furthermore, there is significant evidence that neurological deficits resulting from re-bleeding is more severe than those related to the initial hemorrhage [26].
Additionally, surgery performed by experienced neurosurgeons may presents very low morbidity and mortality rates [10]. A study with 8 years’ follow-up showed that patients with symptomatic brainstem cavernous malformations treated conservatively or with only ventricular shunt insertion (hydrocephalus) presented worse prognosis than patients submitted to microsurgical resection [26]. It also demonstrated that repeated bleedings increased significantly pre-existing neurological deficits and made surgical dissection more difficult and traumatic [26].
Extensive bleeding with deterioration of consciousness level, respiratory or hemodynamic instability, as well as motor deficits are not contraindications for early surgery. These symptoms could even indicate emergent surgical treatment. Early hematoma drainage with subsequent mass-effect relief could provide a better chance to reverse such deficits.
According to the literature, surgery of symptomatic brainstem cavernous malformations is recommended in the following situations [10]:
-
For single-bleeding lesions (acute or sub-acute stages as demonstrated by MRI and in which perilesional hematoma reaches or has a distance <2 mm from the pial or ventricular surface).
-
For multiple-bleeding lesions which present with progressive neurological deficits regardless of the location of the lesion.
In asymptomatic patients, factors that, although are not absolute indications for surgery, suggest a significant benefit in the long-term follow-up are young patients with a single bleeding episode (due to their long life expectancy) and the presence of asymptomatic multiple hemorrhages as demonstrated in serial imaging exams.
The conservative treatment may be a reasonable option in the following situations (in either symptomatic or asymptomatic cases):
-
Single-bleeding deep-located lesions (>2 mm to pial or ventricular surface).
-
Multiple-bleeding but clinically stable lesions (without mass effect) in patients of advanced age or in those with no clinical conditions for surgery. In such cases careful clinical follow-up and serial imaging examinations are recommended. These patients should be advised to seek an emergency neurosurgical department even in the presence of minor suspicious symptoms.
Surgical nuances
The first step of the surgical procedure should be the drainage of the surrounding hematoma followed by exposure and dissection of the lesion. Care is taken to not penetrate the cavernous malformation itself but to dissect it around the borders to minimize bleeding. Cavernous malformations generally present a good cleavage plane. Whereas acute hematoma could facilitate surgical dissection, delayed or sub-acute surgical procedures and multiple hemorrhages could make surgical resection more difficult as the capsule might adhere to the surrounding brain tissue.
After total resection meticulous hemostasis is performed. Removal of the hemosiderin-stained gliotic tissue surrounding the cavity of the hematoma is avoided to not cause additional neurological deficits. In the past these capsules were though to be composed of only hyaline degeneration, fibrous proliferation and even calcifications [10, 28]. A recent study with DTI-MRI tractography demonstrated the presence of viable white matter tracts passing through this hemosiderin rim [28].
Adjuvant needed technologies
Navigation
Navigation plays an essential role in the preoperative planning as well as in the intraoperative localization of brainstem cavernomas. MRI may demonstrate an apparently superficial location but abnormalities of the pial surface of the brainstem may not be visible. According to our experience (as demonstrated in illustrative videos) the surface of the brainstem almost always appears normal after operative exposure and navigation is extremely useful to plan the pial incision [29, 30].
Electrophysiological monitoring and stimulation
The use of intraoperative monitoring with evoked potentials (SSEPs MMEPs) as well as cranial nerve monitoring and subcortical motor tracts stimulation is currently highly recommended in assisting the surgical resection of brainstem cavernous malformations [24]. Moreover, intraoperative electrophysiological stimulation of the floor of the fourth ventricle has proved to be extremely precise in order to localize the so-called “safe entry-zones” and avoid direct damage of cranial nerve nuclei [18, 31].
Radiotherapy
The efficacy of radiation therapy (radiosurgery) for brainstem cavernous malformations (unlike vascular malformations) is still extremely controversial and its benefits are dubious. Some authors have reported a reduction in annual risk of bleeding, as well as a reduction in the rates of seizures in the case of supratentorial cavernous malformations after radiosurgery [32, 33] Radiosurgical and radiotherapy series demonstrated a high incidence of complications in treatment of brainstem cavernomas [34, 35]. According to some authors the maximal permitted marginal dosis to the brainstem (approximately 15 Gy) may limit the potential therapeutic benefits of radiation.
Functional results
Although mortality rates of recent microsurgical series are very low (around 2%), surgery of brainstem cavernous malformations is usually associated with additional transitory morbidity. Experienced skull base neurosurgeons have reported new cranial nerve deficits in approximately 47% patients [1, 7]. Internuclear ophtalmoplegia is a commonly reported neurological deficit [7].
Patients with pontomesencephalic and pontine cavernomas (as well as with multiple preoperative hemorrhages) present higher probability of facial paresis. Higher preoperative Karnofsky Performance Scores, small-volume lesions, early surgery and single bleeding are also factors known to be associated with a better functional prognosis [10].
The results of large series with long-term follow-up demonstrate that more than 50% of the patients who experienced postoperative new neurological deficits improved over time to the previous preoperative condition or even better [36]. The III, V and VII cranial nerves are more prone to completely recover [7]. In our series although 13% of the patients presented postoperatively with new neurological deficits, only 1 patient (2%) remained symptomatic in the 6-month follow-up and 32% improved their preoperative deficits.
Conclusions
According to our experience, surgical resection remains the treatment of choice of brainstem cavernomas if there was previous hemorrhage and the lesion reaches the pial surface of brainstem. An excellent outcome with very low morbidity and no mortality may be achieved if the surgery is performed by experienced neurosurgeons in selected referral centers employing all the currently available technology.
References
Cantore G, Missori P, Santoro A (1999) Cavernous angiomas of the brain stem. Intra-axial anatomical pitfalls and surgical strategies. Surg Neurol 52:84–94
Abla A, Wait SD, Uschold T, Lekovic GP, Spetzler RF (2008) Developmental venous anomaly, cavernous malformation, and capillary telangiectasia: spectrum of a single disease. Acta Neurochir 150:487–489
Pozzati E, Giuliani G, Nuzzo G, Poppi M (1989) The growth of cerebral cavernous angiomas. Neurosurgery 25:92–97
Ramina R, Ingunza W, Vonofakos D (1980) Cystic cerebral cavernous angioma with dense calcification. J Neurosurg 52:259–262
Zimmerman RS, Spetzler RF, Lee KS, Zabramski JM, Hargraves RW (1991) Cavernous malformations of the brainstem. J Neurosurg 75:32–39
Porter RW, Detwiler PW, Spetzler RF, Lawton MT, Baskin JJ, Derksen PT, Zabramski JM (1999) Cavernous malformations of the brainstem: experience with 100 patients. J Neurosurg 90:50–58
Wang CC, Liu A, Zhang JT, Sun B, Zhao YL (2003) Surgical management of brain-stem cavernous malformations: report of 137 cases. Surg Neurol 59:444–454
Vinas FC, Gordon V, Guthikonda M, Diaz FG (2002) Surgical management of cavernous malformations of the brainstem. Neurol Res 24:61–72
Simard JM, Garcia-Bengochea F, Ballinger WE Jr, Mickle JP, Quisling RG (1986) Cavernous angioma: a review of 126 collected and 12 new clinical cases. Neurosurgery 18:162–172
Samii M, Eghbal R, Carvalho GA, Matthies C (2001) Surgical management of brainstem cavernomas. J Neurosurg 95:825–832
Kupersmith MJ, Kalish H, Epstein F, Yu G, Berenstein A, Woo H, Jafar J, Mandel G, De Lara F (2001) Natural history of brainstem cavernous malformations. Neurosurgery 48:47–54
Osborn AG, Salzman KL, Barkovick AJ (2009) Diagnostic imaging: brain, 2nd edn. Lippincott Williams & Wilkins, Philadelphia
Chen X, Weigel D, Ganslandt O, Buchfelder M, Nimsky C (2007) Diffusion tensor imaging and white matter tractography in patients with brainstem lesions. Acta Neurochir 149:1117–1131
Chen X, Weigel D, Ganslandt O, Fahlbusch R, Buchfelder M, Nimsky C (2007) Diffusion tensor-based fiber tracking and intraoperative neuronavigation for the resection of a brainstem cavernous angioma. Surg Neurol 68:285–291
Garrett M, Spetzler RF (2009) Surgical treatment of brainstem cavernous malformations. Surg Neurol 72:3–9
Moringlane JR, Ramina R (1984) Angiographically occult vascular malformations in functional areas of the brain. Diagnosis and treatment. Zentralbl Neurochir 45:268–272
Fahlbusch R, Strauss C, Huk W (1991) Pontine-mesencephalic cavernomas: indications for surgery and operative results. Acta Neurochir Suppl 53:37–41
Kyoshima K, Kobayashi S, Gibo H, Kuroyanagi T (1993) A study of safe entry zones via the floor of the fourth ventricle for brain-stem lesions. Report of three cases. J Neurosurg 78:987–993
Meneses MS (1999) Neuroanatomia aplicada. Guanabara-Koogan, Rio de Janeiro
Strauss C, Lutjen-Drecoll E, Fahlbusch R (1997) Pericollicular surgical approaches to the rhomboid fossa. Part I. Anatomical basis. J Neurosurg 87:893–899
Strauss C, Romstock J, Fahlbusch R (1999) Pericollicular approaches to the rhomboid fossa. Part II. Neurophysiological basis. J Neurosurg 91:768–775
Shimoji K, Miyajima M, Karagiozov K, Yatomi K, Matsushima T, Arai H (2009) Surgical considerations in fourth ventricular ependymoma with the transcerebellomedullary fissure approach in focus. Childs Nerv Syst 25:1221–1228
Bricolo A, Turazzi S (1995) Surgery for gliomas and other mass lesions of the brainstem. Adv Tech Stand Neurosurg 22:261–341
Sandalcioglu IE, Wiedemayer H, Secer S, Asgari S, Stolke D (2002) Surgical removal of brain stem cavernous malformations: surgical indications, technical considerations, and results. J Neurol Neurosurg Psychiatry 72:351–355
Bruneau M, Bijlenga P, Reverdin A, Rilliet B, Regli L, Villemure JG, Porchet F, de Tribolet N (2006) Early surgery for brainstem cavernomas. Acta Neurochir 148:405–414
Mathiesen T, Edner G, Kihlstrom L (2003) Deep and brainstem cavernomas: a consecutive 8-year series. J Neurosurg 99:31–37
Fahlbusch R, Strauss C, Huk W, Rockelein G, Kompf D, Ruprecht KW (1990) Surgical removal of pontomesencephalic cavernous hemangiomas. Neurosurgery 26:449–457
Cauley KA, Andrews T, Gonyea JV, Filippi CG (2010) Magnetic resonance diffusion tensor imaging and tractography of intracranial cavernous malformations: preliminary observations and characterization of the hemosiderin rim. J Neurosurg 112:814–823
Mao Y, Zhou L, Du G, Chen L (2003) Image-guided resection of cerebral cavernous malformations. Chin Med 116:1480–1483
Oiwa Y, Nakai K, Masaki Y, Masuo O, Kuwata T, Moriwaki H, Itakura T (2002) Presigmoid approach for cavernous angioma in the pons—technical note. Neurol Med Chir 42:91–98 (discussion 97-8)
Ishihara H, Bjeljac M, Straumann D, Kaku Y, Roth P, Yonekawa Y (2006) The role of intraoperative monitoring of oculomotor and trochlear nuclei-safe entry zone to tegmental lesions. Minim Invasive Neurosurg 49:168–172
Kondziolka D, Lunsford LD, Flickinger JC, Kestle JR (1995) Reduction of hemorrhage risk after stereotactic radiosurgery for cavernous malformations. J Neurosurg 83:825–831
García-Muñoz L, Velasco-Campos F, Lujan-Castilla P, Enriquez-Barrera M, Cervantes-Martínez A, Carrillo-Ruiz J (2007) Radiosurgery in the treatment of brain cavernomas. Experience with 17 lesions treated in 15 patients. Neurochirurgie 53:243–250
Liscák R, Vladyka V, Simonová G, Vymazal J, Novotny J Jr (2000) Gamma knife radiosurgery of the brain stem cavernomas. Minim Invasive Neurosurg 43:201–207
Pollock BE, Garces YI, Stafford SL, Foote RL, Schomberg PJ, Link MJ (2000) Stereotactic radiosurgery for cavernous malformations. J Neurosurg 93:987–991
Ohue S, Fukushima T, Kumon Y, Ohnishi T, Friedman AH (2010) Surgical management of brainstem cavernomas: selection of approaches and microsurgical techniques. Neurosurg Rev 33(3):315–322 (discussion 323–4)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramina, R., Mattei, T.A., de Aguiar, P.H.P. et al. Surgical management of brainstem cavernous malformations. Neurol Sci 32, 1013–1028 (2011). https://doi.org/10.1007/s10072-011-0477-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-011-0477-8