Abstract
Background
Giant intracranial aneurysms (GIA) are often not eligible for direct clip occlusion. Surgical alternatives include partial clip occlusion or the placement of a cerebrovascular bypass or the combination of both. These alternative indirect strategies are expected to lead to a decrease in GIA volume over time rather than instantaneously. To examine whether this is the case, we analyzed follow-up imaging results 1 year after surgery.
Methods
We retrospectively screened the prospective GIA Registry’s imaging database for anterior circulation GIA treated by surgical strategies other than direct clipping. We measured pre- and 1-year post-treatment GIA volume, lateral ventricle volume (LVV), and mid-line shift (MLS) in 19 cases.
Results
After a mean follow-up of 466 days (standard deviation ±171) GIA volumes decreased from 9.6 cm3 (interquartile range (IQR) 6.1–14.1) to 4.3 cm3 (IQR 2.9–5.7; p < 0.01). Ipsilateral LVV increased from 8.6 cm3 (IQR 6.4–24.9) to 16.0 cm3 (IQR 9.1–27.2; p < 0.01) while contralateral LVV increased from 10.3 cm3 (IQR 7.3–20.1) to 11.7 cm3 (IQR 8.2–19.4; p = 0.02). MLS changed from 0.1 mm (IQR −1.9 to 2.0) to −0.9 mm (IQR −1.8 to 0.4; p = 0.03). The decrease in GIA volume correlated with the increase in ipsilateral LVV (rs = 0.60; p = 0.01) but not with the changes in MLS (rs = 0.41; p = 0.08).
Conclusions
In our patient cohort, surgical strategies other that direct clipping for the treatment of anterior circulation GIA lead to a significant decrease in GIA volume over time. The resulting decrease in mass effect was more sensitively monitored by the measurement of changes in ipsilateral LVV than changes in MLS.
Clinical Trial Registration-URL
http://www.clinicaltrials.gov. Unique identifier: NCT02066493.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Giant intracranial aneurysms (GIA) are defined as aneurysms with a diameter ≥ 25 mm. They are known to exert considerable mass effect on the brain, which differentiates them from most non-giant intracranial aneurysms (IA) [1, 6, 7, 14]. In contrast to the treatment of non-giant IA, GIA treatment is therefore not only directed at aneurysm occlusion but also at the reduction of the mass effect on the brain.
Reaching this twofold goal is complicated by the fact that GIA frequently incorporate thrombus and neighboring vessels, which makes regular aneurysm treatment strategies like endovascular coiling or direct surgical clipping unfeasible. A relevant alternative is the surgical establishment of a cerebrovascular bypass in combination with proximal and/or distal GIA occlusion [9, 17, 18, 20]. This indirect surgical strategy leaves the body of the GIA basically untouched and seeks to alter blood flow patterns in order to reduce hemodynamic stress on the GIA and facilitate GIA shrinkage over time, resulting in a decrease in mass effect. Some authors have questioned the ability of GIA to shrink over time if treated by such an indirect strategy [13].
So far, no scientific evidence exists on postoperative changes in the mass effect of GIA that cannot be clipped directly. Furthermore, there is uncertainty on how to best quantify changes in the mass effect GIA exert on the brain. In the field of intracerebral hemorrhage (ICH) mid-line shift (MLS) or lateral ventricle volume (LVV) have long been used as indicators of changes in mass effect [10, 16].
We designed an analysis to examine changes in cerebral mass effect over time after surgical treatment of GIA that are not eligible for direct clipping. The main goal was to describe changes in GIA volume on follow-up magnetic resonance imaging (MRI) along with changes in MLS and LVV. We also aimed to examine whether changes in GIA volume correlate with those in MLS and LVV.
Methods
Patients
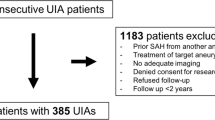
All imaging and clinical data for this analysis were extracted from the Giant Intracranial Aneurysm Registry’s prospective database. This registry is an international observational trial that is currently collecting clinical and imaging data on GIA throughout Europe and Asia [4]. Data collection was approved by the ethics committee of the Charité, Berlin (EA2/052/08). Each patient or their next of kin consented to the participation in this trial. The GIA Registry is listed at clinicaltrials.gov (NCT02066493). Inclusion criteria for the specific analysis presented here, which is a sub-analysis of the GIA Registry, were:
-
1)
diagnosis of an unruptured anterior circulation GIA on MRI,
-
2)
the GIA was not treated by direct clipping but by indirect surgical strategies, as described below, either due to the incorporation of important vessels into the GIA and/or the presence of relevant intra-aneurysmal thrombus or calcification,
-
3)
surgery was conducted without partial GIA resection or thrombectomy,
-
4)
existence of two separate MRI examinations in the registry’s imaging database, of which one was done less than 3 months before treatment and the other one at least 9 months after treatment.
We exclusively included cases located in the anterior circulation since at this location the mass effect is more likely to simultaneously affect both the lateral ventricles and the mid-line of the brain.
Neuroimaging and volumetric analysis
Since T2-weighted images (T2WI) produce optimal contrast between cerebrospinal fluid and neighboring brain structures, data from T2WI (in axial slices) were collected. All imaging was independently analyzed at the GIA Registry’s coordinating center by two separate examiners (N.M. and J.D.). Analyses of GIA volume and lateral ventricle volume (LVV) were performed using the software “iPlan Cranial” (BrainLab, Heimstetten, Germany), which is used in clinical routine mainly for preoperative neuronavigation planning. The circumference of the object of interest was manually marked using the mouse cursor within each slice of the T2WI to create a segmentation. The software then calculated the object’s volume by multiplying the marked areas in each slice with the slice thickness of the T2WI. To quantify MLS the distance between the septum pellucidum and the connecting line between anterior and posterior insertion of the falx cerebri was measured at the level of the third ventricle [21, 22].
Surgical strategies
Surgical strategies were divided according to the following categories: bypass combined with proximal or distal occlusion or aneurysm trapping; only bypass without aneurysm occlusion or only proximal occlusion without bypass. Proximal occlusion refers to the surgical occlusion (either by clip or ligation) of the parent vessel proximal to the GIA. Distal occlusion describes the surgical occlusion of one or more vessel branches directly exiting the GIA.
Statistical analysis
Statistical analysis was performed using SPSS software, Version 22.0.0.0 (IBM Corp., Armonk, NY, USA). The Shapiro–Wilk test was used to test variables for normal distribution. Normally distributed values are given as means with standard deviation, not normally distributed values as medians with interquartile range (IQR). Differences between groups for normally distributed values were analyzed using the independent t test. The Mann–Whitney U test and the Wilcoxon signed-rank test were applied to compare values that were not normally distributed. The relation between changes in GIA volume and those in LVV was examined by Spearman correlation. Inter-observer variabilities were calculated using the two-way random effects model intra-class correlation test.
Results
Nineteen cases of anterior circulation GIA in 16 patients (eight female, eight male) were included into the analysis. Three patients presented with bilateral GIA of which both were treated in separate surgical sessions. All surgical procedures were conducted between March 2009 and February 2012. Mean time to follow-up MRI was 466 days (±171). Mean patient age was 52 years (±13). Patient and treatment characteristics are displayed in Table 1. In 15 cases, cerebrovascular bypass surgery was conducted in combination with proximal and/or distal GIA occlusion. In two cases only, a bypass was placed without GIA occlusion due to relevant deterioration of intra-operative monitoring of motor evoked potentials during the attempt to partially occlude the GIA. In two cases only a proximal occlusion of a cavernous internal carotid GIA was conducted without bypass placement. MRI was performed at nine different institutions. Mean axial slice thickness in the T2WI was 3.8 mm (±1.9). Interobserver agreement for GIA volume, LVV, and MLS was excellent (each with correlation coefficients >0.91, p < 0.05). Figure 1 gives an example of the mass effect of a GIA of the right MCA before and 13 months after treatment.
Magnetic resonance images of a GIA of the right MCA (a) before and (b) 13 months after surgical placement of a radial artery graft high-flow bypass and proximal GIA occlusion. As the GIA has shrunk after treatment, there is an increase in ipsilateral LVV. Also, MLS is reduced after treatment and even slightly skewed towards the ipsilateral hemisphere
Changes in GIA volume, LVV and MLS after treatment
Figure 2 shows the changes in GIA volume, LVV, and MLS. GIA volumes decreased significantly by 55.2 % from 9.6 cm3 (IQR 6.1–14.1) to 4.3 cm3 (IQR 2.9–5.7; p < 0.01). Ipsilateral LVV increased by 86.0 % from 8.6 cm3 (IQR 6.4–24.9) to 16.0 cm3 (IQR 9.1–27.2; p < 0.01). Contralateral LVV showed an increase by 13.6 % from 10.3 cm3 (IQR 7.3–20.1) to 11.7 cm3 (IQR 8.2–19.4; p = 0.02). Before treatment, the MLS was directed to the contralateral hemisphere with a value of 0.1 mm (IQR −1.9 to 2.0). After treatment the MLS changed by a median of 10 mm and was now directed towards the treated GIA with a value of −0.9 mm (IQR −1.8 to 0.4; p = 0.03).
Changes in LVV and MLS in relation to the decrease in GIA volume
Next, we analyzed whether there was a correlation between the reduction in GIA volume after surgery and changes in LVV and MLS. Since the changes in contralateral LVV had been rather small, we exclusively focused on changes in ipsilateral LVV and MLS. We found a correlation between changes in GIA volume and those in ipsilateral LVV with an rs of 0.60 (p = 0.01). In contrast, there was no correlation between changes in GIA volume and those in MLS (rs = 0.41; p = 0.08).
To describe these findings in more detail, we divided our patient cohort into quartiles according to the amount of reduction in GIA volume after surgery. Table 2 shows the reduction in GIA volume, the increase in ipsilateral LVV and the change in MLS for each of those quartiles. Table 3 describes the distribution of GIA volume reductions for each type of surgical strategy. All of the five GIA with the largest reduction in volume (1st quartile) were treated using a proximal occlusion strategy, in most cases with the addition of a bypass. The surgical strategies in the 4th quartile of GIA volume reduction all consisted of a bypass with the addition of a proximal occlusion in one case and a distal occlusion in two cases. In this 4th quartile we observed a decrease in GIA volume in two cases (by 25.0 and 27.3 %). The other two cases showed an increase in GIA volume. One of those cases was a cavernous ICA aneurysm, which was treated by proximal occlusion plus bypass. We observed a 5.5 % increase in GIA volume 11 months after surgery (from 5.4 to 5.7 cm3). The other case was a giant MCA aneurysm, which was treated by the placement of an STA-MCA bypass. Neither proximal nor distal aneurysm occlusion was carried out since we found a significant deterioration of intraoperative neurophysiological monitoring during the test occlusion of proximal and distal MCA segments. After 14 months of follow-up, we observed a 70.1 % increase in GIA volume (from 9.7 to 16.5 cm3).
Complications
Surgical complications were observed in two of 19 cases (10.5 %). In a 58-year-old male patient, a parapharyngeal hemorrhage occurred within 2 h after the placement of a radial graft high-flow bypass from the extracranial ICA to an M2 segment with proximal occlusion of a giant M1/M2 aneurysm. The reason for the hematoma was a leakage from the proximal bypass anastomosis. The hematoma was immediately evacuated and a revision of the proximal bypass anastomosis was conducted. The patient recovered without neurological deficit. In a 65-year-old patient with a giant AcomA aneurysm, which was trapped and bypassed, a postoperative new minor paresis of knee extension and foot extension occurred. On the postoperative MRI, a new partial ischemia in the region supplied by an A3-branch was detected. The patient was able to walk independently at discharge but the pareses remained largely the same at a 1-year follow-up.
Discussion
The main result of our analysis is that GIA volumes decreased over time after surgical treatment even though no direct GIA clipping was conducted. These findings are of special interest since there is an ongoing discussion on whether GIA that are not directly clipped have the ability to shrink over time [13]. Since in none of the cases included in our analysis any thrombectomy or partial aneurysm resections were conducted, our findings suggest that such shrinkage is possible after changing the pattern of blood flow within or around a GIA by surgical means. This is important since GIA frequently display partial thrombosis or calcifications or even incorporate crucial vessels, which causes direct clipping strategies to be associated with greater risks than indirect surgical strategies, which do not directly attack the GIA.
GIA shrinkage is important since it decreases the stress on neighboring brain structures and increases their chances of reexpansion, which may lead to an improvement of the patient’s neurological symptoms. We therefore also examined such changes in mass effect on the brain. We found a significant increase in ipsilateral and contralateral LVV after treatment, as well as a significant reduction in MLS. However, only changes in ipsilateral LVV correlated with the reduction in GIA volume, while changes in MLS did not. This suggests that ipsilateral LVV may be a more sensitive descriptor of actual changes in mass effect than MLS after surgical treatment of GIA of the anterior circulation that are not eligible for direct clipping.
Describing changes in IA mass effect after treatment is usually not necessary since the majority of IA either do not exert relevant mass effect or their mass effect vanishes after direct clip occlusion. As the mass effect caused by GIA can often not be dissolved by simple direct clipping, scientific data on mass effect dynamics after GIA treatment are needed.
In ICH, stroke, and brain tumors, the quantification of intracranial mass effect is common since it was shown to be associated with the overall prognosis [3, 8, 10, 12, 15, 22]. One established method is to measure the volume of the lesion itself over time. However, in GIA, such direct volumetry after treatment is often difficult due to imaging artifacts caused by clips or endovascular implants such as stents, flow-diverters, or coils. As the lateral ventricles are usually not overshadowed by such artifacts, their quantification may be more reliable than direct GIA volumetry. Also, only measuring changes in GIA volume may serve as an indirect assessment of the effects on the brain. If LVV measurements are added, they allow for a direct assessment of how the brain mass reacts to changes in mass effect. If direct GIA volumetry is difficult, alternative modes of mass effect measurement ought to be considered. MLS is one such alternative, as changes in MLS were shown to correlate with morbidity and mortality in stroke, ICH, and brain tumors [5, 11, 22]. In the patients of our analysis, MLS was reliably measurable with excellent inter-observer agreement both on pre- and post-treatment MRI. However, the amount of changes in MLS did not correlate with the amount of decrease in GIA volume. A reason for this may be that GIA shrinkage usually takes place in proximity to the lateral border of the lateral ventricles and not at their medial border close to the mid-line. Therefore, such changes in GIA volume may first be picked up by the lateral border of the ventricle, causing asymmetric LVV expansion. The septum pellucidum at the mid-line may be affected significantly later in this process and only if GIA shrinkage is substantial. Another interesting observation in our patient cohort was that post-treatment MLS even passed the mid-line and was skewed towards the shrinking GIA (Fig. 2). To explain this phenomenon, one may argue that during the time of GIA growth the mass effect may have damaged parts of the ipsilateral brain hemisphere. Once the GIA itself shrinks after treatment, this damaged brain matter may not expand in its entirety and may be drawn into the newly created space, resulting in a mid-line skewed towards the GIA.
Since MLS did not correlate with GIA volume in our analysis, volumetry of the lateral ventricles seems to be an attractive alternative. Nag et al. found the compression of the lateral ventricles to be an independent prognostic factor for poor outcome in ICH [10]. This was true even in cases in which no MLS was observed. Such a possible superiority of volumetric over linear measurements to monitor treatment success was also shown in the field of hydrocephalus therapy [19]. In patients with clinical improvement after ventricular shunt surgery, volumetry was shown to consistently detect decreases in ventricular size, while linear measurements failed to do so [2]. Our results are in line with this, since only volumetry of the lateral ventricles but not MLS correlated with changes in GIA volume.
The strength of this analysis is that it is the first to systematically examine volumetric changes after GIA therapy to describe mass effect dynamics and to compare them to changes in MLS. However, certain limitations need to be mentioned. Owing to the rareness of the disease and to the fact that cases with thrombectomy and partial GIA resection were not included, the overall number of cases (n = 19) was rather limited and therefore all statements on statistical significance ought to be interpreted with caution. Also, three patients presented with bilateral GIA, which may have affected the degree by which LVV and MLS changed after treatment. Another limitation of our analysis may be that LVV expansion alone may not be sufficient to describe the entirety of changes in mass effect after GIA treatment. Since the lateral ventricles compete with other brain compartments for the newly developing space created by GIA shrinkage, future studies should also quantify changes in volume of the ipsilateral brain hemisphere. Furthermore, this analysis is not powered to assess whether certain surgical strategies may have led to a larger decrease in GIA volume than others. Finally, this analysis did not examine whether the changes in mass effect over time may have a clinical effect, since clinical follow-up data will be evaluated in a separate project by the GIA Registry’s study group once the currently still ongoing inclusion phase will be completed in the future.
We conclude that in our patient cohort surgical therapy of anterior circulation GIA that were not eligible for direct clipping lead to a decrease in GIA volume and mass effect on the brain over time. The decrease in mass effect was more sensitively monitored by the measurement of changes in ipsilateral LVV than changes in MLS.
References
Alvarez H (2009) Etiology of giant aneurysms and their treatment. Am J Neuroradiol 30, E8
Anderson RC, Grant JJ, de la Paz R, Frucht S, Goodman RR (2002) Volumetric measurements in the detection of reduced ventricular volume in patients with normal-pressure hydrocephalus whose clinical condition improved after ventriculoperitoneal shunt placement. J Neurosurg 97:73–79
Baschnagel AM, Meyer KD, Chen PY, Krauss DJ, Olson RE, Pieper DR, Maitz AH, Ye H, Grills IS (2013) Tumor volume as a predictor of survival and local control in patients with brain metastases treated with γ knife surgery. J Neurosurg 119:1139–1144
Dengler J, Heuschmann PU, Endres M, Meyer B, Rohde V, Rufenacht DA, Vajkoczy P, Giant Intracranial Aneurysm Study Group (2011) The rationale and design of the Giant Intracranial Aneurysm Registry: a retrospective and prospective study. Int J Stroke 6:266–270
Forsyth PA, Posner JB (1993) Headaches in patients with brain tumors: a study of 111 patients. Neurology 43:1678–1683
Krings T, Alvarez H, Reinacher P, Ozanne A, Baccin CE, Gandolfo C, Zhao WY, Reinges MH, Lasjaunias P (2007) Growth and rupture mechanism of partially thrombosed aneurysms. Interv Neuroradiol 13:117–126
Kulcsár Z, Houdart E, Bonafé A, Parker G, Millar J, Goddard AJ, Renowden S, Gál G, Turowski B, Mitchell K, Gray F, Rodriguez M, van den Berg R, Gruber A, Desal H, Wanke I, Rüfenacht DA (2011) Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. Am J Neuroradiol 32:20–25
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198
Lylyk P, Miranda C, Ceratto R, Ferrario A, Scrivano E, Luna HR, Berez AL, Tran Q, Nelson PK, Fiorella D (2009) Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery 64:632–642
Nag C, Das K, Ghosh M, Khandakar MR (2012) Prediction of clinical outcome in acute hemorrhagic stroke from a single CT scan on admission. N Am J Med Sci 4:463–467
Pullicino PM, Alexandrov AV, Shelton JA, Alexandrova NA, Smurawska LT, Norris JW (1997) Mass effect and death from severe acute stroke. Neurology 49:1090–1095
Rost NS, Smith EE, Chang Y, Snider RW, Chanderraj R, Schwab K, FitzMaurice E, Wendell L, Goldstein JN, Greenberg SM, Rosand J (2008) Prediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC score. Stroke 39:2304–2309
Schebesch KM, Proescholdt M, Ullrich OW, Camboni D, Moritz S, Wiesenack C, Brawanski A (2010) Circulatory arrest and deep hypothermia for the treatment of complex intracranial aneurysms–results from a single European center. Acta Neurochir 152:783–792
Schubiger O, Valavanis A, Wichmann W (1987) Growth-mechanism of giant intracranial aneurysms; demonstration by CT and MR imaging. Neuroradiology 29:266–271
Skeie BS, Skeie GO, Enger PØ, Ganz JC, Heggdal JI, Ystevik B, Hatteland S, Parr E, Pedersen PH (2011) Gamma knife surgery in brain melanomas: absence of extracranial metastases and tumor volume strongest indicators of prolonged survival. World Neurosurg 75:684–691
Strik HM, Borchert H, Fels C, Knauth M, Rienhoff O, Bähr M, Verhey JF (2005) Three-dimensional reconstruction and volumetry of intracranial haemorrhage and its mass effect. Neuroradiology 47:417–424
Sughrue ME, Saloner D, Rayz VL, Lawton MT (2011) Giant intracranial aneurysms: evolution of management in a contemporary surgical series. Neurosurgery 69:1261–1270
Szikora I, Berentei Z, Kulcsar Z, Marosfoi M, Vajda ZS, Lee W, Berez A, Nelson PK (2010) Treatment of intracranial aneurysms by functional reconstruction of the parent artery: the Budapest experience with the pipeline embolization device. Am J Neuroradiol 31:1139–1147
Toma AK, Holl E, Kitchen ND, Watkins LD (2011) Evans’ index revisited: the need for an alternative in normal pressure hydrocephalus. Neurosurgery 68:939–944
Vajkoczy P (2009) Revival of extra-intracranial bypass surgery. Curr Opin Neurol 22:90–95
Vespa PM, O’Phelan K, Shah M, Mirabelli J, Starkman S, Kidwell C, Saver J, Nuwer MR, Frazee JG, McArthur DA, Martin NA (2003) Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology 60:1441–1446
Zazulia AR, Diringer MN, Derdeyn CP, Powers WJ (1999) Progression of mass effect after intracerebral hemorrhage. Stroke 30:1167–1173
Acknowledgments
The Giant Intracranial Aneurysm Registry is funded by the Center for Stroke Research – Berlin.
Statement
All trials based on data from the Giant Intracranial Aneurysm Registry were approved by the ethics committees of all participating centers and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All patients included gave their informed consent prior to their inclusion in the study.
Conflict of interest
None.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Comment
The giant intracranial aneurysm is a complex neurosurgical pathology with extraordinarily high associated morbidity and multiple factors that must be kept in mind during treatment, including both risk of rupture, as well as mass effect, as the authors thoroughly investigate. Through volumetric and linear measurements, the authors utilize the Giant Intracranial Aneurysm Registry to assess the amount of brain compression caused by these aneurysms after treatment by indirect surgical management with or without bypass. Through this retrospective study, with inclusion criteria of at least a 9-month follow-up MRI (although cited in the abstract to be 1 year), the authors correlate decreasing anterior circulation giant aneurysm volume with increasing ipsilateral ventricular volume, thus serving as a measure for the amount of mass effect an aneurysm is imparting onto the surrounding brain and potentially a proxy for aneurysmal involution, which may be obscured on imaging due to metallic artifact post-treatment.
While the authors pose an interesting question, the clinical application of this data is not thoroughly explored, although it is noted that the outcomes will be discussed in another publication. Given that the average modified Rankin scale prior to treatment of the studied patient population is less than 1, the mass effect of the aneurysms is clinically negligible pre-procedurally, so monitoring only the radiographic finding has minimal clinical application. In addition to minimal clinical information being reported, no angiographic imaging is reported, and given that decreasing mass effect of giant aneurysms is not necessarily associated with cessation of blood flow into the lesion, it is unclear how the results of this paper lends to conclusions applicable to patient care (Darsaut 2011).
The authors choose to correlate aneurysm volume to midline shift and both ipsilateral and contralateral lateral ventricular volume, citing that these measurements have been correlated to intracranial hemorrhage outcomes in the past. However, it should be kept in mind the difference in the slow-growing pathologies of a giant intracranial aneurysm, especially in the setting of wall calcifications, and that of an acute intracranial hemorrhage. There is an inverse correlation between ipsilateral ventricular volume and aneurysm volume, but additionally, a paradoxical midline shift towards the treated aneurysm should be more thoroughly investigated, because while a 1-mm pre-treatment midline shift is most often clinically insignificant, typically a 9-mm midline shift, which was found in post-treatment analysis, would need to be intervened on in most neurosurgical settings. Additionally, the patients with bilateral aneurysms should be excluded from analysis because of the significant confounding nature of bilateral compressive pathologies on midline shift and contralateral ventricular volume.
Given the small sample size of patients and the heterogeneity of both aneurysm location and treatment approach in this study, computational flow dynamic studies would be valuable in understanding how these complex aneurysms responded to indirect treatment, and could direct future surgical management of these lesions (Lawton 2011). However, to that end, the likely future of treatment of these highly morbid lesions, often necessitating surgical intervention shown to be fraught with potential for clinical and neurologic complications, will likely be exclusively endovascular, since the advent of the flow-diverting stent. Although the specific locations of these aneurysms are not discussed in this study, it is likely that many, if not all, of the internal carotid artery lesions could have been treated via flow-diverting stent constructs, with not only exceptional aneurysmal occlusion outcomes but also with even more decrease in aneurysmal volume than seen in this trial (Szikora 2013).
As endovascular therapies, including flow-diverter stents, continue to evolve, they will likely become the preferred treatment for not only giant internal carotid artery aneurysms but also for more distal anterior circulation, as well as posterior circulation, aneurysms, where the obstacles related to flow demand to perforators will likely be overcome with further innovation. As with indirect bypass, flow diverters aim for endoluminal remodeling, rather than abrupt aneurysmal occlusion, so involved blood vessels may either maintain patency or develop collateral perfusion, as seen in the ophthalmic artery aneurysm flow-diversion experience (Zanaty 2015).
The authors are to be commended on their thorough objective study of giant intracranial aneurysm mass effect. With inclusion of clinical data and angiographic outcomes, and understanding of the implications resultant from paradoxical midline shift towards the treated aneurysm, this paper will be a fine addition to the literature. As giant aneurysms treated with flow diverters are added to the Giant Intracranial Aneurysm Registry, this paper will serve as a resource on how to compare radiographic outcomes of this pathology with a changing treatment paradigm in the future.
Daniel M. Heiferman, Dustin M. Hayward, Christopher M. Loftus
Illinois, USA
References
Darsaut TE, Darsaut NM, Chang SD, Silverberg GD, Shuer LM, Tian L, Dodd RL, Do HM, Marks MP, Steinberg GK (2011) Predictors of clinical and angiographic outcome after surgical or endovascular therapy of very large and giant intracranial aneurysms. Neurosurgery 68:903–915
Sughrue ME, Saloner D, Rayz VL, Lawton MT (2011) Giant intracranial aneurysms: evolution of management in a contemporary surgical series. Neurosurgery 69:1261–1270
Szikora I, Marosfoi M, Salomváry B, Berentei Z, Gubucz I (2013) Resolution of mass effect and compression symptoms following endoluminal flow diversion for the treatment of intracranial aneurysms. AJNR Am J Neuroradiol 34:935–939.
Zanaty M, Chalouhi N, Barros G, Schwartz EW, Saigh MP, Starke RM, Whiting A, Tjoumakaris SI, Hasan D, Rosenwasser RH, Jabbour P (2015) Flow-diversion for ophthalmic segment aneurysms. Neurosurgery 76:286–290.
No parts of this project have been presented anywhere before.
Rights and permissions
About this article
Cite this article
Maldaner, N., Guhl, S., Mielke, D. et al. Changes in volume of giant intracranial aneurysms treated by surgical strategies other than direct clipping. Acta Neurochir 157, 1117–1123 (2015). https://doi.org/10.1007/s00701-015-2448-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-015-2448-y