Abstract
Background
Intracranial aneurysms (IA) are usually quantified according to their largest diameter. However, volumetry has recently been increasingly conducted as well, especially in giant intracranial aneurysms (GIAs). Since so far the true value of GIA volumetry is unknown, we designed a trial to examine correlations between GIA diameter and volume with special focus on clinical implications.
Methods
Magnetic resonance imaging of 69 unruptured GIAs in 66 patients was retrospectively evaluated. The largest diameter and volume were measured. Also, potential associations to the patients’ clinical conditions were examined.
Results
Comparing GIA sizes of our patient cohort produced different results depending on whether GIA diameter or volume was measured. Measuring the diameter identified posterior circulation GIAs as the largest ones (39.2 mm, IQR 37.3–48.3), while measuring the volume found GIAs of the MCA to be the largest ones (12.3 cm3, IQR 7.2–27.8). A correlation of GIA diameter and volume was only found in anterior circulation GIAs, which were predominantly saccular in shape, but not in those of the posterior circulation, of which most were fusiform. Neither GIA diameter nor GIA volume but only GIA location was associated with neurological deficits.
Conclusion
Diameter and volume measurements are not interchangeable modes of GIA quantification. Our data suggest that the idea of distinguishing different sizes of GIA may be clinically less relevant than examining their location, shape or mass effect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The concept of measuring lesion size by volume instead of diameter has evolved as 3D imaging techniques and dedicated software have increasingly become available. While volumetry has long been part of the clinical routine for pathologies such as brain tumors or abdominal aortic aneurysms (AAAs), intracranial aneurysms (IAs) are still quantified according to their largest diameter as small (<7 mm), medium (7–13 mm), large (14–24 mm) or giant (≥25 mm) [5, 12, 16, 18]. Intracranial aneurysm diameter is viewed as the main risk factor for morbidity and mortality, but there is evidence suggesting that IA volume may be an independent risk factor for poor outcome as well [4, 13, 18]. Since giant intracranial aneurysms (GIAs)—unlike non-giant IAs—usually cause considerable intracranial volume shifts due to their mass effect, some recent trials have begun to adopt the concept of volumetry specifically to quantify GIAs [1, 7]. Nevertheless, the true value of GIA volumetry is still uncertain mainly because so far GIA volumetry has not been systematically correlated with clinical findings, nor has it been compared to measuring GIA diameters.
We therefore designed a trial to compare GIA quantification by means of measuring the diameter and volume with regard to the vessel of GIA origin, the location of mass effect and clinical findings. We aimed to test two hypotheses: (1) Comparing GIA sizes leads to different results depending on whether GIAs are quantified by volume or diameter. (2) Increasing GIA size is associated with more severe neurological deficits independent of whether GIAs are quantified by volume or diameter.
Methods
Patients
Data collection was approved by the ethics committee of the GIA registry’s coordinating center at the Charité, Berlin (EA2/052/08) and by the ethics committees of all participating centers [3]. Each patient or their next of kin consented to participation. Inclusion criteria for the specific trial presented here were the diagnosis of an unruptured GIA (defined as an aneurysm with a diameter ≥25 mm) and the existence of an MRI examination before treatment initiation.
Neuroimaging, volumetric analyses and diameter measurements
As magnetic resonance imaging is superior to digital subtraction angiography concerning the visualization of thrombosed parts of GIAs, all cases were examined using T2-weighted images (T2WIs), which display excellent contrast between the GIA and other brain structures. All T2WIs were analyzed at the GIA registry’s coordinating center at the Charité-Berlin by two experienced blinded examiners (N.M. and J.D.). GIAs were characterized by their vessel of origin using the following categories: the anterior cerebral artery (ACA), cavernous internal carotid artery (ICA), supraclinoid ICA (which included GIAs of the transitional area), middle cerebral artery (MCA) and posterior circulation (including the posterior cerebral artery, cerebellar arteries, basilar artery and vertebral artery). Additionally, GIAs were described according to their location of mass effect using the following categories: frontal, frontotemporal (for simultaneous mass effect on frontal and temporal lobes), temporocentral, temporomesial and brainstem. Mass effect was defined as any displacement of brain parenchyma from its anatomically normal location due to the GIA. Volumetric analyses were performed using the software “iPlan Cranial” (BrainLab, Heimstetten, Germany), which is used in clinical routine mainly for preoperative neuronavigation planning. The examiners manually marked the circumference of the object of interest using the mouse cursor within each slice of the T2WI to create a segmentation. The software then calculated the object’s volume by multiplying the marked areas in each slice with the slice thickness of the T2WI. To measure the largest GIA diameter the three-dimensional reconstruction of the GIA was used and examined for the two points furthest apart from each other within the GIA. Thrombosed or calcified parts of the aneurysm wall were defined as being part of the GIA.
Clinical findings
All patients were assessed prior to any type of treatment according to the modified Rankin Score (mRS). Furthermore, the presence of cranial nerve deficits, hemiparesis or aphasia was documented.
Statistical analysis
The Shapiro-Wilk test was used to test variables for normal distribution. Interobserver variabilities were calculated using the two-way random effects model intraclass correlation test. Since the variables for volume and diameter were not distributed normally, nonparametric tests were used for further analysis. The Kruskal-Wallis test was applied to compare volumes or diameters in relation to GIA location within the entire patient cohort. The Mann-Whitney U test was used to compare volumes or diameters between two specific GIA locations. Spearman correlation was used to describe the relation between diameter and volume for the entire patient cohort. Binary logistic regression analysis was applied to test for associations between GIA volume or diameter and motor deficits, cranial nerve deficits and aphasia. Ordinal regression analysis was used to test for an association between mRS as the dependent variable and GIA volume or diameter or vessel of origin as independent variables.
Results
Sixty-nine unruptured GIAs in 66 patients, which had been included into the GIA registry at 12 participating centers between January 2009 and November 2013, were included in this specific trial. The mean axial slice thickness of the T2WI was 3.4 mm (standard deviation 1.6). Interobserver agreement was excellent with an intraclass correlation coefficient of >0.92 and corresponding p values of <0.001 for both the measurement of GIA diameters and volumes. Patient and GIA characteristics are summarized in Table 1. Most GIAs were located at the cavernous ICA and MCA. All posterior circulation GIAs were located at vertebrobasilar vessel segments. A mass effect was observed most frequently at the temporal lobe, followed by the frontal lobe and brainstem.
GIA diameter and volume in relation to the vessel of GIA origin
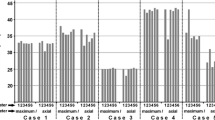
When GIAs were described by their vessel of origin, measurements of diameters and volumes produced significantly different results (Fig. 1a and b). Diameter measurements found GIAs in the posterior circulation to be the largest (39.2 mm, IQR 37.3–48.3), while volumetry found GIAs of the MCA to be the largest (12.3 cm3, IQR 7.2–27.8). Measuring GIA diameters resulted in three pairs of GIA locations with significant size differences (posterior circulation, MCA and cavernous ICA) while volumetry only found one such pair (MCA and cavernous ICA).
Box plots of GIA diameters and volumes according to the vessel of GIA origin (a, b) and the location of mass effect (c, d). ACA anterior cerebral artery, cav. ICA cavernous internal carotid artery, supraclin. ICA supraclinoid internal carotid artery, MCA middle cerebral artery, post. posterior circulation. *p < 0.05
GIA diameter and volume in relation to location of GIA mass effect
Very similar findings were made when GIAs were characterized by the location of their mass effect. Figure 1c and d shows that measuring diameters identified GIAs at the brainstem to be the largest (39.6 mm, IQR 37.2–48.6) while volumetry found the largest sizes in frontotemporal GIAs (13.3 cm3, IQR 7.7–35.0). Measuring diameters resulted in five pairs of significant size differences involving all locations, while volumetry resulted in only two such pairs between the frontal and frontotemporal and frontotemporal and temporomesial location.
Correlating GIA diameter and volume
Figure 2 depicts the GIA diameters and the corresponding GIA volumes of our patient cohort. For the entire patient cohort the Spearman correlation coefficient (rs) between diameter and volume was 0.72 (p < 0.001), representing moderate correlation. When we performed separate subanalyses for each group of GIA vessel origin, we found even more pronounced correlations in GIAs of the MCA (r s = 0.96, p < 0.001) and ACA (r s = 1.00, p < 0.001). GIAs of the cavernous ICA showed an rs of 0.70 with a p-value < 0.001. No correlation between volume and diameter was found in GIAs of the posterior circulation (r s = 0.20, p = 0.48) and the supraclinoid ICA (r s = 0.43, p = 0.34).
Clinical findings
Clinical data were available in 63 cases. Motor deficits were present in 20.6 %, cranial nerve deficits in 44.4 % and aphasia in 3.2 % of cases. For both the entire patient cohort and each GIA location group neither GIA diameter nor GIA volume was associated with the occurrence of motor deficits, cranial nerve deficits or aphasia (Table 2). The distribution of mRS values in relation to the location, diameter and volume of the GIA is shown in Table 3. For the entire patient cohort there was no association between mRS and GIA diameter (p = 0.77) or GIA volume (p = 0.38). A separate analysis of each GIA location also showed no such associations. Significant associations were only found between mRS and GIA location, as exemplified in Fig. 3. GIAs of the posterior circulation and those with mass effect on the brainstem showed significantly higher mRS values than GIAs of all other locations with odds ratios for higher mRS ranging between 8.7 and 24.5 and corresponding p-values between 0.001 and 0.013.
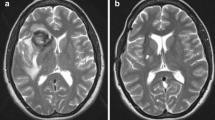
Magnetic resonance images giving examples for an association between neurological deficits and GIA location but not GIA size. a Giant MCA aneurysm (volume: 27.0 cm3; diameter: 43.0 mm) with left temporal mass effect in a 34-year-old male patient without any symptoms (mRS 0). b Giant basilar tip aneurysm (volume: 15.9 cm3; diameter: 36.8 mm) with mesencephalic mass effect in a 51-year-old male patient who displayed cranial nerve deficits, dysphasia and an mRS 3
Discussion
Measuring GIA volume instead of diameter is a recently introduced concept of GIA quantification [2, 7]. However, so far GIA volumetry has neither been compared to the traditional quantification by measuring GIA diameter nor has GIA volume been correlated to clinical features. This trial was undertaken to systematically analyze these points.
The results of our trial confirm our first hypothesis that measuring GIA diameter produces different results than measuring GIA volume when comparing GIA sizes of different locations. Ranking the GIA of our patient cohort by their median size led to different orders depending on which mode of measurement was used (Fig. 1). For measuring diameter, this ranking order (from largest to smallest GIA and independent of statistical significance) was posterior circulation > MCA > ACA > supraclinoid ICA > cavernous ICA. For measuring GIA volume, the size ranking order was MCA > ACA > posterior circulation > cavernous ICA > supraclinoid ICA. This difference between both modes of GIA quantification became even more evident when only statistically significant size differences were considered: measuring diameter revealed posterior circulation GIAs to be significantly larger than those of the cavernous and supraclinoidal ICA. In contrast, volumetry did not find any significant size difference between posterior circulation GIAs and GIAs of any other locations. The only agreement between both modes of GIA quantification was that GIAs of the MCA were significantly larger than those of the cavernous ICA and that GIAs with simultaneous mass effect on the frontal and temporal lobes were larger than those with only frontal or only temporomesial mass effect. Our findings are the first to show in detail that diameter and volume measurements are not interchangeable modes of GIA quantification.
When examining whether both modes of measurement are related to each other, we found a correlation only in GIAs of the anterior circulation, while no such correlation existed in GIAs of the posterior circulation. This finding may be explained by differences in GIA shapes. The majority of anterior circulation GIAs in our patient cohort was more saccular in shape (77 %), while posterior circulation GIAs were predominantly fusiform (62 %). These findings are in line with the results of a recently published series of GIAs, which found the prevalence of fusiform GIAs to be three times higher in the posterior than in the anterior circulation [10]. The morphology of all GIAs in our patient cohort was skewed toward a more fusiform shape, as depicted in Fig. 2. The only anterior circulation GIAs that showed no correlation between measuring diameter and volume were those of the supraclinoid ICA. This group was characterized by rather inhomogeneous shapes and aneurysm wall pouches, which may explain why the volumes and diameters did not correlate. Therefore, our results suggest that presenting information on the shape of a GIA is crucial to interpret data on GIA diameter or volume, which otherwise may have little value for GIA quantification.
Concerning the second hypothesis our results showed that increasing GIA size was not associated with more severe neurological deficits independent of whether GIAs were quantified by volume or diameter. In our patient cohort, neither of the two modes of GIA quantification was related to clinical findings at all. To explain this, it seems reasonable to speculate that once an IA becomes a GIA its sheer size may already have caused a level of clinical symptoms that may not be significantly exacerbated with further GIA growth. Our results suggest that within the group of GIAs the idea of distinguishing different sizes may be clinically less relevant than in non-giant IAs [18]. Furthermore, size differences in GIAs may not only be less relevant for the clinical condition at initial diagnosis but also for decision-making on whether to treat a GIA or not. We feel that GIAs, even more so than non-giant IAs, should be reviewed individually based not only on diameter or volume, but also on various non-size-related characteristics such as location, angioarchitecture, shape, mass effect and the patient’s clinical condition.
In our patient cohort, the only characteristic that was associated with neurological deficits was the location of the GIA. Posterior circulation GIAs showed significantly higher mRS values than those at other locations, which is in agreement with findings on non-giant intracranial aneurysms [18]. This is most likely explained not so much by GIA size or shape but by the relatively narrow space within the posterior fossa or the foramen magnum, which does not allow vital structures, most of all the brainstem, to change their position to be less affected by the mass effect caused by the GIA.
The concept of volumetry instead of measuring diameters is more established in other pathologies, such as AAAs or brain tumors, where it has become a part of clinical routine [12, 14, 15]. However, a prognostic value superior to measuring diameter was only established for tumor volumetry [8–10, 12, 14]. In AAAs, volumetry does not correlate with outcome, while increases in AAA diameter were shown to predict AAA rupture [11]. Another disadvantage of volumetry is that it requires more time and resources, especially dedicated software, than measuring the diameter [5, 15, 16]. However, some reports are in favor of AAA volumetry as they found that certain changes in AAA size and shape over time are detected more sensitively by volumetry than by measuring AAA diameter [15, 17]. Furthermore, diameter measurements alone may underestimate the true growth of a lesion. For example, if a spherical tumor with a diameter of 40 mm grows in all directions by only 25 % in diameter, its volume increases by about 100 % [12]. A supposed advantage of volumetry is that it comprises data from the entire body of the lesion, while measuring diameter describes only a small part of the lesion [16]. This argument may be relevant for lesions of irregular shapes such as GIAs, which grow anisotropically, which means that some areas grow more actively than others [2, 6]. However, GIA volumetry remains limited since it is only one single descriptor that can mean different things depending on the aneurysm shape or location.
The strength of our trial is that it is the first to systematically compare GIA diameters and volumes. Nevertheless, certain limitations should be mentioned. First of all, case inclusion in this imaging project was not consecutive because MRI was not available in all of the consecutively included patients from the GIA registry. Also, MRI slice thickness was not standardized, which resulted in volumetry being of higher resolution in some cases than in others. Furthermore, the number of included cases is rather limited, which may limit the generalizablitiy of our findings. Nevertheless, GIAs are rare entities and therefore a multicenter approach was necessary to even gather the number of cases presented here.
We conclude that in our patient cohort GIA diameter and volume measurements were not interchangeable modes of GIA quantification. Furthermore we found no association between different GIA sizes and the patient’s clinical condition, independent of whether diameter or volume was used to quantify the GIA. Our data therefore suggest that comparing different sizes may be more important for non-giant IAs than for GIAs. These findings are clinically relevant since they promote the idea that decision-making on GIA treatment should not be mainly based on the actual size of the GIA but rather on other individual characteristics such as location, shape, the region affected by the mass effect and the patient’s overall clinical condition.
References
Carneiro A, Rane N, Küker W, Cellerini M, Corkill R, Byrne JV (2014) Volume changes of extremely large and giant intracranial aneurysms after treatment with flow diverter stents. Neuroradiology 56:51–58
Cebral JR, Raschi M (2013) Suggested connections between risk factors of intracranial aneurysms: a review. Ann Biomed Eng 41:1366–1383
Dengler J, Heuschmann PU, Endres M, Meyer B, Rohde V, Rufenacht DA, Vajkoczy P (2013) The rationale and design of the Giant Intracranial Aneurysm Registry: a retrospective and prospective study. Int J Stroke 6:266–270
de Rooij NK, Velthuis BK, Algra A, Rinkel GJ (2009) Configuration of the circle of Willis, direction of flow and shape of the aneurysm as risk factors for rupture of intracranial aneurysms. J Neurol 256:45–50
Kitagawa A, Mastracci TM, von Allmen R, Powell JT (2013) The role of diameter versus volume as the best prognostic measurement of abdominal aortic aneurysms. J Vasc Surg 58:258–265
Krings T, Alvarez H, Reinacher P, Ozanne A, Baccin CE, Gandolfo C, Zhao WY, Reinges MH, Lasjaunias P (2007) Growth and rupture mechanism of partially thrombosed aneurysms. Interv Neuroradiol 13:117–126
Larrabide I, Geers AJ, Morales HG, Aguilar ML, Rüfenacht DA (2014) Effect of aneurysm and ICA morphology on hemodynamics before and after flow diverter treatment. J Neurointerv Surg. doi:10.1136/neurintsurg-2014-011171
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Mandonnet E, Delattre JY, Tanguy ML, Swanson KR, Carpentier AF, Duffau H, Cornu P, Van Effenterre R, Alvord EC Jr, Capelle L (2013) Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol 53:524–528
Nurminen V, Lehecka M, Chakrabarty A, Kivisaari R, Lehto H, Niemelä M, Hernesniemi J (2014) Anatomy and morphology of giant aneurysms–angiographic study of 125 consecutive cases. Acta Neurochir 156:1–10
Parr A, Jayaratne C, Buttner P, Golledge J (2011) Comparison of volume and diameter measurement in assessing small abdominal aortic aneurysm expansion examined using computed tomographic angiography. Eur J Radiol 79:42–47
Rees J, Watt H, Jäger HR, Benton C, Tozer D, Tofts P, Waldman A (2009) Volumes and growth rates of untreated adult low-grade gliomas indicate risk of early malignant transformation. Eur J Radiol 72:54–64
Sadato A, Hayakawa M, Tanaka T, Hirose Y (2011) Comparison of cerebral aneurysm volumes as determined by digitally measured 3D rotational angiography and approximation from three diameters. Interv Neuroradiol 17:154–158
van den Bent MJ, Wefel JS, Schiff D, Taphoorn MJ, Jaeckle K, Junck L, Armstrong T, Choucair A, Waldman AD, Gorlia T, Chamberlain M, Baumert BG, Vogelbaum MA, Macdonald DR, Reardon DA, Wen PY, Chang SM, Jacobs AH (2011) Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol 12:583–593
van Keulen JW, van Prehn J, Prokop M, Moll FL, van Heerwaarden JA (2009) Potential value of aneurysm sac volume measurements in addition to diameter measurements after endovascular aneurysm repair. J Endovasc Ther 16:506–513
von Allmen RS, Powell JT (2013) Part two: against the motion. External diameter for AAA size. Eur J Vasc Endovasc Surg 46:6–8
Wever JJ, Blankensteijn JD, Th M, Mali WP, Eikelboom BC (2000) Maximal aneurysm diameter follow-up is inadequante after endovascular abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 20:177–182
Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, Forbes GS, Thielen K, Nichols D, O’Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JC (2003) Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362:103–110
Acknowledgments
The Giant Intracranial Aneurysm Registry is funded by the Center for Stroke Research-Berlin. The grant number is “CS-2009-12”
Clinical Trial Registration-URL:
http://www.clinicaltrials.gov. Unique identifier: NCT02066493.
Conflicts of interest
None.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Comment
This is a concise report on analyses of 2D diametric and volumetric measurement of intracranial giant aneurysms and their correlation with patients’ symptoms. Although this study does not alter in any way or shape the clinical management of those complex aneurysms, it shows the discrepancy between size and volume in posterior circulation giant aneurysms (most likely due to more fusiform shape) and the fact that location but not the size or volume is the only indicator of symptomatic aneurysms in this population.
Amir Dehdashti
NY,USA
No parts of this project have been presented anywhere before.
The Giant Aneurysm Study Group (November 2014)
The Giant Aneurysm Study Group (November 2014)
Vajkoczy P, Uebelacker A, Maldaner N, Dengler J
Department of Neurosurgery, Charité – Universitaetsmedizin Berlin
Endres M
Department of Neurology, Charité – Universitaetsmedizin Berlin
Bohner G, Wiener E, Bauknecht HC
Department of Neuroradiology, Charité – Universitaetsmedizin Berlin
Heuschmann PU, Malzahn U
Institute of Clinical Epidemiology and Biometry, University of Würzburg
Glaesker S, Zentner J, Van Velthoven V
Department of Neurosurgery, University Hospital Freiburg, Germany
Guhl S, Schroeder HWS
Department of Neurosurgery, University of Greifswald, Germany
Strowitzki M
Department of Neurosurgery, Trauma Center Murnau, Murnau, Germany
Etminan N, Haengghi D, Eicker S, Turowski B
Department of Neurosurgery, University of Düsseldorf, Germany
Schebesch KM, Brawanski A
Department of Neurosurgery, University of Regensburg, Germany
Wrede K, Sure U
Department of Neurosurgery, University of Essen, Germany
Schmidt NO, Regelsberger J, Westphal M
Department of Neurosurgery, University Medical Center, Hamburg Eppendorf, Germany
Mielke D, Rohde V
Department of Neurosurgery, Georg-August-University Goettingen, Germany
Hosch H, Moskopp D
Department of Neurosurgery Vivantes-Klinikum im Friedrichshain, Berlin, Germany
Joedicke A
Department of Neurosurgery Vivantes-Klinikum Neukoelln, Berlin, Germany
Hohaus C, Meisel HJ
Department of Neurosurgery, BG-Clinic Bergmannstrost, Halle, Germany.
Wostrack M, Meyer B, Lehmberg J
Department of Neurosurgery, Technical University of Munich, Germany
Musahl C, Hopf N
Department of Neurosurgery, Klinikum Stuttgart, Stuttgart, Germany
Winkler G, Spetzger U
Department of Neurosurgery, Klinikum Karlsruhe, Germany
Graewe A, Meier U
Department of Neurosurgery, Unfallkrankenhaus Berlin, Germany
Hong B, Nakamura M, Krauss J
Department of Neurosurgery, Hannover Medical School, Hannover, Germany
Grote A, Simon M, Schramm J
Department of Neurosurgery, University Hospital Bonn, Bonn, Germany
Kursumovic A, Rath SA
Department of Neurosurgery and Interventional Neuroradiology, Donau-Isar-Klinikum,
Deggendorf, Germany
Walter J, Kalff R
Department of Neurosurgery, Friedrich-Schiller-University, Jena, Germany
Marbacher S, Fathi A, Fandino J
Department of Neurosurgery, Kantonsspital Aarau, Aarau, Switzerland,
Familiari P, Raco A
Department of Neurosurgery, University of Rome “Sapienza”, Rome, Italy
Bijlenga P, Schaller K
Service de Neurochirurgie, Faculté de Médecine de Genève and Hôpitaux Universitaire de Genève, Switzerland
Gruber A, Wang WT, Knosp E
Department of Neurosurgery, Medical University Vienna, Vienna, Austria
Hoffmann KT, Boxhammer E
Department of Neuroradiology, University of Leipzig, Leipzig, Germany
Rüfenacht DA
Department of Neuroradiology, Klinik Hirslanden, Zurich, Switzerland
Boccardi E, Piano M
Department of Neuroradiology, Ospedale Niguarda Ca’ Granda, Milano, Italy
Niemelä M, Nurminen V, Lehecka M, Hernesniemi J
Department of Neurosurgery, Helsinki University Central Hospital, Helsinki, Finland
Burkhardt JK, Bozinov O, Regli L
Department of Neurosurgery, University Hospital of Zurich, Switzerland
Shekhtman OD, Eliava SS
Burdenko Neurosurgical Institute, Russian Academy of Medical Sciences, Moscow, Russia
Kato N, Irie K, Nishimura K, Kaku S, Arakawa H, Yuki I, Ishibashi T, Murayama Y
Department of Neurosurgery, Jikei University School of Medicine, Tokyo, Japan
Fiss I, Kombos T
Department of Spine Surgery and Neurosurgery, Helios Klinikum Hildesheim, Hildesheim, Germany
van der Zwan A, van Doormaal TP
Department of Neurosurgery, University Medical Center Utrecht, Utrecht, Netherlands
Cognard C, Gawlitza M
Department of Neuroradiology, Toulouse University Hospital, Toulouse, France
Rights and permissions
About this article
Cite this article
Dengler, J., Maldaner, N., Bijlenga, P. et al. Quantifying unruptured giant intracranial aneurysms by measuring diameter and volume—a comparative analysis of 69 cases. Acta Neurochir 157, 361–368 (2015). https://doi.org/10.1007/s00701-014-2292-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-014-2292-5