Abstract
Purpose
Radiosurgery (RS) is a well-established treatment in selected patients with brain metastasis. The aim of this study is to compare the differences between CyberKnife (CK) and TomoTherapy (HT) treatment plans of RS of single brain metastasis (BM) to define when HT should be used in cases beyond Cyberknife—when both systems are readily available for the radiation oncologist.
Methods and materials
Nineteen patients with single brain metastasis treated with CK were re-planned for radiosurgery using TomoTherapy Hi-ART system. Two planning approaches have been used for TomoTherapy plans: the classical one (HT) and the improved conformity (icHT) that produces dose distributions more similar to those of RS plans. PTV coverage, Conformity Index (CI), Paddick Conformity Index (nCI), Homogeneity Index (HI), Gradient Index (GI), and beam on time of CK, HT, and icHT plans were evaluated and compared.
Results
A good coverage was found for CK, HT, and icHT plans. A difference between mean HI of CK and icHT plans was observed (p = 0.007). Better dose gradients compared to both icHT and HT modalities were observed in CK plans. icHT modality showed improved mean CI respect to HT modality, similar to that obtained in CK plans.
Conclusions
CK plans show higher conformity and lower GI than icHT and HT plans. TomoTherapy demonstrates the advantage of being a device capable to reach different clinical objectives depending on the different planning modality employed. CyberKnife and TomoTherapy are both optimal RS devices, the choice to use one over another has to be clinically guided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Brain metastases are the most frequent intracranial neoplasms in adults. In patients suffering of cancer, BMs will appear in 20–40% of cases, and in 10–15%, BMs are present at the first diagnosis [1].
Considering the longer survival of cancer patients related to the early detection of disease and treatment improvements, the incidence of brain metastatic disease will increase. For this reason, the interest to control the brain disease, to preserve functions, to enhance the quality of life, and to avoid the death due to neurologic causes and not only palliate the symptoms is becoming a clinical priority.
The treatment options for brain metastatic patients are whole brain radiotherapy (WBRT), surgical resection, and stereotactic radiosurgery (RS). The choice of a local treatment alone or in association to whole brain radiotherapy is usually done depending on performance status, number of BMs, controlled systemic disease, and histology [2]. Recently, two large randomized studies have shown similar survival benefits and functional independence between patients with 1–3 BMs treated with RS alone and RS plus WBRT [3, 4].
RS may result in a better local control in patients with radioresistant histology, and it can delay WBRT, such as salvage treatment. The ideal candidate for RS is the patient with controlled extra-cranial metastases and a good performance status [5–7]. The use of RS as the exclusive treatment of BMs is affirmed in emerging studies employing this approach; recently, a Japanese prospective observational study reported that the outcome of patients with five to ten BMs treated with RS without WBRT is non-inferior to that of patients with two to four BMs in terms of overall survival [8].
RS treatments were originally performed with GammaKnife, a device equipped with hundreds of 60Co sources focused to a single point, and after few years also with linear accelerators. Several differences exist between the two systems, but the most significant is the approach followed for patient immobilization and target localization. While, in the first case, invasive stereotactic frames are used for both target localization and patient immobilization; in the second one, thermoplastic mask coupled with imaging techniques as CBCT is usually employed. Despite the differences between the two approaches, no randomized trials have been carried out so far to show whether one modality could provide clinical advantages over the other. For this reason, the choice of the device to be used for RS treatments is left up to physician’s preferences and expertise and to device availability. In the last few years, new systems, such as CyberKnife (Accuray Incorporated, Sunnyvale, CA, USA) and Helical TomoTherapy (Accuray Incorporated, Sunnyvale, CA, USA), capable of delivering highly conformed dose distribution to targets with complex shapes have been proposed and used to deliver RS treatments non-invasively. Despite the high dose conformity, that both systems can provide their performances are different. CyberKnife plans are, in fact, characterized by inhomogeneous highly conformal dose distribution to the target, while TomoTherapy plans can provide both homogeneous and typical radiosurgery dose distributions [9] to the target.
In both cases, no invasive head frame is used as patients are immobilized with thermoplastic masks and targets are localized using images: a couple of orthogonal Kilovoltage (KV) X-ray images in CyberKnife treatments and MegaVoltage CT images in Helical TomoTherapy cases.
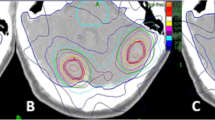
The localization and immobilization approaches, together with the high dose conformity that both CyberKnife and Helical TomoTherapy are capable to deliver, make them suitable to perform radiosurgery treatments. To understand if one device shall be preferred to the other and to analyze possible advantages of one modality over the other, we performed a dosimetric comparison between the plans of 19 single brain metastases, originally treated with CyberKnife, and subsequently re-planned for Helical TomoTherapy. In the latter case, two approaches were followed: the classical one and the improved conformity suggested by Soisson et al. [9].
Methods
Patients
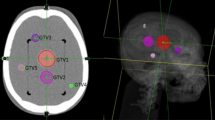
Nineteen patients with brain metastasis treated with single fraction RS using a CyberKnife G4 system were re-planned using the TomoTherapy Hi-ART system. A Brilliance CT Big Bore (Philips, Amsterdam, The Netherlands) was used as a CT simulator acquiring a contrast-enhanced CT scan (1.25 mm slice thickness) for planning purposes. A thermoplastic mask was used to immobilize patients during CT acquisition and throughout treatment delivery. A T1-weighed magnetic resonance study was registered on the planning CT to define the GTV as the enhancing lesion. A 1-mm uniform expansion was applied to create a clinical target volume capable of considering a possible infiltrative growth beyond the visible lesion [10]. The same Planning Target Volume (PTV) used for Cyberknife, obtained adding a further 1-mm uniform margin to CTV, was also employed in Tomotherapy planning. We are aware that due to the image guidance [11], 1-mm margin is considered safe for CyberKnife brain treatments and not for Tomotherapy treatment unless more invasive patient immobilization devices are employed [12]. The aim of this work is just a dosimetric comparison between two modalities, and for this reason, the same PTVs have to be considered. A comparison between plans created on PTVs tailored on each specific treatment modality goes beyond the goal of this study.
Mean and median PTVs were 6.32 and 4.63 cm3, respectively (range 0.69–18.35 cm3). The prescription doses ranged from 12 to 22 Gy, depending on the lesion size and tumor location, according to RTOG 90-05 protocol. [13].
CyberKnife planning
CyberKnife (CK) plans were created using either fixed collimators or the Iris variable aperture collimator and adopting a stepwise multicriteria optimization in the sequential module of the Multiplan dedicated treatment planning system (v. 3.5.4) [13]. Doses were prescribed at the 80% isodose value. Together with PTV coverage, conformity indexes were also considered during plan optimization. Doses to healthy tissue were minimized during the optimization process by means of automatic shell volumes around the PTV. A detailed description of the parameters used to index the treatment plan conformity and dose fall-off outside the treatment volume is given in the following session. The Treatment planning optimization resulted in an arrangement of non-isocentric non-coplanar beams of various sizes: a trade-off was accepted between the total beam number (which is correlated to the plan delivery time) and the plan conformity and dose gradient, obtaining on average 141 beams (range 99–160).
TomoTherapy planning
Two planning strategies have been followed to prepare TomoTherapy plans: the classical one (HT), which consists in delivering a highly homogeneous dose distribution to the target and the improved conformity which, on the contrary, forces the system to deliver higher doses inside the target to obtain the steepest dose gradient outside it. The improved conformity mode, originally proposed by Soisson et al. [9], creates a dose distribution comparable to that of RS plans using additional non-anatomic planning structures, both internal and external with respect to PTV, according to a specific prescription. In the following part, we will refer to the Soisson’s planning approach as icHT. In the HT mode, the prescription dose was required to cover 100% of the PTV and the maximum dose was also controlled. In the icHT mode, the 125% of the prescription dose was required to cover an inner non-anatomic planning structure, while the maximum dose to the PTV was released. All plans were calculated with “Fine” dose grid, 10-mm field width, 0.215 pitch, and modulation degrees between 2 and 3 using a TomoTherapy Hi-Art treatment planning system (v. 4.2.2).
Plan Comparison: Treatment plan quality was scored for both CyberKnife and TomoTherapy in terms of: PTV coverage, Conformity Indexes, Gradient Index, Homogeneity Index, and beam on time. PTV coverage was defined as the percentage of volume inside the prescription isodose line. Two conformity indices: CI and nCI were considered. CI = V TV/V PTV with V TV the treated volume enclosed by the prescription isodose surface and V PTV the planning target volume. nCI = (V PTV × V TV)/(TVPV)2 with TVPV, the portion of PTV within the prescription isodose surface [14, 15]. GI = PIVhalf/PIVwith PIVhalf the volume encompassed by the 50% isodose line and PIV the volume of the prescription isodose and the Homogeneity Index (HI) = MD/PD with MD the maximum dose within the target volume and PD the prescribed dose.
Statistical analysis
The Friedman test was used to detect differences in treatment parameters among the three planning modalities. When a statistically significant difference was found, a further investigation among the three treatment modalities was performed using the Wilcoxon signed rank test. Post hoc analysis with Wilcoxon signed ranks test was conducted with a Bonferroni correction applied, resulting in a significance level at p < 0.017. The Statistical Package for the Social Sciences (IBM Corporation, New York, USA) was used.
Results
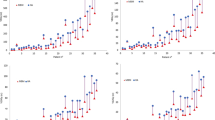
In Table 1, the mean values and standard deviations for: PTV coverage, Conformity Indexes, Gradient Index, Homogeneity Index, and beam on time for CK, icHT, and HT plans are summarized. All the planning modalities reached a good coverage. In terms of PTV, coverage differences between HT and icHT were not statistically significant (p = 0.061); on the other hand, for CK, a better coverage compared to HT (p = 0.006) and icHT (p = 0.0001) was observed. Significant differences of HI between the different modalities were found. As foreseen, HT resulted in more homogenous dose distribution inside the PTV (mean HI = 1.06 ± 0.05) than CK (mean HI = 1.25 ± 0.00, p = 0.0001).
icHT planning modality showed similar CI and nCI values compared to HT modality (p = 0.036 and p = 0.14, respectively), while a statistically significant difference between CK, HT, and icHT was observed in both CI and nCI. CK planning showed a better conformity in PTV coverage [mean CI = 1.05 (±0.02) and mean nCI = 1.08 (±0.04)], significantly superior to that of icHT (p = 0.002 and p = 0.0001, respectively) and HT (p = 0.0001 and p = 0.0001, respectively). Better dose gradients compared for both icHT and HT modalities were observed for CK plans (p = 0.0001 and p = 0.0001, respectively), even though an improvement in the GI was observed for icHT in respect to HT plans (p = 0.0001). Statistically significant differences in treatment time between icHT and CK (p = 0.0001) and icHT and HT (p = 0.003) were observed. In particular, the lower delivery time was obtained for icHT: 19 vs 22 min for HT and 33 min for CK.
Discussion
The first device developed for RS treatments was GammaKnife, a frame-based system characterized by inhomogeneous dose distribution inside the target with high target conformity values. The progress in radiotherapy technology of the last few years has permitted to obtain similar dose distribution also with Helical TomoTherapy and CyberKnife systems with the advantage of a non-invasive approach.
The aim of our study is to compare CyberKnife and TomoTherapy plans of single brain metastasis to report potential advantages of one modality over the other. For this purpose, 19 plans of single brain metastasis planned with CyberKnife and re-planned with TomoTherapy were compared evaluating the conventional index used to score RS plans, namely CI, HI, GI, and beam on time. Mean CI resulted 1.06 ± 0.03, 1.19 ± 0.07, and 1.27 ± 0.10 for CK, icHT, and HT plans respectively; the differences between CK, icHT, and HT were statistically significant (p = 0.001). Differences in CI between CK and both icHT and HT modalities are attributable to the different dose delivering and beam collimation systems. In CK, the irradiation is performed using non-isocentric non-coplanar beams coming from different angles, while in Helical TomoTherapy treatments, dose is delivered by a collimated fan beam along an helical pattern. Moreover, when a Helical TomoTherapy system with fixed jaws is considered (as the one here used), additional doses superior and inferior to the target volume are observed [14].
The same behavior we observed for CI is pointed out in GI analysis. Better dose gradients compared to both icHT (5.35 + 1.31) and HT (7.18 + 2.10) modalities were obtained for CK plans (3.62 + 0.58), even though an improvement in the GI was observed for icHT respect to HT plans.
Dosimetric evaluations were an issue of different studies.
Recently, Lomax et al. [15] evaluated 551 plans of both malignant and benign brain lesions treated with GammaKnife reporting a median CI of 1.24.
A median CI of 1.67 was reported by Nakamura et al. [15] for the treatment of 1338 lesions using GammaKnife. Kubo et al. [16] reached a median CI of 1.4 for the treatment of 408 lesions with GammaKnife and a CI of 1.8 in 12 lesions treated with Linac. The results reported in literature showed that GammaKnife plans obtained worst median CI than the ones we obtained for all the devices we used (CK median CI: 1.06, icHT median CI: 1.15, and HT median CI: 1.17).
This finding is confirmed comparing our results with the results obtained with Linac or others Tomotherapy dosimetric plan evaluations. In fact, in a recent work [17], Han et al. compared 16 intracranial lesions planned with step-and-shoot IMRT generated using coplanar and non-coplanar beams and Helical TomoTherapy. The average nCI values were 1.53 ± 0.38 in coplanar IMRT beams, 1.35 ± 0.15 in no coplanar IMRT beams, and 1.26 ± 0.10 in TomoTherapy plans.
The treatment time in RS is very important particularly in multiple BMs treatment. A double beam on time in CK plans respect to icHT plans (statistically significant p = 0.0001) was observed. HT plans with a mean beam on time of 22 ± 6 min resulted significantly longer than icHT (p = 0.0001). Considering that the volume of the target could limit the use of radiosurgery, HT could be advantageous in cases of multiple BMs that have to be treated in the same RS session or in case of WBRT with integrated boost to the lesions [18]. It must be pointed out that the newest CyberKnife M6 system equipped with a Multileaf collimator has been reported to reduce treatment delivery time significantly [19]. Although comparison with CK MLC plans may be an interesting development of this study; at the moment, brain metastases are still treated in the majority of CK centers using circular collimating apertures as examined in the present work.
The homogeneity was the last dosimetric factor analyzed: both CK and icHT reached a high heterogeneity (mean HI 1.25 ± 0.00 and 1.28 ± 0.03 for CK and icHT, respectively), while a more homogenous dose distribution (mean HI 1.06 ± 0.05) was observed for HT.
The choice to give high relevance to conformity and low-dose spillage outside the target derived from the historical radiosurgery experience with GammaKnife that using multiple overlapping shots may produce hotspots within targets. However, as demonstrated by Nakamura et al. [20], hotspots inside targets could not necessarily result in a significant risk of complications compared to more homogenous dose distribution.
The importance of homogeneity in radiosurgery plans is still controversial; in fact, if the heterogeneity is considered from some authors, a factor that could influence the local control [21] in particular in radioresistant metastasis [22]; on the other hand, the heterogeneity could be related with higher probability of toxicities [23]. It is worthwhile to mention that besides dose heterogeneity, other important predictors of radionecrosis are: prescription dose, tumor size, and location, and the brain volume that receive at least 12 Gy (V12) [24–26].
Conclusions
In the last few years, an improvement in clinical outcome of brain metastatic patients, not correlated to radiotherapy devices used to perform RS, was observed. For this reason, considering also the impressive diffusion of new technologies, such as CyberKnife and TomoTherapy, it is important to guide the use of a specific device keeping into account its characteristic performances. If we consider that the endpoint of RS treatments is to give high dose to the target sparing healthy surrounding tissue, CK better respects these objectives. On the other side, the capability of TomoTherapy in delivering both homogeneous and heterogeneous dose distribution could be useful in case of disease localized in critical areas or in case of retreatment where an homogenous dose distribution could reduce the risk of radionecrosis.
Finally, the diffusion of RS treatments for multiple BMs can benefit from the shorter treatment time of TomoTherapy in comparison to CyberKnife.
In conclusion, CyberKnife and TomoTherapy are both optimal RS devices and the choice to use one over another has to be clinically guided.
References
Eichler AF, Loeffler JS (2007) Multidisciplinary management of brain metastases. Oncologist 12(7):884–898
Sperduto PW, Chao ST, Sneed PK et al (2010) Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4259 patients. Int J Radiat Oncol Biol Phys 77:655–661
Aoyama H, Shirato H, Tago M et al (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295:2483–2491
Kocher M, Soffietti R, Abacioglu U et al (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 29:134–141
Tsao MN, Rades D, Wirth A et al (2012) Radiotherapeutic and surgical management for newly diagnosed brain metastasis (es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol 2:210–225
Brown PD, Brown CA, Pollock BE et al (2002) Stereotactic radiosurgery for patients with “radioresistant”brain metastases. Neurosurgery 51:656–665
Chang EL, Selek U, Hassenbusch SJIII et al (2005) Outcome variation among so called “radioresistant” brain metastases treated with stereotactic radiosurgery. Neurosurgery 56:936–945
Yamamoto M, Serizawa T, Shuto T et al (2014) Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901):a multi institutional prospective observational study. Lancet Oncol 15(4):387–395
Soisson ET, Hoban PW, Kammeyer T et al (2011) A technique for stereotactic radiosurgery treatment planning with helical TomoTherapy. Med Dosim 36(1):46–56
Baumert BG, Rutten I, Dehing-Oberije C et al (2006) A pathology-based substrate for target definition in radiosurgery of brain metastases. Int J Radiat Oncol Biol Phys 66(1):187–194
Hoogeman MS, Nuyttens JJ, Levenda G et al (2008) Time dependence of intrafraction patient motion assessed by repeat stereoscopic imaging. Int J Radiat Oncol Biol Phys 70:1313–1319
Drabick DM, MacKenzie MA, Fallone GB (2007) Quantifying appropriate PTV setup margins: analysis of patient setup fidelity and intrafraction motion using post-treatment megavoltage computed tomography scans. Int J Radiat Oncol Biol Phys 68:1222–1228
Shaw E, Scott C, Souhami L et al (2000) Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brainmetastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 47(2):291–298
Gladwish A, Oliver M, Craig J et al (2007) Segmentation ad leaf sequencing for intensity modulated arc therapy. Med Phys 34(5):1779–1788
Lomax NJ, Scheib SG (2003) Quantifying the degree of conformity in radiosurgery treatment panning. Int J Radiat Oncol Biol Phys 55(5):1409–1419
Kubo HD, Wilder RB, Pappas CTE (1999) Impact of collimator leaf width on stereotactic radiosurgery and 3D conformal radiotherapy treatment plans. Int J Radiat Oncol Biol Phys 44:937–945
Han C, Liu A, Schultheiss TE et al (2006) Dosimetric comparisons of Helical Tomotherapy treatment plans and step-and-shoot intensity modulated radiosurgery treatment plans in intracranial stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 65(2):608–616
Bauman G, Yartsev S, Fisher B et al (2007) Simultaneous infield boost with helical TomoTherapy for patients with 1 to 3 brain metastases. Am J Clin Oncol 30(1):38–40
McGuinnes CM, Gottschalk AR, Lessard E et al (2015) Investigating the clinical advantages of a robotic linac equipped with a multileaf collimator in the treatment of brain and prostate cancer patients. J Appl Clin Med Phys 16(5):284–295
Nakamura JL, Verhey LJ, Smith V et al (2001) Dose conformity of gamma knife radiosurgery and risk factors for complications. Int J Radiat Oncol Biol Phys 51(5):1313–1319
Leith JT, Cook S, Chougule P et al (1994) Intrinsic and extrinsic characteristics of human tumors relevant to radiosurgery: comparative cellular radiosensitivity and hypoxic percentages. Acta Neurochir Suppl 62:18–27
Tome W, Fowler J (2000) Selective boosting of tumor sub-volumes. Int J Radiat Oncol Biol Phys 48(2):593–599
Kohutek ZA, Yamada Y, Chan TA et al (2015) Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol 125(1):149–156
Flickinger JC, Lunsford LD, Kondziolka D et al (1992) Radiosurgery and brain tolerance: an analysis of neurodiagnostic imaging changes after gamma knife radiosurgery for arteriovenous malformations. Int J Radiat Oncol Biol Phys 23:19–26
Blonigen BJ, Steinmetz RD, Levin L et al (2010) Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 77:996–1001
Minniti G, Clarke E, Lanzetta G et al (2011) Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol 6:48
Acknowledgements
The abstract was presented in the poster session of the ESTRO 33 Congress, 4–8 April 2014, Barcelona, Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Funding
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Greto, D., Pallotta, S., Masi, L. et al. A dosimetric comparison between CyberKnife and tomotherapy treatment plans for single brain metastasis. Radiol med 122, 392–397 (2017). https://doi.org/10.1007/s11547-017-0735-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-017-0735-9