Abstract
Background
As increasing numbers of deep brain stimulation (DBS) procedures are performed, rare abnormal findings on postoperative images that are not attributable to well-known complications are reported. Between 2005 and 2012, we encountered several symptomatic patients with transient abnormal low-attenuation lesions on postoperative computed tomography (CT) scans. The aim of this study was to clarify this rare phenomenon using chronological observations and to suggest a feasible mechanism.
Results
In this period, seven (3.2 %) patients displayed transient increased low-attenuation signals, circumferentially surrounding the DBS electrodes and extending into the subcortical white matter. All these patients suffered from unexpected but transient neurological symptoms during the postoperative period. The abnormal low-attenuation lesions only disappeared completely a considerable time after the clinical symptoms had disappeared, without treatment in most patients.
Conclusions
We report here our chronological observations of acute brain reactions after DBS procedures, which we believe are neither infectious nor vascular, but are possibly caused by the mechanical breakdown of the blood–brain barrier by microelectrode recordings or by anchored DBS electrodes. These lesions are thought to constitute a self-limiting disorder requiring no further treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Deep brain stimulation (DBS) is a well-established treatment for movement disorders such as advanced Parkinson’s disease (PD). With the increasing number of DBS procedures performed, many complications have been reported in the literature [7, 13–15, 18, 25, 26]. DBS-related complications can be categorized into stereotactic-procedure-related, hardware-related, and stimulation-procedure-related complications.
In the past few years, some authors have reported rare and peculiar abnormal findings on postoperative magnetic resonance (MR) images that cannot be attributed to the aforementioned complications [8, 22]. In 2004, Ryu et al. demonstrated asymptomatic T2-weighted hyperintensity surrounding DBS electrodes during the first three months after their implantation in 39 % of patients [22]. Recently, another study reported similar imaging findings for the early postoperative period [8]. The authors observed that these interesting, typically unilateral findings were rare, with an incidence of 6.3 %, and had an asymptomatic clinical course in most patients. However, there is still little precise and detailed information about this rare phenomenon, including its incidence, natural course, clinical significance, or mechanism.
We have also encountered several patients with similar imaging findings on serial computed tomography (CT) during the postoperative period. The aim of this study was to clarify this rare phenomenon through a chronological series of observations, with careful radiological and clinical follow-ups, and to suggest a feasible mechanism. Investigating the similarities and differences between these patients should allow better recognition of this rare phenomenon and its appropriate management.
Materials and methods
Between March 2005 and July 2012, 221 patients underwent 243 DBS procedures at the Seoul National University Hospital. Among the 221 DBS patients, there was a diagnosis of PD in 173 patients, dystonia in 31 patients, essential tremor in 15 patients, and obsessive–compulsive disorder in two patients. All DBS patients routinely underwent an immediately postoperative CT on the DBS procedure day and had their next CT one month later. However, if judged necessary, additional CT was performed during their period of hospitalization.
We retrospectively reviewed the radiological and clinical data collected during the perioperative period for all of these patients. Patients who met the following criteria were included in the study: (1) transient low attenuation on CT images during the early postoperative period that had been absent on previous images, including on the immediately postoperative images; and (2) changes in both the images and the clinical features of patients not associated with DBS-procedure-related complications, such as hemorrhage or infection.
In the DBS procedures used for all patients, a stereotactic Leksell®-G frame (Elekta Instruments AB, Stockholm, Sweden) was mounted on the head of the patient under local anesthesia. The subthalamic nucleus (STN) was localized by a combination of direct visualization by magnetic resonance imaging (MRI), microelectrode recording (MER), and a stimulation technique. The quadripolar DBS electrode model 3389 (Medtronic Sofamor Danek, Minneapolis, MN, USA), with four platinum iridium cylindrical surfaces (1.27 mm in diameter, 1.5 mm in length, with 0.5 mm electrode spacing), and the Soletra model 7428 implantable pulse generator (Medtronic Neurological Division, Minneapolis, MN, USA) were implanted in a single session, as described elsewhere [16, 17, 19–21]. Electrical stimulation was commenced one day after surgery. The stimulation parameters and medication were progressively adjusted using the N’vision® programmer (Medtronic) [16, 17, 19–21].
Results
We identified seven patients (3.2 %) with abnormal findings on CT images, displaying increased low attenuation circumferentially surrounding parts of the electrodes and extending into the subcortical white matter. Serial CT, including immediately postoperative images, showed no evidence of the well-known complications, such as hemorrhage, infarction, or infection. The patients included four female and three male patients, whose mean age at the time of the DBS procedure was 56 years (range, 49–67 years). The diagnosis for all patients was idiopathic PD and they had undergone bilateral DBS procedures with STN targets. The low-attenuation lesions on the CT scans were unilateral in four patients and bilateral in three patients. The patient characteristics are shown in detail in Table 1.
All the patients recovered immediately and fully after the DBS procedure, with no focal neurological deficit. There were no abnormal findings associated with hemorrhage or infarction on the CT images obtained immediately after surgery. However, these patients experienced newly developed neurological deterioration after a median period of four days (range, 2–11 days) after DBS. Three patients showed neurological deterioration that was assumed to be a frontal lobe dysfunction, including irritability, confusion, perseveration, and repetitive behavior. Three other patients suffered focal neurological deficits, such as mild motor weakness, motor dysphasia, or swallowing difficulty. The other patient experienced her first seizure. After these sudden and unexpected events, all the patients underwent CT. There were no abnormal findings associated with hemorrhage or infarction, but newly developed low-attenuation lesions around the electrodes were observed.
Depending on the improvement in the patients’ symptoms, serial follow-up CT was performed, with further clinical evaluations. These included closely monitored body temperature and blood tests, such as white blood count (WBC, normal range: 4000–10,000/μL), erythrocyte sedimentation rate (ESR, normal range: 0–9 mm/h), and high-sensitivity C-reactive protein (hs-CRP, normal range: 0–0.5 mg/dL) assays.
Most patients underwent close observation with no medical treatment (such as corticosteroid and antibiotics), except for two patients in the early part of the study period. One patient (case #1) underwent short-term corticosteroid treatment and another patient (case #2) was treated with empirical antibiotic therapy for unexplained neurological deterioration, although there was no evidence of any central nervous system infection. However, after these experiences, subsequent patients were managed with only clinical observation, without treatment, including antibiotics or corticosteroids.
The median duration of the clinical symptoms was 10 days (range, 5–16 days). After the clinical symptoms had disappeared, the abnormal findings on CT images persisted for a certain period. The median period between the initial detection and the complete disappearance of the low-attenuation lesions was 25 days (range, 23–37 days). During the period of observation (3–4 months), a mean of three CT scans and one MRI scan were performed per patient. More detailed information regarding the patients is given in Table 1.
Illustrative cases
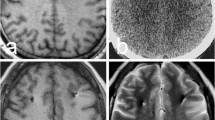
A 54-year-old man (case #2) with a 14-year history of PD underwent bilateral STN DBS. The immediately postoperative CT revealed no abnormalities such as hemorrhage or infarction (Fig. 1a). Three days after DBS, his anti-PD medication was discontinued, the DBS turned on, and the stimulation parameters adjusted. The patient showed a dramatic improvement in his PD-related motor and sensory symptoms. On postoperative day (POD) 3, a routine blood test showed slightly increased ESR (42 mm/h) and elevated hs-CRP (5.49 mg/dL), but he was afebrile, with a normal WBC count (6000/μL). On POD 5, the patient showed abrupt neurological deterioration, with symptoms of frontal lobe dysfunction, such as confusion, perseveration, and disinhibition. CT images showed bilateral, round, low-density lesions in the white matter along the DBS electrodes (Fig. 1b). However, the patient’s hs-CRP had decreased slightly (1.13 mg/dL), his WBC count was within the normal range (6400/μL), and he remained afebrile. Although there was no evidence of infection, we decided to commence empirical antibiotic therapy, because we could not completely rule out postoperative infection based on the abnormal CT findings. The patient’s motor performance was excellent without any anti-PD medication, but his frontal lobe symptoms continued for a few days. From POD 9, his abnormal behavior decreased gradually and had almost returned to normal on POD 18. Whereas the extent of the low-attenuation lesions on the CT images was maintained until POD 8, they decreased gradually after POD 18 and had completely disappeared by POD 29 (Fig. 1c–e). MRI on POD 39 showed no abnormal findings (Fig. 1f).
a Immediately postoperative computed tomography (CT) scan revealed no abnormal findings in the white matter around the electrodes. b and c Serial CT images taken on postoperative day (POD) 5 (b) and POD 8 (c) after deep brain stimulation (DBS) of the subthalamic nucleus (STN) demonstrated newly developed, round, low-attenuation lesions surrounding both DBS electrodes. d On POD 18, CT images showed the reduced extent of the low-attenuation lesions. e and f The low-attenuation lesions that had gradually decreased from POD 18 had completely disappeared on both a CT scan (POD 29) and MR image (POD 39)
Another patient (case #4) fully recovered from the STN DBS procedure with no discernible problems (Fig. 2a). However, on POD 2, he abruptly displayed neurological deterioration, including confusion, irritability, and repetitive behavior. CT on POD 3 showed a low-attenuation lesion around a right-side electrode (Fig. 2b). The patient was managed conservatively with clinical observation only. During the follow-up period, he was still afebrile, and his serial blood tests and clinical course did not suggest infection. Thereafter, his neurological symptoms gradually decreased and had almost disappeared on POD 18. The low-attenuation lesion gradually decreased after POD 9 and had completely disappeared on a CT image acquired on POD 24 (Fig. 2c and d).
a An immediately postoperative noncontrast brain CT scan showed no parenchymal lesion. b A noncontrast brain CT scan taken on POD 3 after STN DBS surgery showed a round, low-attenuation lesion on the right DBS electrode. c On POD 9, CT images showed the reduced extent of the low-attenuation lesion. d The low-attenuation lesion on a CT scan had completely disappeared on POD 24
Discussion
In a total of 221 patients who had undergone 243 DBS procedures, we identified seven patients (3.2 %) with a transient low-attenuation lesion along the DBS electrodes that had not been found in the immediately postoperative period. However, the incidence in our study is possibly underestimated because of our policy of CT based on clinical signs and symptoms during the early postoperative period. The incidence in two previous studies was reported as 6.3 % and 39 % [8, 22].
The differential diagnosis of this abnormal imaging finding would normally presume a vascular origin, such as hemorrhage or infarction related to the DBS procedure. However, there were no abnormal findings on the immediately postoperative and serial follow-up CT scans, and the lesions disappeared completely without treatment. Furthermore, the pattern of round, low-attenuation lesions along the white matter tract of the DBS electrode leads did not correlate with any arterial or venous territory. Therefore, it is unlikely that these low-attenuation lesions had a vascular etiology. Infectious conditions, such as acute infectious cerebritis, should be considered as an alternative cause. However, the aberrant signal appeared too soon after the procedure for any offending organism to have colonized the brain parenchyma, causing infectious symptoms, and the simultaneous occurrence of acute infectious cerebritis in both hemispheres of three patients could not be easily explained. Moreover, the lesions completely disappeared within several weeks and most patients recovered fully without antibiotic treatment. Another possible cause might have been an allergic reaction to the DBS electrodes. However, four patients had only unilateral lesions, while the other patients had bilateral lesions. Therefore, an acute allergic reaction to an unknown antigen in the surface of the electrode is an unlikely cause of these acute brain reactions.
Several experimental studies provided clues to the possible mechanism of this rare and peculiar phenomenon [3, 10, 23]. In an immunohistochemical study, Spataro et al. identified a reactive cellular response and broken blood vessels around the insertion sites of the electrodes [23]. They observed a gradual increase in the laminin staining intensity in the large blood vessels by day 7, after which the signal decreased to the control level. The researchers showed that these increases in laminin staining in the microvascular structures reflected a response to focal parenchymal injury and the activation of repair processes, and that the phenomenon could last for as long as six weeks [23]. They also identified the cellular process that stimulated the brain’s response to injury [3, 10, 23]. When the blood–brain barrier (BBB) is damaged by a needle-stab injury or the transplantation of electrodes, various cells can produce and secrete cytokines, including activated mononuclear phagocytes, microglia, and blood-borne macrophages. The cytokines aggravate further inflammatory processes and angiogenesis within hours of the insult [10, 23]. A breakdown of the BBB by mechanical injury resulting from the insertion of needles for MER or the implantation of electrodes into the brain parenchyma during surgery could result in an acute inflammatory cerebral reaction, secondary to vasogenic edema and the aggregation of inflammatory blood cells. Such an acute brain parenchymal reaction caused by the breakdown of the BBB might be a self-limiting process that resolves spontaneously over several weeks. These experimental data correlate well with the chronological observational data on our patients, in whom peculiar findings developed during the early postoperative period, tended to be transient, and showed a self-limiting clinical course without treatment.
The mechanical BBB breakdown process might also be exacerbated by indwelling and anchoring DBS electrodes in particular cases. Biran et al. reported that the method of anchoring silicon microelectrode arrays to the skull significantly influences the brain tissue reaction including greater microglial activation and increased astrogliosis [4, 5]. They suggested that shearing and/or compression of the adjacent tissue, which arises from the stiffness mismatch between the implant and brain tissue and is caused by the relative motion between the skull and the brain parenchyma, could stimulate an inflammatory tissue response [4, 5]. Based on these studies, we believe that the acute mechanical BBB breakdown resulting from brain parenchymal damage during the surgery associated with MER recordings or by the anchored DBS electrodes is a plausible explanation for the acute brain reaction observed along the white matter tracts of the DBS electrodes.
Recently, Englot et al. reported abnormal T2-weighted MR signal changes after the DBS procedure [8]. No differences in age, diagnosis, or lead locations were noted between the patients with and without these T2 signal changes. The authors suggested vasogenic edema surrounding the DBS electrodes as a possible explanation. From this perspective, that study seems to share many similarities with ours.
However, the incidence of symptomatic patients was much higher in our study than in the study by Englot et al. (20 %, three of 15 patients) [8]. Although direct comparisons with previous studies are complicated by the rarity of this phenomenon and the different treatment regimens used, this difference in incidence might result from different follow-up imaging strategies, such as the modalities used (CT versus MRI) or the initial check time. Another possible explanation is that different DBS procedures were used for MER. We used quadripolar DBS electrodes, which have a greater contact surface with the normal brain parenchyma than do single electrodes. This is a plausible explanation of the higher incidence of symptomatic acute brain reactions in our study. MR images seem to be superior to CT images in precisely detecting small lesions around the electrodes because their resolution is higher. However, when the clinical or economic aspects of imaging are considered, the effectiveness of repeated applications of MRI for chronological observations, such as the initial detection and disappearance of rare abnormal phenomena, is limited.
For acute brain reactions with vasogenic edema, short-term corticosteroid therapy seems to be effective for severely symptomatic patients. However, we still cannot confirm that patients with these peculiar images require prompt treatment. Apart from two patients identified in the initial portion of our study period, when we did not recognize these peculiar signals, our patients recovered fully without treatment.
Although recognition of this rare phenomenon has gradually increased, little is yet known about it. Further well-designed and multicenter studies with long-term follow-up are required to ensure better recognition of this disorder, its natural course, and appropriate management. On the other hand, it has been our endeavor to clarify the possible mechanism. A more sophisticated evaluation using advanced tools such as MRI and specialized MRI protocols may contribute to a better understanding of the causes of the changes after DBS procedures [5, 11, 24, 25]. Together with this proposed study, a large-scale and detailed analysis of whether this rare phenomenon results from differences associated with DBS procedures such as the laterality, the number of microelectrodes and DBS lead paths, the locations of the best contact of the electrodes, operation time (including the required time for MER), and early stimulation or not, is necessary to clarify the causes [1, 2, 6, 9, 11]. Moreover, analysis of the measured impedance of the electrodes between the time of symptom detection and the time of aberrant finding detection may provide a clue as to the possible mechanism of this rare phenomenon [1, 6, 9, 12].
Limitations of the present study include its small sample size using a case series and retrospective study design. However, considering the scant detailed information regarding this rare phenomenon, we believe that the present study provides further information regarding its incidence, natural course, clinical significance, and possible mechanism.
Conclusions
We have reported our chronological observations of seven patients with acute brain reactions along the electrodes after DBS procedures. The cause of this rare phenomenon is considered to be neither infectious nor vascular, but possibly the result of a mechanical breakdown of the BBB during MER recording or the anchored DBS electrodes. Although these lesions are thought to constitute a self-limiting disorder requiring no additional treatment, further study is needed to obtain more detailed information.
Abbreviations
- DBS:
-
deep brain stimulation
- PD:
-
Parkinson’s disease
- MR:
-
magnetic resonance
- CT:
-
computed tomography
- STN:
-
subthalamic nucleus
- MER:
-
microelectrode recording
- BBB:
-
blood brain barrier
- POD:
-
postoperative day
References
Abosch A, Lanctin D, Onaran I, Eberly L, Spaniol M, Ince NF (2012) Long-term recordings of local field potentials from implanted deep brain stimulation electrodes. Neurosurgery 71:804–814
Allert N, Markou M, Miskiewicz AA, Nolden L, Karbe H (2011) Electrode dysfunctions in patients with deep brain stimulation: a clinical retrospective study. Acta Neurochir (Wien) 153:2343–2349
Arvin B, Neville LF, Barone FC, Feuerstein GZ (1996) The role of inflammation and cytokines in brain injury. Neurosci Biobehav Rev 20:445–452
Biran R, Martin DC, Tresco PA (2007) The brain tissue response to implanted silicon microelectrode arrays is increased when the device is tethered to the skull. J Biomed Mater Res A 82:169–178
Biran R, Martin DC, Tresco PA (2005) Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol 195:115–126
Butson CR, Cooper SE, Henderson JM, McIntyre CC (2007) Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage 34:661–670
Chou YC, Lin SZ, Hsieh WA, Lin SH, Lee CC, Hsin YL, Yen PS, Lee CW, Chiu WT, Chen SY (2007) Surgical and hardware complications in subthalamic nucleus deep brain stimulation. J Clin Neurosci 14:643–649
Englot DJ, Glastonbury CM, Larson PS (2011) Abnormal T2-weighted MRI signal surrounding leads in a subset of deep brain stimulation patients. Stereotact Funct Neurosurg 89:311–317
Farris S, Vitek J, Giroux ML (2008) Deep brain stimulation hardware complications: the role of electrode impedance and current measurements. Mov Disord 23:755–760
Giulian D, Woodward J, Young DG, Krebs JF, Lachman LB (1988) Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. J Neurosci 8:2485–2490
Gross RE, McDougal ME (2013) Technological advances in the surgical treatment of movement disorders. Curr Neurol Neurosci Rep 13:371
Guridi J, Rodriguez-Oroz MC, Alegre M, Obeso JA (2012) Hardware complications in deep brain stimulation: electrode impedance and loss of clinical benefit. Parkinsonism Relat Disord 18:765–769
Hamani C, Lozano AM (2006) Hardware-related complications of deep brain stimulation: a review of the published literature. Stereotact Funct Neurosurg 84:248–251
Hariz MI, Rehncrona S, Quinn NP, Speelman JD, Wensing C (2008) Multicenter study on deep brain stimulation in Parkinson's disease: an independent assessment of reported adverse events at 4 years. Mov Disord 23:416–421
Kenney C, Simpson R, Hunter C, Ondo W, Almaguer M, Davidson A, Jankovic J (2007) Short-term and long-term safety of deep brain stimulation in the treatment of movement disorders. J Neurosurg 106:621–625
Kim HJ, Jeon BS, Lee JY, Paek SH, Kim DG (2012) The benefit of subthalamic deep brain stimulation for pain in Parkinson disease: a 2-year follow-up study. Neurosurgery 70:18–23
Lee JY, Kim JW, Lim YH, Kim C, Kim DG, Jeon BS, Paek SH (2010) Is MRI a reliable tool to locate the electrode after deep brain stimulation surgery? Comparison study of CT and MRI for the localization of electrodes after DBS. Acta Neurochir (Wien) 152:2029–2036
Lyons KE, Wilkinson SB, Overman J, Pahwa R (2007) Surgical and hardware complications of subthalamic stimulation: a series of 160 procedures. Neurology 63:612–616
Paek SH, Kim HJ, Yoon JY, Heo JH, Kim C, Kim MR, Lim YH, Kim KR, Kim JW, Han JH, Kim DG, Jeon BS (2011) Fusion image-based programming after subthalamic nucleus deep brain stimulation. World Neurosurg 75:517–524
Paek SH, Kim JW, Lim YH, Kim MR, Kim DG, Jeon BS (2010) Data management system in a movement disorder center: technical report. Stereotact Funct Neurosurg 88:216–223
Paek SH, Lee JY, Kim HJ, Kang D, Lim YH, Kim MR, Kim C, Jeon BS, Kim DG (2011) Electrode position and the clinical outcome after bilateral subthalamic nucleus stimulation. J Korean Med Sci 26:1344–1355
Ryu SI, Romanelli P, Heit G (2004) Asymptomatic transient MRI signal changes after unilateral deep brain stimulation electrode implantation for movement disorder. Stereotact Funct Neurosurg 82:65–69
Spataro L, Dilgen J, Retterer S, Spence AJ, Isaacson M, Turner JN, Shain W (2005) Dexamethasone treatment reduces astroglia responses to inserted neuroprosthetic devices in rat neocortex. Exp Neurol 194:289–300
Uitti RJ, Tsuboi Y, Pooley RA, Putzke JD, Turk MF, Wszolek ZK, Witte RJ, Wharen RE Jr (2002) Magnetic resonance imaging and deep brain stimulation. Neurosurgery 51:1423–1428
Umemura A, Jaggi JL, Hurtig HI, Siderowf AD, Colcher A, Stern MB, Baltuch GH (2003) Deep brain stimulation for movement disorders: morbidity and mortality in 109 patients. J Neurosurg 98:779–784
Voges J, Waerzeggers Y, Maarouf M, Lehrke R, Koulousakis A, Lenartz D, Sturm V (2006) Deep-brain stimulation: long-term analysis of complications caused by hardware and surgery–experiences from a single centre. J Neurol Neurosurg Psychiatry 77:868–872
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors’ contributions are as follows.
Jin Wook Kim: surgery, making the manuscript
Jae Ha Hwang: surgery and management of patients
In Kyeong Kim: surgery and management of patients
Young Eun Kim: neurological evaluation and management of patients
Hui-Jun Yang: neurological evaluation and management of patients
Gwanhee Ehm: neurological evaluation and management of patients
Han-Joon Kim: neurological evaluation and management of patients
Dong Gyu Kim: consultation for the manuscript
Sun Ha Paek: surgery and co-corresponding author 1
Beom S. Jeon: neurological evaluation
Comment
The authors describe a possibly overlooked complication of DBS, although previous reports exist (1).It would be interesting to determine if this effect is related to the microthalamotomy effect due to the micrelectrode trajectories, or to the fact that stimulation was started very early after the implantation of the electrodes.
Perhaps the use of MR to follow the changes,or the measure of the electrodes impedance would be useful to clarify the related mechanism.
A prospective study to further characterize this phenomenon would be welcome.
Reference:
1. Englot DJ, Glastonbury CM, Larson PS (2011) Abnormal T2-weighted MRI signal surrounding leads in a subset of deep brain stimulation patients. Stereotact Funct Neurosurg 89:311-317.
Fernando Alonso
Juan A. Barcia
Madrid
Rights and permissions
About this article
Cite this article
Kim, J.W., Hwang, J.H., Kim, I.K. et al. Acute brain reaction to DBS electrodes after deep brain stimulation: chronological observation. Acta Neurochir 155, 2365–2371 (2013). https://doi.org/10.1007/s00701-013-1853-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-013-1853-3