Abstract
Background
The objective of this study was to correlate various radiological parameters with clinical outcome in patients who had undergone lumbar total disc replacement (TDR). Lumbar TDR is one possible treatment option in patients with low back pain (LBP), offering an alternative to lumbar fusion. Favourable clinical outcome hinges on a number of radiological parameters, such as mobility, sintering, and—most importantly—accurate positioning of the implant.
Methods
A total of 46 patients received a prosthetic disc because of degenerative lumbar disc disorders. Follow-up evaluation included analysis of radiographs and subjective rating of the clinical status by the patient using the North American Spine Society (NASS) patient questionnaire, visual analogue scale (VAS) for pain and state of health, and the EuroQol EQ-5D. Radiological follow-up took place after 2 years. Coronal and sagittal positions of the prosthesis, intervertebral disc height, facet joint pressure, mobility, sintering, and calcification were evaluated. Optimal positioning of the prosthesis was defined as a central coronal position and a most dorsal position in the sagittal plane. Based on the radiologically determined placement of the prosthesis, the patient population was divided into three groups, i.e., prosthesis ideally placed (<2 mm), discretely shifted (2–3 mm), or suboptimally placed (>3 mm).
Results
Overall, 81 % of patients stated that they would undergo the operation again. Health status was stable at a VAS score of 7.04 points 2 years after TDR, compared to 3.97 points before TDR. Mean working capacity had increased from 53 % preoperatively to 88 % 2 years after TDR. Overall, 39 % of the prostheses were rated as ideally positioned, while 13 % were discretely shifted and 48 % were suboptimally placed with respect to one of the radiological criteria. In 80.4 % of patients, follow-up assessment after ≥2 years indicated good mobility at the operated segment, while calcification was noted in 4 % and sintering was detected in 15 % of the implants.
Conclusions
Our data indicate poor correlation between clinical outcome and position of the prosthesis. Although 48 % of the implants were suboptimally placed in either the coronal or sagittal plane, most of the patients reached a very good clinical outcome. However, suboptimally placed devices appeared to cause significantly more neurological symptoms in long-term follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Up to 84 % of adults experience low back pain (LBP) at some time in their lives [4, 7]. There are different aetiologies of LBP, which appears beside benign myofascial or posture induced as discogenic, spondylotic, or osteochondrotic pain. Many of these incidents can be treated nonsurgically, but conservative therapy is frequently ineffective, necessitating surgical stabilization.
Lumbar fusion of the degenerated segments has been the gold standard until recently. The main goal of lumbar fusion is to restore disc height and the sagittal balance, which enables the decompression of the neuroforamen and the spinal canal. However, lumbar fusion may be associated with pertinent problems, such as accelerated degeneration of the adjacent lumbar segments, pseudoarthrosis, and persistent LBP [1].
In recent years, lumbar total disc replacement (TDR) has been gaining acceptance as an alternative treatment in patients with lumbar disc disorder [13, 15, 25–27]. The primary goal of TDR is dynamic stabilization, preserving segment mobility and restoration of natural disc function while relieving the pain [5, 16]. TDR restores disc height and the range of motion without disrupting the sagittal balance [27].
In this study, we correlated various radiological parameters with the clinical outcome at the 2-year follow-up assessment in patients with TDR.
Materials and methods
Patient selection
A total of 46 prostheses were implanted in 46 patients (women: 27; men: 19; mean age: 49 years, range 18–60 years) between May 2005 and August 2010. All patients underwent surgery due to symptomatic lumbar disc disorders. Follow-up assessment took place 2 or more years after surgery. The treated segments included L3/L4 (2 prostheses), L4/L5 (23 prostheses), and L5/S1 (21 prostheses). Hybrid procedure with TDR combined with anterior lumbar interbody fusion (ALIF) in the adjacent segment was done in 13 patients. Only patients with monosegmental TDR were included in this study.
To qualify for TDR, patients had to be between 18 and 60 years of age and had been suffering from ongoing LBP, with or without leg pain. The patients’ age range was based on the guidelines of the Swiss Spine Society (SGS) [24]. The age limit was set in response to an expert recommendation with respect to the bone density that was considered acceptable for the surgery [22]. Bone densitometric measurements were done in doubtful cases. Only patients without history of osteoporosis underwent surgery. Furthermore, no patient showed a reduced bone density during surgery. In all cases, conservative treatment such as medication and physiotherapy, and interventional pain treatment such as epidural infiltration had failed. Lumbar TDR was indicated if facet joint injections had been unsuccessful, qualifying LBP as vertebrogenic or discogenic pain. In doubtful cases, discogenic pain was confirmed by provocative discography. Intact facet joints or minor spondylarthrosis was a prerequisite. Monosegmental or bisegmental lumbar discopathies were confirmed by standing a.p. and lateral X-rays in combination with sagittal views in extension and flexion as well as with magnetic resonance imaging (MRI).
Contraindications for TDR were osteoporosis, spinal canal stenosis, progressive scoliosis, degenerative facet joints, spondylolisthesis, fractures, malignancies, and previous retro- or severe intraperitoneal surgeries.
All patients with TDR who had undergone a clinical follow-up assessment after ≥2 years were included in this study (Table 1). The follow-up assessment took place after a median of 756 days (range 608 to 897 days) after surgery.
Surgical procedure for lumbar TDR
All patients underwent disc replacement surgery performed by two senior surgeons. The affected lumbar segments were accessed by the standard retroperitoneal approach. Depending on the patient’s anatomy and affected segment, we implanted either the A-Maverick® (anterior insertion) or the O-Maverick® (35° oblique insertion) prosthesis (Medtronic, Sofamor Danek, Memphis, USA) in all 46 cases.
Preoperative planning
Preoperative standing X-ray and MRI of the lumbar spine were done, comprising axial, sagittal, and coronal planes. The pictures indicated whether a workable trajectory could be achieved and ruled out severe facet joint degeneration, Tarlov’s cyst, low aortic bifurcation, enlarged midline vessels, or other vascular anomalies.
Surgical procedure
The “French” position, with the legs spread apart was used. A paramedian incision was made, and the retroperitoneal space was accessed by blunt dissection between the abdominal wall muscles and peritoneum. The L5/S1 segment was exposed within the bifurcation of the iliac vessels whereas the L4/L5 disc was approached from the left side by retracting the left iliac artery and vein to the right. Depending on the vascular anatomy, a segmental ascending vein had to be clipped in some cases. Great care was taken to preserve as much as possible of the sympathetic nervous fibres running with the anterior ligaments. To avoid vascular insult especially of the left leg, oxygen saturation was monitored at the left big toe.
After exposing the disc by the retractors the midline was defined under a.p. fluoroscopic view. Following discectomy, clearing the vertebral endplates, and removal of the posterior ligament and osteophytes, the midline was confirmed again. This was necessary to rule out any possible rotation or slight dislocation of the vertebral bodies after release of the posterior structures. The midline was defined by the superior and inferior pedicels and not by the spinous processes (as they are often off-midline and not perpendicular). We chose an implant with the largest possible footprint and positioned it as dorsally as possible to maintain the physiological centre of rotation. In addition, we endeavoured to restore lordosis and sagittal balance by optimal placement of the implant.
Follow-up assessments
In 2004, the Swiss Federal Office of Public Health included TDR in the catalogue of benefits. This required the establishment of a national register to monitor implantation of all artificial disc devices as well as clinical follow-up of patients. Prospective clinical evaluation included physical examination, which assessed spine mobility, motor function, and peripheral nerve conduction. The patients rated their condition subjectively using the North American Spine Society (NASS) patient questionnaire [6, 20, 23], visual analogue scale (VAS) for pain and state of health [8], and the EuroQol EQ-5D. At the follow-up visit, patients’ profession, working capacity, and their need for analgesics were recorded as well as incidence and specification of new events, radiculopathy including localisation, circulatory disorders, sympathectomic effects, and retrograde ejaculation.
Radiological interpretation
Standing a.p. views and lateral scanning as well as functional radiographs in flexion and extension were analysed retrospectively. Radiographic films were analysed manually, while digital pictures were analysed using a software programme (Synedra®).
To avoid interpatient variability, all assessments were done twice by a single observer. The arithmetic mean was used if the two values obtained differed.
Radiographic measurement
Parameters assessed were intervertebral disc height, coronal and sagittal position of the prosthesis, facet joint pressure, flexion/extension range of motion (ROM) of the prosthesis, sintering, and calcification.
-
Intervertebral disc height was measured in the middle at the operative level using the vertebral endplates as margins [18]. Graduation was classified (in comparison with the other vertebrae interspaces) as ideal (<2 mm), discretely too high (2–5 mm), and too high (>5 mm).
-
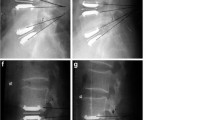
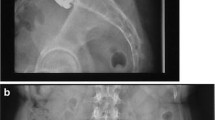
Coronal centering (Fig. 1) of the prosthesis was applied to the midline, measured from the pedicles of the two vertebras. Placement was classified as ideal (<2 mm), discretely shifted (2–3 mm), slightly shifted (3–5 mm), and markedly shifted (>5 mm).
-
Sagittal placement (Fig. 2) was determined at the operative level from the dorsal border of the vertebra defining graduation as ideal (<2 mm), discretely shifted (2–3 mm), and suboptimally placed (>3 mm).
-
Facet joint pressure was assessed indirectly by determining intervertebral disc height, angle of the two vertebrae at the operated level, and joint properties. Graduation was classified as no pressure (no joint alteration), low pressure (joint space narrow), and high pressure (no joint space/signs of arthrosis) [12].
-
Mobility was rated by angle measurements on dynamic (flexion/extension) lateral radiographs. Flexion/extension at the operated level was calculated from lines drawn on the vertebral surfaces. These lines could include either the operative or adjacent endplates, depending on radiograph clarity [18]. Alternatively, angle measurement was done between the posterior wall of the upper and lower vertebra of the operated motion segment [21]. Harrop et al. reported no prevalence of adjacent segment degeneration in patients with motion of ≥5° [11]; therefore a good range of motion was defined as a flexion/extension angle of >5°. Putzier et al. defined segmental mobility of ≥3° as mobile [21], with poor mobility graded as 3–5°. Segmental movement <3° was regarded as immobile.
-
Sintering and calcification were classified in a binary fashion, i.e., determining the presence or absence of signs.
Statistical analyses were done external by a statistician using the t-test and were calculated with STATA 11.0.
Results
All 46 patients had a clinical and radiological follow-up after 2 years or more. Table 1 shows the patient characteristics at baseline.
Clinical indices
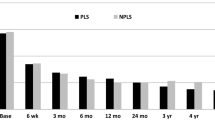
The clinical indices not related to disc positioning were significantly better after than before TDR (p < 0.05). Working capacity increased from 53 % before TDR to 88 % at the follow-up assessment. In addition, the patients’ need for analgesics decreased from 2.34 (95 %—CI: 2.18; 2.51) to 1.4 after >2 years (95 %—CI: 1.06; 1.28, p < 0.05). VAS scores were significantly better after TDR. Mean VAS score for back pain decreased from 6.93 (95 %—CI: 6.35; 7.52) to 3.34 (95 %—CI: 2.61; 4.06) after 2 or more years (p < 0.05). Similarly, mean VAS scores for radicular pain decreased from 5.51 (95 %—CI: 4.65; 6.38) to 2.42 (95 %—CI: 1.68; 3.16) (p < 0.05). As expected, mean VAS scores for health state increased from 3.97 (95 %—CI: 3.35; 4.6) to 7.04 (95 %—CI: 6.35; 7.72) after long-term follow-up (p < 0.05). Figure 3 shows all clinical indices.
Health state in the follow-up assessment was comparable to that of the average population in Switzerland. Data from 2007, registered by the Swiss Federal Statistical Office, show “good to very good” health state in 86.6 % of the Swiss population aged between 45 and 54 years. This finding corresponds to a VAS score of 7 to 10. Overall, 9.5 % of subjects described their health state as mediocre, while 4 % reported a poor state of health.
Preoperative neurological radicular deficits disappeared in 72.7 % of patients and improved in 9.1 % of the patients after TDR. A mean of 18.2 % patients had unchanged neurological status at the follow-up assessment.
Radiological findings
At follow-up after ≥2 years, 18 (39.2 %) prostheses were rated as ideally placed, 6 (13 %) as discretely shifted, and 22 (47.8 %) as suboptimally placed with respect to one of the criteria mentioned above. In total, 37 (80.4 %) of the implants were associated with good segment mobility, with 6 (13 %) resulting in poor mobility, and 3 (6.6 %) were completely immobile. We found sintering in 15.2 % of patients and signs of calcification in 4.35 % of patients. High facet joint pressure with signs of arthrosis was found in 6 (13 %) patients, low facet joint pressure in 32 (69.6 %) patients, and no indication of increased pressure in 8 (17.4 %) patients.
Overall, 18.2 % prostheses were misplaced in the lateral and 68.2 % in the coronal (a.p.) view. A total of 13.6 % of prostheses were displaced in either the sagittal or the coronal plane.
Correlation of clinical indices and disc positioning
Figure 4 shows the changes in clinical indices based on prosthesis positioning after TDR.
To identify the suboptimally placed prostheses, we compared the clinical changes in one group (ideally placed/discretely shifted) with those in a second group (suboptimally placed). Surprisingly, most clinical indices were comparable in the two groups. Only NASS for lumbar neurology indicated a significant difference after TDR based on prosthesis positioning. The score changed from −2.04 (95 %—CI: −2.56; −1.52) at baseline to −.82 (95 %—CI: −1.33; −.3) after ≥2 years (p < 0.05).
The significant difference of the NASS score for lumbar neurology is important, as it is the only value indicating a difference between ideally and suboptimally placed prostheses. If the NASS score is disregarded, prosthesis position and clinical outcome did not correlate in our study.
Discussion
Lumbar TDR is a treatment option for chronic LBP caused by lumbar disc disorders. In the last 50 years, considerable efforts have gone into the development of new approaches to treating disc diseases. Initially, prosthetic intervertebral disc devices have shown some benefit [1]. Nevertheless, the need for improved treatment has emerged from studies showing the limitations of present treatment modalities, especially spinal fusion [3, 10, 14]. Numerous studies have shown ambiguous results when comparing the clinical outcome after lumbar TDR and spinal fusion [9, 19, 29]. Yajun et al. showed that lumbar TDR results in slightly better functioning and improved back or leg pain status than does spinal fusion, but clinical outcome after 5 years was not significantly different between TDR and those with spinal fusion [28]. However, there was significantly greater patient satisfaction at the 2-year follow-up assessment in patients with TDR [28].
While lumbar TDR is increasingly accepted in the treatment of lumbar disc disorders [13, 15, 26], the factors influencing clinical outcome in these patients remain largely unknown. For this reason, we aimed to investigate whether there are radiological parameters predictive of good clinical outcome in patients with lumbar TDR. In contrast to the CHARITÉ™ study [18] that looked at the disc height, we selected prosthesis positioning in the sagittal and coronal plane as the key parameter assumed to influence clinical outcome. Thus, we correlated radiologically determined prosthesis positioning with different clinical indices.
The literature on surgical procedures supports the anterior approach as an option to treat initial or recurrent lumbar disc herniation or segmental collapse with osteochondrosis. Compared to the posterior approach, the anterior route allows a much better access to the lumbar disc with a wider entry to the intervertebral disc space. In addition, the anterior approach allows total removal of disc tissue without affecting the facets and paravertebral muscles [17].
Although access to the disc and discectomy are ideal whilst preserving the anatomical landmarks, accurate placement of the artificial disc device remains difficult. Often, the vertebral bodies are not symmetrically shaped and may be slightly rotated. This may even increase after discectomy and the release of the anterior or posterior ligament. Therefore, defining the midline in the coronal plane during surgery is highly complex. Anatomical structures such as the spinous process or vertebral ground plate cannot be used as benchmarks because of rotation or osteophytic processes. We used the pedicles of the vertebral body, because they seemed best suited for defining the midline in the coronal plane. Using this method, we expected accurate prosthesis placement.
Thus, the finding that more than 50 % of the prostheses were considered suboptimally placed was highly surprising. We reasoned that our system of classifying the three groups based on disc positioning (i.e., ideally placed, discretely shifted, and suboptimally placed) was too narrow, thus generating a large number of poorly placed prostheses. Prostheses misplaced during the surgery were adjusted immediately.
Figures 5 and 6 show ideally placed prostheses in the coronal and sagittal plane.
Although more than half of the implants were suboptimally placed in either the coronal or sagittal plane, most of the patients achieved a very good clinical outcome. All clinical indices were significantly better after lumbar TDR than before. We showed that lumbar TDR markedly improved the patients’ well-being and general state of health. Moreover, working capacity increased from 53 % before TDR to 88 % after TDR, which has important economic consequences.
In a recent paper, Schmidt et al. [25] reported that discs implanted by both the anterior and oblique route significantly increase segmental lordosis while retaining total lordosis. The segmental increase was lower in the oblique implanted group, which is probably due to the remaining anterior longitudinal ligament.
Our analysis revealed a single clinical index, namely the NASS score for neurologic assessment, which differed significantly between patients with ideally placed discs and patients with suboptimally placed discs. This score was the only variable assessing paraesthesia and paresis. Although we did not detect any marked differences in clinical outcome between patients with ideally positioned discs and those with poorly placed discs, there was a significant difference in neurological symptoms recorded at the 2-year follow-up assessment. This finding indicates that prosthesis positioning may indeed influence neurological functioning, although patient satisfaction, back pain, social life, and mobility did not appear to be affected. Thus, we conclude that accurate positioning of the disc may ensure better neurological condition. It would be of considerable interest to re-evaluate this finding after 5 or 10 years to establish whether or not the neurological deficits persist in patients with suboptimally placed prostheses.
Although our rather stringent criteria to classify positioning of the prostheses let to a large proportion of poorly positioned discs, the use of strict criteria is necessary to ensure the best possible placement of the prosthesis, especially because of the long-term neurological and clinical outcome.
The described results are based on and extracted of a more heterogeneous patient collective, which includes multiple treated segments and different types of prosthesis [2]. Results are nearly identical. The principle of lumbar TDR either way suggests a good clinical outcome independent from prosthesis choice, number of treated segments or prosthesis positioning.
We are aware, that there are some limitations of the study. Interpretation of the pictures was subjective, thus introducing a bias for variability. Furthermore, some parameters, e.g., mobility, were influenced by the patient’s collaboration. In 13/46 a hybrid procedure with an ALIF was achieved. In addition, adjacent segment degeneration (ASD) was not investigated specifically. However, in the radiological reviews severe ASD was rolled out. Even though most patients reached an excellent clinical outcome, persistent or new pain symptoms could be based on adjacent segment degeneration.
Conclusions
In our study, we showed that lumbar TDR is a good treatment option in selected patients with LBP due to lumbar disc disorders. TDR leads to a significantly better clinical outcome, increasing patient satisfaction and well-being, mobility, and working capacity.
Because of the strict criteria we applied when classifying prosthesis placement, many prostheses were rated as suboptimally placed. However, prosthesis positioning did not influence clinical outcome to any extent, except that ideally placed or discretely shifted prostheses caused less neurological symptoms than did poorly positioned prostheses after ≥2 years.
Further studies to identify radiological parameters other than prosthesis positioning that influence clinical outcome are needed.
References
Bertagnoli R, Kumar S (2002) Indications for full prosthetic disc arthroplasty: a correlation of clinical outcome against a variety ofindications. Eur Spine J 11(Suppl 2):S131–S136
Boss O, Bäurle B, Sgier F, Hausmann O (2011) Lumbar total disc replacement: correlation of clinical outcome and radiological parameters. Eur Spine J 20:2054
Brodsky AE (1976) Post-laminectomy and post-fusion stenosis of the lumbar spine. Clin Orthop Relat Res 130–139
Cassidy JD, Carroll LJ, Cote P (1998) The Saskatchewan health and back pain survey. The prevalence of low back pain and related disability in Saskatchewan adults. Spine (Phila Pa 1976) 23:1860–1866, discussion 1867
Cunningham BW, Dmitriev AE, Hu N, McAfee PC (2003) General principles of total disc replacement arthroplasty: seventeen cases in a nonhuman primate model. Spine (Phila Pa 1976) 28:S118–S124
Daltroy LH, Cats-Baril WL, Katz JN, Fossel AH, Liang MH (1996) The North American spine society lumbar spine outcome assessment instrument: reliability and validity tests. Spine (Phila Pa 1976) 21:741–749
Deyo RA, Tsui-Wu YJ (1987) Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine (Phila Pa 1976) 12:264–268
Gould D, Kelly D, Goldstone L, Gammon J (2001) Examining the validity of pressure ulcer risk assessment scales: developing and using illustrated patient simulations to collect the data. J Clin Nurs 10:697–706
Guyer RD, McAfee PC, Hochschuler SH, Blumenthal SL, Fedder IL, Ohnmeiss DD, Cunningham BW (2004) Prospective randomized study of the Charite artificial disc: data from two investigational centers. Spine J 4:252S–259S
Harris RI, Wiley JJ (1963) Acquired spondylolysis as a sequel to spine fusion. J Bone Joint Surg Am 45:1159–1170
Harrop JS, Youssef JA, Maltenfort M, Vorwald P, Jabbour P, Bono CM, Goldfarb N, Vaccaro AR, Hilibrand AS (2008) Lumbar adjacent segment degeneration and disease after arthrodesis and total disc arthroplasty. Spine (Phila Pa 1976) 33:1701–1707
Kotsenas AL (2012) Imaging of posterior element axial pain generators facet joints, pedicles, spinous processes, sacroiliac joints, and transitional segments. Radiol Clin N Am 50:705–730
Le Huec JC, Mathews H, Basso Y, Aunoble S, Hoste D, Bley B, Friesem T (2005) Clinical results of Maverick lumbar total disc replacement: two-year prospective follow-up. Orthop Clin N Am 36:315–322
Lee CK (1988) Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine (Phila Pa 1976) 13:375–377
Lemaire JP, Carrier H, el Sariali H, Skalli W, Lavaste F (2005) Clinical and radiological outcomes with the Charite artificial disc: a 10-year minimum follow-up. J Spinal Disord Tech 18:353–359
Link HD (2002) History, design and biomechanics of the LINK SB Charite artificial disc. Eur Spine J 11(Suppl 2):S98–S105
Mathews HH, Lehuec JC, Friesem T, Zdeblick T, Eisermann L (2004) Design rationale and biomechanics of Maverick Total Disc arthroplasty with early clinical results. Spine J 4:268S–275S
McAfee PC, Cunningham B, Holsapple G, Adams K, Blumenthal S, Guyer RD, Dmietriev A, Maxwell JH, Regan JJ, Isaza J (2005) A prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part II: evaluation of radiographic outcomes and correlation of surgical technique accuracy with clinical outcomes. Spine (Phila Pa 1976) 30:1576–1583, discussion E1388–1590
McAfee PC, Fedder IL, Saiedy S, Shucosky EM, Cunningham BW (2003) SB Charite disc replacement: report of 60 prospective randomized cases in a US center. J Spinal Disord Tech 16:424–433
Pose B, Sangha O, Peters A, Wildner M (1999) Validation of the North American Spine Society Instrument for assessment of health status in patients with chronic backache. Z Orthop Ihre Grenzgeb 137:437–441
Putzier M, Funk JF, Schneider SV, Gross C, Tohtz SW, Khodadadyan-Klostermann C, Perka C, Kandziora F (2006) Charite total disc replacement—clinical and radiographical results after an average follow-up of 17 years. Eur Spine J 15:183–195
Quirno M, Goldstein J, Bendo J, Yong K, Spivak J (2011) The incidence of potential candidates for total disc replacement among lumbar and cervical fusion patient populations. Asian Spine J 5(4):213–219
Sangha O, Wildner M, Peters A (2000) Evaluation of the North American Spine Society Instrument for assessment of health status in patients with chronic backache. Z Orthop Ihre Grenzgeb 138:447–451
Schluessmann E, Diel P, Aghayev E, Zweig T, Moulin P, Röder C (2009) SWISSspine: a nationwide registry for health technology assessment of lumbar disc prostheses. Eur Spine J 18:851–861
Schmidt R, Obertacke U, Nothwang J, Ulrich C, Nowicki J, Reichel H, Cakir B (2010) The impact of implantation technique on frontal and sagittal alignment in total lumbar disc replacement: a comparison of anterior versus oblique implantation. Eur Spine J 19:1534–1539
Siepe CJ, Mayer HM, Wiechert K, Korge A (2006) Clinical results of total lumbar disc replacement with ProDisc II: three-year results for different indications. Spine (Phila Pa 1976) 31:1923–1932
Tournier C, Aunoble S, Le Huec JC, Lemaire JP, Tropiano P, Lafage V, Skalli W (2007) Total disc arthroplasty: consequences for sagittal balance and lumbar spine movement. Eur Spine J 16:411–421
Yajun W, Yue Z, Xiuxin H, Cui C (2010) A meta-analysis of artificial total disc replacement versus fusion for lumbar degenerative disc disease. Eur Spine J 19:1250–1261
Zigler JE, Burd TA, Vialle EN, Sachs BL, Rashbaum RF, Ohnmeiss DD (2003) Lumbar spine arthroplasty: early results using the ProDisc II: a prospective randomized trial of arthroplasty versus fusion. J Spinal Disord Tech 16:352–361
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boss, O.L., Tomasi, S.O., Bäurle, B. et al. Lumbar total disc replacement: correlation of clinical outcome and radiological parameters. Acta Neurochir 155, 1923–1930 (2013). https://doi.org/10.1007/s00701-013-1774-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-013-1774-1