Abstract
A retrospective clinical–radiological study to evaluate the long-term outcome after artificial disc replacement was performed. The objective is to investigate long-term results after implantation of a modular type artificial disc prosthesis in patients with degenerative disc disease (DDD). Total disc replacement (TDR) is a surgical procedure intended to save segmental spinal function, and thus replace spondylodesis. Short-term results are promising, whereas long-term results are scarce. The Charité TDR is the oldest existing implant, therefore, the longest possible follow-up is presented here. Seventy-one patients were treated with 84 Charité TDRs types I–III. Indication for TDR was moderate to severe DDD. Fifty-three patients (63 TDRs) were available for long-term follow-up of 17 years. Evaluation included Oswestry disability index, visual analog scale, overall outcome score, plain and extension/flexion radiographs. Implantation of Charité TDR resulted in a 60% rate of spontaneous ankylosis after 17 years. No significant difference between the three types of prostheses was found concerning clinical outcome. Reoperation was necessary in 11% of patients. Although no adjacent segment degeneration was observed in the functional implants (17%), these patients were significantly less satisfied than those with spontaneous ankylosis. TDR, nowadays, is an approved procedure. Proof that long-term results of TDR implantation in DDD are at least as good as fusion results is still missing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years many attempts to replace spine fusion surgery through other, more functional procedures have been of clinical and experimental interest. One of them is the adaptation of endoprosthetic knowledge to the anterior intervertebral joint. Different types of artificial disc replacements have passed experimental status and are nowadays frequently used by spine surgeons instead of the previously performed spondylodesis [11, 13, 27, 42, 44, 47]. The Charité artificial disc was the first available total disc replacement (TDR) system [30]. Therefore, this artificial disc implant gives the spine surgeon’s community the opportunity to learn about the long-term results of TDR.
The purpose of this retrospective study is to analyze the clinical and radiological long-term results of the Charité TDR in the surgical treatment of patients with degenerative disc disease (DDD) of the lumbar spine. Due to the fact that the first TDRs ever implanted are part of this study, the presented data represent the longest currently possible follow-up for TDRs.
Patients and methods
Patients
Between 1984 and 1989 71 consecutive patients were treated surgically using 84 Charité total disc prostheses.
After an average follow-up of 17.3 years (14.5–19.2 years) 53 patients (follow-up rate 74.6%) were available for clinical and radiological examination. Patient population consisted of 20 males and 33 females with an average age of 44 years (30–59 years). Overall 63 TDRs (follow-up rate 75.0%) had been performed in the patients available for follow-up. Treated levels included L3/4 in 2 patients, L4/5 in 25 patients, L5/S1 in 16 patients, and L4–S1 in 10 patients. Fifteen patients operated between 1984 and 1985 had been treated with 16 type I, 22 patients operated between 1985 and 1987 had received 25 type II and 16 patients operated between 1987 and 1989 had received 22 type III Charité total disc prostheses (Table 1). In all 53 patients, indication for surgery was a mono- or bisegmental DDD of the lumbar spine with moderate to severe osteochondrosis of the treated segments. Eight patients had had previous disc surgery and three patients suffered from additional spondylolisthesis grade I according to Meyerding [35].
Implants
While type I and II Charité total disc prostheses were manufactured in the former German Democratic Republic (GDR) and never commercially available, type III is still in use, and in the meantime distributed by Johnson and Johnson.
Type I
Type I Charité total disc prostheses consisted of two highly polished metal endplates and an ultra high molecular weight polyethylene (UHMWPE) sliding core articulating between these endplates. The endplates of this type were 1 mm thick, had 5 respectively later 11 teeth for bony anchorage and were made of URX2CrNiMoN 18.12 steel. The sliding core was manufactured from Chirulen UHMWPE [30].
Type II
Type II Charité total disc prostheses also consisted of two highly polished metal endplates and an UHMWPE sliding core. The endplates were made of stainless steel. In contrast to type I, endplates were enlarged resulting in bilateral wings with three anterior and two posterior anchoring teeth [30].
Type III
Type III Charité total disc prostheses followed the same technical principle as types I and II. The endplates were changed to cast CoCrMo alloy with three anterior and three posterior anchoring teeth. During the study period only three endplate sizes either in parallel or 5° angulation were available [30]. A porous coating of the endplates consisting of titanium and calcium phosphate was introduced later on to promote osteointegration, but was not available at the time of surgery of the reported patients.
Surgical technique
All operations were performed by senior spine surgeons experienced with the anterior approach including the designers of the prosthesis. Under protection of the great abdominal vessels, the intervertebral disc space was exposed using a pararectal extraperitoneal approach. The anterior longitudinal ligament was incised and the degenerated disc tissue was removed after radiological verification of the correct level. The size for each part of the prosthesis was measured, and after preparation of the endplates the artificial disc replacement was implanted. The anterior longitudinal ligament was then thoroughly sutured and afterwards the wound closure was performed in layers.
Postoperative treatment
A 24-h postoperative observation of neurologic function and airway control in the intensive care unit was standard in the Charité hospital at the time of primary surgery. Intravenous antibiotics were regularly given intraoperatively and for 3 days after surgery. Oral diet was usually started on the third postoperative day. Trunk immobilization was not necessary. Sitting, bending, and heavy lifting were prohibited for 8 weeks.
Outcome measurement
Clinical parameters
Clinical examination was performed at follow-up. Evaluation included the German version of the Oswestry disability index (ODI) [19]. Additionally, the entire quantity of pain was evaluated according to Chapman [10]. Pain measurement was assessed with a 10-point visual analog scale (VAS) with endpoint anchors of “no pain” (0 points) and “severe pain” (10 points). The subjective perception of overall outcome with the procedure was graded by the patients at follow-up as excellent, good, fair, or poor according to Odom’s criteria [37]. The four criteria were graded 1 (excellent) to 4 (poor) to show a grade point average for each type of prosthesis.
Radiological parameters
Radiographic evaluation including plain X-rays (anterior–posterior and lateral) and flexion/extension views of the lumbar spine was performed. Operated levels were evaluated on X-rays with regard to functional, implant-related, and degenerative parameters. The amount of segmental mobility was determined on flexion/extension films in degrees and defined as the angle between the posterior wall of the upper and lower vertebra of the operated motion segments [23]. A segmental mobility of 3° or less was graded as fused. A segmental mobility of more than 3° was graded as mobile. Heterotopic ossification, respectively fusion at the operated level was assessed according to the classification of McAfee [33]. Implant failure was recorded and divided into fracture, dislocation, and subsidence.
Additionally, adjacent segments were evaluated to determine any progression of disc degeneration in comparison to preexisting X-rays. Therefore, disc space height was measured according to Pope [40], and dynamic translation was evaluated according to Boden [6].
Statistical analysis
Comparison of data was performed using Mann–Whitney U test and Wilcoxon rank sum test. Statistical significant differences were defined at a 95% confidence level. The values are given as mean ± standard deviation; SPSS (release 10.0 SPSS Inc. Chicago, IL, USA) software supported statistical evaluation.
Results
Clinical results
There was no statistical significant difference between the three types of Charité TDR regarding baseline demographic parameters such as patient age and gender, follow-up period, number of implanted TDRs, and operated levels (Table 1).
Clinical evaluation at follow-up showed no significant difference of the ODI and the VAS between the different types of Charité artificial discs (Table 2). Additionally, overall outcome of the patients in relation to the type of prosthesis showed no significant differences either (Table 3).
In summary, there was no significant difference between the three types of Charité TDR for all clinical parameters.
Radiological results
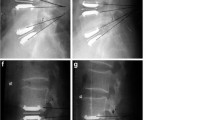
Out of the 53 patients available for long-term follow-up 12 (23%) had a segmental fusion during follow-up due to implant failure or pain (Table 4). Seven (13%) of these 12 were results of implant fractures (Fig. 1).
Five (9%) had to undergo secondary operative instrumented spondylodesis. The indications for secondary fusion operations were implant fracture (n=1), implant subsidence (n=2), implant dislocation (n=1), and persisting pain due to progressive degeneration (n=1) (Fig. 2).
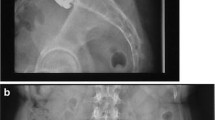
Out of the remaining 41 (77%), 9 patients (17%) showed no signs of heterotopic ossification or ankylosis (Fig. 3).
Two (4%) out of these nine patients had no radiological signs of segmental motion impairment representing McAfee types 0 and I (Table 5). Seven (13%) patients showed signs of possible or likely motion impairment graded as types II and III according to McAfee. The other 32 (60%) patients had definitive signs of ankylosis and were graded as type IV according to McAfee (Table 5). In these 32 cases no radiological distinguishable reason for the ossifications resulting in spontaneous ankylosis was found (Figs. 4, 5).
The evaluation of adjacent segments, as described above, showed nine (17%) cases with significant degenerative changes (Fig. 6).
Adjacent segment alterations were found only in cases where spontaneous ankylosis of the treated segments, spondylodesis, or fusion after implant failure occurred. In the nine cases without signs of ankylosis, spondylodesis, or implant failure (as mentioned above) no signs of adjacent segment degeneration were found. The different types of prostheses did not influence this parameter nor did the kind of fusion (spondylodesis vs ankylosis) as shown in Table 6.
Correlation of clinical parameters and radiological results
As Table 7 shows, the subjective clinical parameters (ODI, VAS, and Odom’s criteria) do not correlate with the functional status of the treated segments as evaluated radiologically.
Discussion
For more than 20 years TDRs have been implanted by spine surgeons. Short- and mid-term results after TDR of different study groups have shown promising results [3, 7, 11–13, 17, 20, 22, 32, 34, 45, 46, 48]. However, long-term results with a follow-up of up to 13 years published by van Ooij as well as a review of literature were less favorable [25, 39]. Therefore, the purpose of this retrospective study was to analyze the clinical and radiological long-term results of the Charité TDR in the surgical treatment of patients with DDD of the lumbar spine. Due to the fact, that the first TDRs ever implanted are part of this study, the presented data represent the longest currently possible follow-up of the Charité TDR.
In summary, this long-term follow-up study demonstrates dissatisfying results after artificial disc replacement in the majority of the evaluated cases concerning clinical as well as radiological outcome. The large percentage (83%) of radiologically confirmed fusions of the treated segments shows that the Charité artificial disc replacement cannot guarantee long-term near to normal function of the spinal motion segment in patients with moderate to severe DDD.
Multiple causes for the high rate of spontaneous fusions have to be taken into consideration. Whereas the anterior approach is carried out in the same way nowadays as during the time of these reported operations, the preservation of the anterior longitudinal ligament performed during that time, nowadays is known to be a trigger for unwanted ossifications [36].
The reparative processes, which are induced by the incision of the anterior longitudinal ligament, have not been examined in detail so far, but might also have contributed to the high rate of unintended segmental fusions [31].
Additionally, for a correct implantation of artificial disc prostheses a nearly complete removal of the remaining disc tissue is mandatory [5, 8, 12, 21]. This process implies the decortication of the vertebral endplates. Consecutively, osteoinductive substances are regularly released into the disc space due to the interruption of the integrity of the bone–cartilage border [1]. In operative spondylodesis, this effect is intended to enhance and promote the osseous consolidation; however, it is unintended in TDR. This undesired process might also have contributed to the high rate of spondylodesis. Finally, since all of the reported patients suffered preoperatively from moderate to severe DDD, a progression of these processes after surgery can be assumed. This may be another cause of ongoing ossification as a result of degenerative changes of segment-related tissue such as bone and ligaments.
This study included patients who were treated with three different types of the Charité artificial disc prostheses. The history of the development of this first TDR device was previously described in detail by Link [30]. The major changes from the first prototype to the currently commercially available Charité artificial disc replacement are summarized in the material section of this study. While the first implant was given up due to an insufficient bone implant contact area, the second was replaced because of a high rate of fractures in the wing area of the endplate. However, the third implant—the Charité III TDR—is still in use today with only minor changes. Although the basic principle based on the “low-friction” concept known from endoprosthetic joint replacements remained the same throughout the years, these three different TDR types demonstrated considerable differences regarding design, material combination, endplate geometry, endplate thickness, and size. However, interestingly, there was no significant difference in the clinical or radiographical long-term outcome between these three different TDR types. This is especially surprising, because the first two implant types were given up due to obvious design problems, which leads to the conclusion that even considerable design modifications of this TDR do not seem to have a significant input on the overall clinical and radiological long-term results.
All TDRs are promoted with the theoretical advantage to prevent an adjacent level degeneration. In fact, none of the few (n=9; 17%) functional TDRs evaluated in this study, showed significant adjacent level degeneration. However, the overall percentage of adjacent segment degeneration (17%) after implantation of a TDR was comparable to results of follow-up studies concerning adjacent segment degeneration after fusion surgery [2, 16, 18, 24, 26, 28, 41]. For example, the studies of Etebar and Kumar demonstrated 4 and 30 years after surgery a rate of adjacent segment degeneration of 14.4 and 14.2%, respectively. Due to the high rate of spontaneously ankylosed segments in this study, the high percentage of adjacent segment degenerations shown is not very astonishing.
Further, it is demonstrated in this study that the patients with preserved segmental mobility after an average follow-up of 17 years, were significantly less satisfied with the long-term outcome of the surgery than patients with spontaneous ankylosis or fused motion segments after implant failure. Due to the fact that in these cases no adjacent segment degeneration was evident, the clinical problem must be located in the operated motion segment itself. For the successful treatment of DDD with a TDR implant, anatomical reposition of the complete motion segment is as important as in fusion surgery [8]. However, even with an ideal implantation technique the Charité TDR can only achieve the repositioning of the anterior column, while the degenerative disorders of the posterior elements are not addressed. Furthermore, it can be assumed that the occurrence of anterior and posterior degenerative changes of the lumbar motion segments is closely related to each other [9, 38, 43]. It has been postulated by Dunlop that in cases of moderate to severe DDD facet joint arthritis always can be found as accompanying degenerative alteration of the posterior spine elements [15]. Studies have further demonstrated that after implantation of a TDR, loads in the facet joints might increase 2.5-fold in comparison to the normal loading conditions [14, 29]. Therefore, preexisting changes in the posterior column might persist or even progress after TDR implantation resulting in deterioration of patients’ low back pain (LBP) symptoms over the years. For example, an unintended slight hyperlordosis of the motion segment after TDR implantation might lead in the long run to LBP due to progressive facet joint degeneration, and leg pain due to narrowing of the intervertebral foramen with consecutive nerve root compression. This might explain why the spontaneously ankylosed motion segments did better at follow-up than the functional and mobile segments. To prevent these problems, an optimal implantation of the TDR is mandatory. Additionally, degenerative changes of the facet joints should be evaluated very carefully prior to the implantation of a disc prosthesis [3, 4].
Conclusion
In summary, implantation of Charité TDR resulted in a high rate (60%) of spontaneous ankylosis after an average follow-up of 17 years. There was no significant difference in the clinical outcome between the three types of prostheses. Although no adjacent segment degeneration was observed in the few functional implants (17%), these patients were significantly less satisfied with the long-term outcome of the surgery than the patients with spontaneously ankylosed motion segments or fusion after implant failure. Although the Charité TDR nowadays is an approved implant, the evidence that long-term results of TDR implantation in DDD are as good as or even better than fusion results is still missing.
References
Adams MA, Freeman BJC, Morrison HP, Nels IW, Dolan P (2000) Mechanical initiation of intervertebral disc degeneration. Spine 25(13):1625–1636
Aota Y, Kumano K, Hirabayashi S (1995) Postfusion instability at the adjacent segments after rigid pedicle screw fixation for degenerative lumbar spinal disorders. J Spinal Disord 8(6):464–473
Bertagnoli R, Kumar S (2002) Indications for full prosthetic disc arthroplasty: a correlation of clinical outcome against a variety of indications. Eur Spine J 11(Suppl 2):S131–S136
Blumenthal SL, Ohnmeiss DD, Guyer R, Hochschuler S, McAfee P, Garcia R, Salib R, Yuan H, Lee C, Bertagnoli R, Bryan V, Winter R (2002) Artificial intervertebral discs and beyond: a North American Spine Society Annual Meeting symposium. Spine J 2(6):460–463
Blumenthal SL, Ohnmeiss DD, Guyer RD, Hochschuler SH (2003) Prospective study evaluating total disc replacement: preliminary results. J Spinal Disord Tech 16(5):450–454
Boden SD, Wiesel SW (1990) Lumbosacral segmental motion in normal individuals. Have we been measuring instability properly? Spine 15(6):571–576
Buettner-Janz K, Schellnack K, Zippel H, Conrad P (1988) [Experience and results with the SB-Charité lumbar intervertebral endoprosthesis]. Z Klin Med 43:1785–1789
Buettner-Janz K, Hahn S, Schikora K, Link HD (2002) [Basic principles of successful implantation of the SB Charite model LINK intervertebral disk endoprosthesis]. Orthopäde 31(5):441–453
Butler D, Trafimow JH, Andersson GB, et al (1990) Discs degenerate before facets. Spine 15:111–113
Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE (1985) Pain measurement: an overview. Pain 22:1–31
Cinotti G, David T, Postacchini F (1996) Results of disc prosthesis after a minimum follow-up period of 2 years. Spine 21(8):995–1000
David T (1993) Lumbar disc prosthesis. Surgical technique, indications and clinical results in 22 patients with a minimum of 12 months follow-up. Eur Spine J 1:254–259
Delamarter RB, David M, Kanim A, Linda E, Bae H (2003) ProDisc artificial total lumbar disc replacement: introduction and early results from the United States clinical trial. Spine 28(Suppl 20):S167–S175
Dooris AP, Goel VK, Grosland NM, Gilbertson LG, Wilder DG (2001) Load-sharing between anterior and posterior elements in a lumbar motion segment implanted with an artificial disc. Spine 26(6):E122–E129
Dunlop R, Adams MA, Hutton WC (1984) Disc space narrowing and the lumbar facet joints. J Bone Joint Surg Br 66:706–710
Eck JC, Humphreys SC, Hodges SD (1999) Adjacent-segment degeneration after lumbar fusion: a review of clinical, biomechanical, and radiologic studies. Am J Orthop 28:336–340
Enker P, Steffee A, Mcmillin C, Keppler L, Biscup R, Miller S (1993) Artificial disc replacement. Preliminary report with a 3-year minimum follow-up. Spine 18(8):1061–1070
Etebar S, Cahill DW (1999) Risk factors for adjacent-segment failure following lumbar fication with rigid instrumentation for degenerative instability. J Neurosurg 90:163–169
Fairbank JCT, Couper J, Davies J, O’Brien J (1980) The oswestry low back pain disability questionnaire. Physiotherapy 8:271
Griffith SL, Shelokov AP, Buttner-Janz K, LeMaire JP, Zeegers WS (1994) A multicenter retrospective study of the clinical results of the LINK SB Charite intervertebral prosthesis. The initial European experience. Spine 19(16):1842–1849
Hochschuler SH, Ohnmeiss DD, Guyer RD, Blumenthal SL (2002) Artificial disc: preliminary results of a prospective study in the United States. Eur Spine J 11(Suppl 2):S106–S110
Hopf C, Heeckt H, Beske C (2002) [Disc replacement with the SB Charite endoposthesis—experience, preliminary results and comments after 35 prospectively performed operations]. Z Orthop Ihre Grenzgeb 140(5):485–491
Kandziora F, Pflugmacher R, Scholz M, Schnake K, Lucke M, Schröder R, Mittlmeier T (2001) Comparison between sheep and human cervical spines. An anatomic, radiographic bone mineral density, and biomechanical study. Spine 26(9):1028–1037
Kumar MN, Jacquot F, Hall H (2001) Long-term follow-up of functional outcomes and radiographic changes at adjacent levels following lumbar spine fusion for degenerative disc disease. Eur Spine J 10:309–313
de Kleuver M, Oner FC, Jacobs WC (2003) Total disc replacement for chronic low back pain: background and a systematic review of the literature. Eur Spine J 12(2):108–116
Lee CK (1988) Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine 13:375–377
Lee CK, Parsons JR, Langrana LA, Zimmermann MC (1992) Relative efficacy of the artificial disc versus spinal fusion. In: Weinstein JN (ed) Clinical efficacy and outcome in the diagnosis and treatment of low back pain. Raven Press, New York, pp 237–244
Lehmann TR, Spratt KF, Tozzi JE, et al (1987) Long-term follow-up of lower lumbar fusion patients. Spine 12:97–104
Lemaire JP, Skalli W, Lavaste F, Templier A, Mendes F, Diop A, Sauty V, Laloux E (1997) Intervertebral disc prosthesis. Results and prospects for the year 2000. Clin Orthop 337:64–76 (Review)
Link HD (2002) History, design and biomechanics of the LINK SB Charité artificial disc. Eur Spine J 11(Suppl 2):S98–S105
Manzini CU, Spina V, Mascia MT, Magistro R, Carpenito G, Ferri C (2004) [Diffuse post-traumatic calcification of the anterior longitudinal ligamentum of cervical and dorsal spine]. Reumatismo 56(2):114–117
Mayer HM, Wiechert K, Korge A, Qose I (2002) Minimally invasive total disc replacement: surgical technique and preliminary clinical results. Eur Spine J 11(Suppl 2):S124–S130
McAfee PC, Cunningham BW, Devine J, Williams E, Yu-Yahiro J (2003) Classification of heterotopic ossification (HO) in artificial disk replacement. J Spinal Disord Tech 16(4):384–389
McAfee PC, Fedder IL, Saiedy S, Shucosky EM, Cunningham BW (2003) SB Charite disc replacement: report of 60 prospective randomized cases in a US center. J Spinal Disord Tech 16(4):424–433
Meyerding HW (1956) Spondylolisthesis; surgical fusion of lumbosacral portion of spinal column and interarticular facets; use of autogenous bone grafts for relief of disabling backache. J Int Coll Surg 26:566–591
Miyamoto S, Kuratsu S, Yonenobu K, Ono K (1992) Evaluation of cell proliferating potentials in ossification of the spinal ligaments by argyrophilic nucleolar organizer region (AgNOR) staining. In: Kurokawa T (ed) Annual report of the investigation committee for ossification of the spinal ligaments under the auspices of Japanese ministry of health and welfare. Department of Orthopaedics, University of Tokyo, Japan, pp 160–165
Odom GL, Finney W, Woodhall B (1958) Cervical disc lesions. JAMA 166:23–28
Oegema TR Jr, Bradford DS (1991) The interrelationship of facet joint osteoarthritis and degenerative disc disease. Br J Rheumatol 30:16–20
van Ooij A, Oner FC, Verbout AJ (2003) Complications of artificial disc replacement a report of 27 patients with the SB Charite disc. J Spinal Disord Tech 16(4):369–383
Pope MH, Wilder DG, Matteri RE, Frymoyer JW (1977) Experimental measurements of vertebral motion under load. Orthop Clin North Am 8(1):155–167
Rahm MD, Hall MD (1996) Adjacent-segment degeneration after lumbar fusion with instrumentation: A retrospective study. J Spinal Disord 9(5):392–400
Ray CD (1992) The artificial disc. In: Weinstein JN (ed) Clinical efficacy and outcome in the diagnosis of low back pain. Raven Press, New York, pp271–278
Schwarzer AC, Aprill CN, Derby R, et al. (1994) The relative contributions of the disc and zygapophyseal joint in chronic low back pain. Spine 19:801–806
Steffee AD (1992) The Steffee artificial disc. In: Weinstein JN (ed) Clinical efficacy and outcome in the diagnosis of low back pain. Raven Press, New York, pp 245–257
Tropiano P, Huang RC, Girardi FP, Marnay T (2003) Lumbar disc replacement: preliminary results with ProDisc II after a minimum follow-up period of 1 year. J Spinal Disord Tech 16(4):362–368
Zeegers WS, Bohnen LM, Laaper M, Verhaegen MJ (1999) Artificial disc replacement with the modular type SB Charite III: 2-year results in 50 prospectively studied patients. Eur Spine J 8(3):210–217
Zigler JE, Burd TA, Vialle EN, Sachs BL, Rashbaum RF, Ohnmeiss DD (2003) Lumbar spine arthroplasty: early results using the ProDisc II: a prospective randomized trial of arthroplasty versus fusion. J Spinal Disord Tech 16(4):352–361
Zippel H (1991) “Charité modular”: conception, experience and results. In: Weinstein JN, Mayer HM, Weigel K (eds) The artificial disc. Springer, Berlin Heidelberg New York, pp 69–78
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Putzier, M., Funk, J.F., Schneider, S.V. et al. Charité total disc replacement—clinical and radiographical results after an average follow-up of 17 years. Eur Spine J 15, 183–195 (2006). https://doi.org/10.1007/s00586-005-1022-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-005-1022-3