Abstract
Background
There is as yet little knowledge as to the arachnoid architecture within the velum interpositum. The aim of this study was to clarify the distribution of the arachnoid membrane within the velum interpositum and its relationship with the arachnoid envelope over the pineal region.
Methods
In seven adult cadaver heads, histological sections of the third ventricle roof, stained with Masson’s trichrome stains, were studied under light microscopy.
Results
Within the velum interpositum, there are two arachnoid layers. The dorsal layer of arachnoid membrane envelops the internal cerebral veins and fixes them to the surrounding tela choroidea as well as the ventral arachnoid layer. The ventral layer of arachnoid membrane is a direct anterior extension of the arachnoid envelope over the pineal region and covers the midline inferior layer of tela choroidea. Both arachnoid layers end near the foramen of Monro.
Conclusions
The membranous roof of the third ventricle comprises two layers of the tela choroidea and two arachnoid layers. These two arachnoid layers are derived from the arachnoid envelope over the pineal region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditional teaching described that the third ventricle roof extends from the foramen of Monro anteriorly to the suprapineal recess posteriorly. It has four layers: one neural layer formed by the fornix, two thin membranous layers of tela choroidea demarcated a potential space called the velum interpositum (VI) and a layer of blood vessels within the VI [7]. In our previous anatomical study, we have found that the arachnoid envelope over the pineal region (AEPR) not only surrounds the vein of Galen and its tributaries but also the suprapineal recess and pineal gland [9]. The anterior end of the AEPR seems attaching directly onto the dorsal surface of the suprapineal recess under the junction of crus and body of fornix superiorly, and on the lateral surfaces of the suprapineal recess laterally. The internal cerebral vein (ICV) coursing within VI had its own secondary arachnoid envelope arising from the AEPR. However, the length of the secondary arachnoid envelope surrounding the ICV could not be determined solely by anatomical study because it is too thin.
The importance of pia-arachnoid architecture of the membranous third ventricle roof lies with the fact that the arachnoid cap cell within VI is presumably the origin from which the third ventricle meningiomas arise, and some operative approaches to the third ventricle are directed through the VI with the risk of vascular injury. Therefore, a detailed study seemed warranted in order to obtain an in-depth understanding of the membranous third ventricle roof.
Material and methods
Of seven adult cadaveric formalin-fixed heads used for histological study, the cranial vault covering the cerebrum and the suboccipital surface of cerebellum was removed. Then a large sample including the pineal region and third ventricle roof was removed from each head en bloc. Serial histological sections were obtained in sagittal (four specimens) and coronal (three specimens) directions at 5-μm intervals. All these sections were stained with Masson’s trichrome stains. Observation was made under the Olympus DP71 light microscope (Olympus, Tokyo, Japan). The Olympus SZ61 stereomicroscope (Olympus, Tokyo, Japan), with a Canon EOS 600D digital camera (Canon, Tokyo, Japan) attached was used for photographic documentation of relevant structures.
Results
The membranous third ventricle roof extends from the foramen of Monro anteriorly to the suprapineal recess posteriorly. The superior layer of the tela choroidea covers the lower surface of the fornix and the hippocampal commissure, while the inferior layer of the tela choroidea covers the third ventricle, thalamus and the pineal gland. The VI is the potential space between the superior and inferior layers of tela choroidea that contains the internal cerebral veins and medial posterior choroidal arteries.
The arachnoid membrane within the VI is derived from the AEPR. Therefore, the observations was made first at the anterior part of the AEPR (aAEPR). The results obtained with the histological methods coincide with our previous anatomical observations in the sense that the aAEPR encloses both the pineal gland and the suprapineal recess (Fig. 1a), and the terminal segment of internal cerebral vein may course within the aAEPR (Fig. 1b) [9]. However, in this study, it was found that the aAEPR is further subdivided into two compartments by an arachnoid ridge arising from the inner surface of the aAEPR at the junction between the suprapineal recess and the pineal gland (Fig. 2a-c). At the level of the pineal stalk, the inferior compartment of the aAEPR ends and the superior compartment continues to extend anteriorly (Fig. 2d-i).
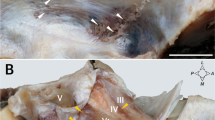
Sagittal (a) and coronal (b) histological sections through the pineal gland (Masson trichrome stain with original magnification ×2). a Anterior part of the arachnoid envelope over pineal region (aAEPR, black arrows) and its contents. b The terminal segment of the ICV on the left side, coursing within the aAEPR (black arrows); note that there is no arachnoid separation (black arrowheads) between the left ICV and the pineal gland. ICV internal cerebral vein, PG pineal gland, SPR suprapineal recess
Serial coronal histological sections from pineal gland posteriorly to the foramen of Monro anteriorly (Masson trichrome stain with original magnification ×2 in a, b, c and e, ×1.5 in d, ×2.5 in f, ×4.5 in g and i, and ×3.5 in h). a Through the posterior part of pineal gland; (b) through the middle part of pineal gland; (c) through the anterior part of pineal gland; (d) through the level of upper orifice of cerebral aqueduct; (e) through the level just anterior to the upper orifice of cerebral aqueduct; (f) through the posterior middle part of third ventricle roof; (g) local magnification of f; (h) through the anterior middle part of third ventricle roof; (i) through the anterior part of third ventricle roof just posterior to the foramen of Monro. Note that the aAEPR (black arrows) is subdivided into two compartments by an arachnoid ridge (black arrowheads) and the inferior compartment containing the pineal gland ends at the base of pineal stalk. The arachnoid membrane demarcating the superior compartment (black arrows) continues to extend anteriorly within the velum interpositum (VI) and terminates at the level of foramen of Monro. Above the suprapineal recess, a layer of arachnoid trabeculae (green arrows) arises from the dorsal surface of the aAEPR and interconnected the bilateral arachnoid envelopes surrounding internal cerebral vein. Within the VI, various strands of arachnoid trabeculae fix the vascular structures on the surrounding tela choroidea and the ventral arachnoid layer. CP choroids plexus, F fornix, ICV internal cerebral vein, MPChA medial posterior choroid artery, PG pineal gland, PR pineal recess, SPR suprapineal recess, Tha thalamus, TV third ventricle
Above the suprapineal recess, the superior part of aAEPR further separates into two layers (Fig. 2b, c). The superior layer, derived from the inner surface (inner reticular layer) of the arachnoid membrane, interconnects the two secondary arachnoid envelopes surrounding the ICVs, which together make up the central part of the dorsal arachnoid membrane within the VI (Fig. 2c-i). The inferior layer alone forms the ventral arachnoid membrane within the VI and extends anteriorly along with and above the midline inferior layer of tela choroidea (Fig. 2d-i). In the entire length from the habenular commissure to the foramen of Monro, the ventral arachnoid membrane attaches laterally to the lateral borders of the paired strands of choroids plexus projecting downward into the third ventricle (Fig. 2d-i). In serial sections, the ventral arachnoid membrane is complete without interruption, due to its outer arachnoid membrane origin. The dorsal arachnoid membrane has two components: the central part, as mentioned above, and the peripheral part, which comprises various strands of arachnoid trabeculae stretching among the ventral arachnoid membrane, the central part of dorsal arachnoid membrane and the surrounding tela choroidea (Fig. 2c-i). The dorsal arachnoid membrane is mainly inner arachnoid membrane origin. The arachnoid envelopes surrounding the bilateral ICVs are progressively thinner as it extents anteriorly. So does the density of arachnoid trabeculae within the VI.
Discussion
The VI is a potential space demarcated by the tela choroidea, a double-layered fold of pia mater intervening between the fornix above, the roof of the third ventricle and thalamus below, and the choroid plexuses of the lateral ventricles laterally. As described by Lozier and Bruce [3], the posterior displacement of the hippocampus carries a layer of pia-arachnoid on the inferior surface of the fornix and ultimately overlies and fuses to the original single layer of pia-arachnoid roofing the third ventricle to form the VI. The separation between superior and inferior layer of tela choroidea may allow for the formation of the cistern of VI. However, the distribution of arachnoid membrane within the VI received only passing mention in most anatomical and surgical discussions of the subject [2, 4–7, 10–12]. Rhoton [6] described that the broad envelope of arachnoid tissue surrounding the vein of Galen and its tributaries is applied to the lower surface of splenium and is continuous anteriorly with the VI. Within the VI, the two layers of tela choroidea are interconnected by loosely organised trabeculae. Vinas et al. [11] mentioned that within the VI, groups of dense trabeculaes wrap the medial posterior choroidal arteries and the internal cerebral veins. According to the statement of Yasargil [12], the VI lies between the pulvinar thalami, the arachnoid margins blending with the tela choroidea.
In the current study, we used histological methods to clarify the distribution of arachnoid membrane within the VI. Interestingly, we found that the superior anterior end of the AEPR extends far anteriorly than we previously expected and the concept of arachnoid architecture within the VI should be redefined. Between the well-known superior and inferior layers of tela choroidea, there are two layers of arachnoid membrane (Fig. 3). The dorsal arachnoid membrane has two parts: the central part comprises arachnoid envelopes surrounding the ICVs, interconnected by a layer of arachnoid trabeculae; the peripheral part comprises loosely organised arachnoid trabeculae stretched between the vascular structures within the VI and the surrounding membranous layers. The ventral arachnoid membrane is a direct anterior extension of the aAEPR over the midline interior layer of tela choroidea and attaches to the lateral borders of the paired strands of choroids plexus of third ventricle.
Schematic drawings showing the origin and distribution of the arachnoid membrane within the VI. a Coronal section through the anterior part of third ventricle roof near the foramen of Monro; (b) coronal section through the middle part of third ventricle roof; (c) coronal section through the pineal gland; (d) sagittal section through the midline. CC corpus callosum, CP choroids plexus, F fornix,; ICV internal cerebral vein, PG pineal gland, SPR suprapineal recess, Tha thalamus, TV third ventricle, VG vein of Galen
Meningiomas of the ventricular system are rare, accounting for less than 1% of all intracranial meningiomas [3, 8]. Meningiomas originate from the arachnoid cap cells that line the outer surface (outer barrier layer) of arachnoid membrane. Arachnoid cap cells are not only confined to the outer barrier layer of arachnoid mater. They are also found in the stroma of the normal choroid plexus, which may be explained by the invagination of leptomeninges that forms part of the tela choroidea [1, 3]. As described in our previous study, the inner surface of the AEPR is continuous with the outer layer of arachnoid membrane, which converges at the tentorial apex [9]. The AEPR and its anterior extension within the VI provide the source of the arachnoid cap cells from which third ventricle meningiomas arise.
In various surgical approaches adopted in dealing with third ventricle or pineal region tumours, the membranous third ventricle roof has to be opened or dissected from the tumour. Protection of the vascular structures within the membranous third ventricle roof, especially the ICV, is one of the main concerns. Based on our previous and current study, we found that there are two most dangerous sites along the ICV course from the foramen of Monro to the origin of the vein of Galen. One is at or near the site of junction between the ICV and the vein of Galen, if the terminal segment of ICV enters the aAEPR before the paired ICVs join to form the vein of Galen (type I configuration classified in our previous anatomical study [9]). In this situation, there is no intervening arachnoid layer between the pineal gland and the ICV (Fig. 1b). Tumours arising from the pineal parenchyma are more prone to adhere with the ICV, adding the risk of venous injury during dissection. The other site is near the level of the foramen of Monro, where the secondary arachnoid envelope surrounding the ICV almost disappeared (Fig. 2i). When dissection is performed in these two areas, extra caution should be paid to avoid venous injury.
Parts of the arachnoid layers within the VI have to be opened in certain surgical exposure. For example, the arachnoid trabeculae interconnecting the arachnoid envelopes of ICVs (central part of the dorsal arachnoid layer) must be opened in the interforniceal approach. Both the central and peripheral parts of the dorsal arachnoid layer are opened in a transchoroidal or subchoroidal approach if an inter-ICV route was used. If the tumour has already displaced the ICVs laterally, only the peripheral part of the dorsal arachnoid layer needs to be opened to reduce tension on the vascular structures within the VI. In all these approaches, when the inferior layer of tela choroidea has to be opened to gain access into the third ventricle, the ventral arachnoid layer would be opened simultaneously with the inferior layer of tela choroidea.
Conclusion
To our knowledge, the detailed arachnoid distribution within the membranous third ventricle roof, as described herein, has not been reported in previous studies. In comparison with previous descriptions that only arachnoid trabeculaes are present within the VI, we found that there are two arachnoid layers lying between the superior and inferior tela choroidea in the entire length from the habenular commissure to the foramen of Monro. The ventral layer is an intact outer arachnoid membrane and derived form arachnoid envelope over the pineal region. The dorsal layer comprises secondary arachnoid envelopes surrounding the ICVs and arachnoid trabeculaes derived from the ventral layer.
References
Cockerham KP, Kennerdell JS, Maroon JC, Bejjani GK (2004) Tumors of the meninges and related tissue: meningiomas and sarcomas. In: Miller NR, Newman NJ, Biousse V, Kerrison JB (eds) Walsh and Hoyt’s Clinical neuro-opthalamolgy, 6th edn, vol 2. Lippincott Williams & Wilkins, Philadelphia, pp 1483–1529
Inoue K, Seker A, Osawa S, Alencastro LF, Matsushima T, Rhoton AL Jr (2009) Microsurgical and endoscopic anatomy of the supratentorial arachnoidal membranes and cisterns. Neurosurgery 65:644–665
Lozier AP, Bruce JN (2003) Meningiomas of the velum interpositum: surgical considerations. Neurosurg Focus 15:E11
Lü J, Zhu XL (2007) Cranial arachnoid membranes: some aspects of microsurgical anatomy. Clin Anat 20:502–511
Nagata S, Rhoton AL Jr, Barry M (1988) Microsurgical anatomy of the choroid fissure. Surg Neurol 30(1):3–59
Rhoton AL Jr (2000) The posterior fossa cisterns. Neurosurgery 47(3 Suppl):S287–S297
Rhoton AL Jr (2002) The lateral and third ventricle. Neurosurgery 51(4 Suppl):S207–S271
Rozario R, Adelman L, Prager RJ, Stein BM (1979) Meningiomas of the pineal region and third ventricle. Neurosurgery 5:489–495
Songtao Q, Xi-an Z, Jun F, Guanglong Huang, Jun P, Binghui Q (2011) Anatomical study of the arachnoid envelope over the pineal region. Neurosurgery 68(1 Suppl Operative):7–15
Tubbs RS, Louis RG Jr, Wartmann CT, Loukas M, Shoja MM, Apaydin N, Oakes WJ (2008) The velum interpositum revisited and redefined. Surg Radiol Anat 30:131–135
Vinas FC, Dujovny M, Fandino R, Chavez V (1996) Microsurgical anatomy of the arachnoidal trabecular membranes and cisterns at the level of the tentorium. Neurol Res 18:305–312
Yaşargil MG (1984) Microneurosurgery, vol 1. Thieme, Stuttgart
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
Many intracranial surgical procedures can be accomplished without injuries to the brain by working in the subarachnoid space. This is the principle of microneurosurgery that is well known to all neurosurgeons. The understanding of the microanatomy of the arachnoid membranes and the subarachnoid cisterns, therefore, remains a fundamental issue of microneurosurgery. Nevertheless, anatomical studies usually provide descriptions of the subarachnoid cisterns, mostly emphasising the anatomy of the cerebral blood vessels and the cranial nerves within the cisterns, whereas the arachnoid membranes have not yet been detailedextensively. In this article the authors provide an elegant microscopic anatomy study describing the distribution of the arachnoid membrane of the roof of the third ventricle. In surgical approaches to the third ventricle or pineal regions, the roof of third ventricle has to be opened or dissected in specific situations. Authors emphasise the role of the arachnoid membrane as a fundamental plane for safe dissection of the vascular structures lying within the membranous third ventricle roof, especially the internal cerebral veins. Information provided by this study contributes to our understanding of the microscopic anatomy of the velum interpositum and roof of the third ventricle.
Alfredo Conti,
Messina, Italy
Rights and permissions
About this article
Cite this article
Zhang, Xa., Qi, S., Fan, J. et al. The distribution of arachnoid membrane within the velum interpositum. Acta Neurochir 154, 1711–1715 (2012). https://doi.org/10.1007/s00701-012-1436-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-012-1436-8