Abstract
Purpose
The cerebellopontine angle (CPA) is a frequent region of skull base pathologies and therefore a target for neurosurgical operations. The outer arachnoid is the key structure to approach the here located lesions. The goal of our study was to describe the microsurgical anatomy of the outer arachnoid of the CPA and its pathoanatomy in case of space-occupying lesions.
Methods
Our examinations were performed on 35 fresh human cadaveric specimens. Macroscopic dissections and microsurgical and endoscopic examinations were performed. Retrospective analysis of the video documentations of 35 CPA operations was performed to describe the pathoanatomical behavior of the outer arachnoid.
Results
The outer arachnoid cover is loosely attached to the inner surface of the dura of the CPA. At the petrosal surface of the cerebellum the pia mater is strongly adhered to the outer arachnoid. At the level of the dural penetration of the cranial nerves, the outer arachnoid forms sheath-like structures around the nerves. In the midline, the outer arachnoid became detached from the pial surface and forms the base of the posterior fossa cisterns. In pathological cases, the outer arachnoid became displaced. The way of displacement depends on the origin of the lesion. The most characteristic patterns of changes of the outer arachnoid were described in case of meningiomas, vestibular schwannomas, and epidermoid cysts of the CPA.
Conclusion
The knowledge of the anatomy of the outer arachnoid of the cerebellopontine region is essential to safely perform microsurgical approaches as well as of dissections during resection of pathological lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cerebellopontine angle (CPA) is a frequent region for lesions which require neurosurgical treatment. The development of microsurgical techniques opened a new area for the treatment of these lesions. Despite of the crowded anatomy of the posterior fossa, deep seated and complex pathologies could be treated in this way with favorable results. The introduction of microsurgery led to the development of different surgical approaches to the CPA such as the retro-, presigmoid, or transpetrosal approaches [39, 40, 42, 44]. Minimally invasive techniques, the use of endoscopes, and the application of keyhole concept reduced the surgery-related traumatization of these sometimes complex and time consuming skull base approaches and further improved the operative results [6, 18, 45]. The development of modern stereotactic radiosurgical methods changed the surgical decision strategies and radicality of resections in case of complex neoplastic lesions of the posterior fossa [8, 13, 43]. The difficulty in the treatment of CPA pathologies lies on the complex anatomical relations of the region. Therefore, it has to be always considered that the surgery-related risks cannot exceed the potential biological risks of these most often benign lesions such as meningiomas or vestibular schwannomas. The knowledge of the surgical anatomy is still the most crucial factor of an effective and safe operation despite of the new technical advances. However, the surgical anatomy of the cerebellopontine angle is well described, and morphological studies are still continuously published [2, 4, 5, 28, 35]. The ongoing development of technical tools requires continuously applied and detailed knowledge of the anatomical relations.
The significance of the anatomy of the subarachnoid cisterns and the surrounding arachnoid membranes was first popularized by Yasargil [50]. His statement “The keypoint of microsurgery is the arachnoidal approach and dissection” on the first pages of his reference work “Microneurosurgery” describes well the important role of these membranous structures [50]. Nearly 30 years later, the detailed knowledge of the anatomy of the arachnoid relations continues to stay essential to safely and effectively perform microsurgical operations on the base of the skull. Historically, the first description of these arachnoid membranes was published by Key and Retzius [3]. Later Liliequist et al. [22] were among the first authors who introduced clinical relevance of these structures. In the nineties and early 2000s, several publications dealt with the very detailed study of the individual arachnoid membranes separating the basal cisterns [12, 24-27, 29, 34, 46-49]. Nowadays, the anatomy of these highly organized and interrelated construct of fine sheets and trabeculae located between the outer arachnoid cover and the pia mater on the base of the skull was stated to be fully described [23]. Despite of the high number of publications dealing with the anatomy of the inner arachnoid membranes, the description of the outer arachnoid cover was always marginal in these studies. After opening the dura, the outer arachnoid plays, however, the most important role to atraumatically access the deep regions of the skull base along the subdural spaces. It serves as direct entrance to the basal cisterns in case of most approaches to the supratentorial space or posterior fossa. To our knowledge, there is no dedicated study dealing with the detailed topographic and surgical anatomy of the outer arachnoid cover on the base of the skull, especially in the posterior fossa.
The goal of our examinations was to study the most relevant topographic and surgical anatomical relations of the outer arachnoid in the complex region of the cerebellopontine angle. Microsurgical and endoscopic approaches require detailed knowledge of this structure to safely and purposefully get access into the targeted cisterns of the CPA. We aimed to describe important anatomical landmarks of the outer arachnoid cover which relieve the orientation during surgical approach of the CPA cisterns. During resection of neoplastic lesions of the CPA such as petroclival meningiomas or vestibular schwannomas, the outer arachnoid is the key structure to protect the sensitive cranial nerves and vessels of the CPA. Consequently, it defines the neurological outcome of the patient after surgery. Beside the description of the undisturbed microsurgical anatomy of the outer arachnoid cover of the CPA, we aimed to specify the pathoanatomical characteristic of the outer arachnoid cover in case of the most frequent space-occupying lesions: CPA meningiomas, vestibular schwannomas, and epidermoid cysts.
Methods
Cadaveric observations
Our dissections were performed on a total of 35 fresh human cadaveric specimens (20 male, 15 female) postmortem not more than 72 h. The cadavers were donated for research and medical education purpose to the Department of Anatomy, Histology and Embryology, Semmelweis University, Budapest. The examined specimens were between the ages of 51 and 87 years and without significant intracranial pathology. The intracranial arterial system of the specimens was injected with red gelation solution. The brains of 7/35 cadavers were removed completely with intact outer arachnoid for macroscopic examinations. In 28/35 cadavers, microscopic and endoscopic dissections were performed through the retrosigmoid craniotomy to describe the relations of the outer arachnoid cover to the dural and bony structures of the cerebellopontine angle as well as the here located neurovascular structures.
The microsurgical cadaveric examinations were performed under an operating microscope (OPMI I, Carl Zeiss, Oberkochen, Germany). Zero- and 30-degree rigid, rod-lens endoscopes (B. Braun/Aesculap AG, Tuttlingen, Germany) with a diameter of 4 mm were used for endoscopic examinations in addition to the operating microscope.
Intraoperative observations
Selected intraoperative video recordings of the last 35 operated clinical cases of cerebellopontine angle lesions matching our diagnostic criteria (Table 1) were retrospectively analyzed to verify the cadaveric anatomical findings and describe the pathoanatomical relations of the outer arachnoid cover. All of the selected cases in this study were operated by the first author (P.K.) between July 2020 and September 2022 through the retrosigmoid approach using endoscope-assisted microsurgical technique.
Results
Topographic anatomy
The outer arachnoid is loosely attached to the inner surface of the posterior fossa dura and follows its geometry rather than the surface anatomy of the here located neurovascular structures. On the petrosal surface of the cerebellum, the outer arachnoid is strongly attached to the pia mater (Fig. 1A, B). Medial from the level of the neuroforamina, around the midline, the outer arachnoid became detached from the pia mater of the cerebellum and stays adhered to the dura of the petrous apex, clivus, and dorsum sellae as well as around the tentorium. In this way, it forms the base of the superior cerebellar, cerebellopontine, prepontine, and premedullary cisterns. The continuity of the outer arachnoid cover is interrupted only by the penetrating structures of the skull base: the cranial nerves and the superior petrosal vein. The in situ examinations clearly showed that the cranial nerves do not perforate the outer arachnoid, but they are followed distally by arachnoid sheaths from the level of the neuroforamina within the bony canals of the skull base formed by the outer arachnoid base of the basal cisterns. In the examined region, we could observe these arachnoid sheaths at the dural exit of the oculomotor and trochlear nerve into the cavernous sinus, at the entrance of the trigeminal nerve to the Meckel’s cave, at the exit of the abducent nerve to the Dorello’s canal, and within the internal auditory meatus along the vestibulocochlear and facial nerves, as well as at the jugular foramen around the caudal cranial nerves. In contrast to the cranial nerves, the superior petrosal vein complex was the only structure which truly penetrated the outer arachnoid in the examined region.
Topographic overview of the outer arachnoid cover of the cerebellopontine angle in fresh cadaveric specimens on the right side. A In situ depiction of the outer arachnoid membranes. The cerebrum and the tentorium were carefully removed to allow an axial view of the outer arachnoid cover at the upper portion of the cerebellopontine angle from above. A dissector was placed in the subdural space and the cerebellum gently retracted to better visualize the topographic relations. The arrowhead indicates the outer arachnoid cover of the cerebellum attached strongly to the pia mater. The double arrowhead points to the detached outer arachnoid cover at the level of the cerebellopontine fissure. This portion forms the lateral wall of the cerebellopontine cistern. The black arrows indicate the outer arachnoid cover around the dural exit of the cranial nerves and their continuity within the neuroforamina. B Right side of a removed brain with intact outer arachnoid membranes around the tentorium photographed from right anterolateral direction slightly below the level of the removed tentorium. The tentorial surface of the cerebellum is covered by the outer arachnoid which is strongly adhered to the pia mater. At the level of cerebellomesencephalic fissure, the outer arachnoid is detached from the pial surface of the cerebellum (black arrowhead) and forms the cranial border of the superior cerebellar cistern. The superior cerebellar artery is often attached to the inner surface of the outer arachnoid cover (black arrow). Around the tentorial edge, the outer arachnoid has a folding and forms the base of the lateral mesencephalic and ambient cisterns. Along this folding, the trochlear nerve is embedded into the outer arachnoid membrane (black double arrowhead). List of abbreviations: BA, basilar artery; cereb., cerebellum; MF, middle fossa; nIII, oculomotor nerve; nIV, trochlear nerve; nV, trigeminal nerve; SCA, superior cerebellar artery; SPV, superior petrosal vein; OL, occipital lobe
Microsurgical anatomy
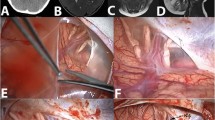
The retrosigmoid craniotomy offers the most extensive approach to the cerebellopontine angle and is considered to be the workhorse approach of the cadaveric examinations and intraoperative video analysis for the description of the microsurgical and endoscopic anatomy of the here located outer arachnoid membranes. Following the release of cerebellospinal fluid (CSF) from the cerebellomedullary cistern, the cerebellum became relaxed. Due to the strong adhesion of the outer arachnoid to the petrosal surface of the cerebellum, the subdural space became wide opened along the petrous dura until the level of the neuroforamina. Microsurgical dissection of the outer arachnoid on the petrosal surface of the cerebellum was possible only along the petrosal fissure of the cerebellum (Fig. 2A). In case of relaxed cerebellum, the outer arachnoid became detached from the petrosal surface of the cerebellum at the level of the cerebellopontine and cerebellomedullary fissures and extended to the junction of the superior petrosal vein and the superior petrosal sinus (Fig. 2B), to the internal auditory meatus as well as the jugular foramen (Fig. 2C). This outer arachnoid wall serves as the lateral wall of the cerebellopontine and premedullary cisterns. At this level, several microvessels could be observed embedded into the outer arachnoid wall (Fig. 2D). Some of these vessels arising from the surface of the vestibulocochlear and the caudal cranial nerves attached to the cerebellar vessels or perforate the dura around the neuroforamina. Forced retraction of the cerebellum prior to the microsurgical dissection of this portion of the outer arachnoid could lead to rupture of these small vessels or injury of the intracisternal portion of the related cranial nerves. The content of the cerebellopontine and premedullary cisterns are only partially visible through the semitransparent outer arachnoid cover. The tentorium, the suprameatal, and jugular tubercle were the primary landmarks to orientate along the rostro-caudal axis. The dural attachment of the superior petrosal vein complex as well as the vestibulocochlear and caudal cranial nerves is shining through the outer arachnoid. Dissection of the outer arachnoid between the superior petrosal vein and the vestibulocochlear nerve led to the cerebellopontine cistern and provided access to the trigeminal nerve (Fig. 2E). After opening the lateral wall of the cerebellopontine cistern, the outer arachnoid base of the cistern became detached from the petrous apex, petroclival, and clival region. The trigeminal and abducent nerves fixed the outer arachnoid at the level of the entrance of the Meckel’s cave as well as the Dorello’s canal. Cranial to the intracisternal portion of the trigeminal nerve, the outer arachnoid of the dorsum sellae could be observed. Depending on the position of the head, the subdural collection of air could detach the outer arachnoid from the dorsum sellae (Fig. 2F). These deep relations could be observed most effectively with the additional use of endoscopes (Fig. 2D–F). Microsurgical dissection of the outer arachnoid at the level of the jugular foramen led into the premedullary cistern. Similarly to the cerebellopontine cistern, the medially located cranial nerve—the hypoglossal nerve—fixed the outer arachnoid base of the premedullary cistern to the inner surface of the dura at the level of the hypoglossal canal. Due to the dense and narrow anatomical relations of the cistern, a detachment of the outer arachnoid from the inner surface of the dura could not be observed compared to the cerebellopontine cistern. Endoscopes were primarily used to observe these near-midline structures. In case of relaxed cerebellum, its tentorial surface became detached from the tentorium similarly to the petrosal surface. This resulted in a wide undisrupted view through the subdural space until the tentorial edge. At the level of the cerebellomesencephalic fissure, the outer arachnoid became detached from the pial surface of the cerebellum and spread to the tentorial edge (Fig. 2F). At this level, the outer arachnoid sheet formed the lateral wall of the superior cerebellar cistern. The lateral pontomesencephalic segment of the superior cerebellar artery was attached to the inner surface of the outer arachnoid membranes (Fig. 1B). The cisternal portion of the trochlear nerve is embedded into the outer arachnoid wall of the superior cerebellar cistern. These important neurovascular structures made the opening of outer arachnoid cover challenging. In this region the outer arachnoid was fixed by the dural penetration of the trochlear nerve into the cavernous sinus between the anterior and posterior petroclinoid folds. Contents of the superior cerebellar cistern and the neighboring lateral mesencephalic and ambient cisterns could be observed most effectively with the use of angled endoscopes due to the obstruction of the straight visual axis by the tentorial edge, the posterior petroclinoid fold, and the posterior clinoid process.
Photographic depiction of the normal, undisrupted surgical anatomical relations of the outer arachnoid cover of the cerebellopontine angle on the left side through the retrosigmoid craniotomy in supine position. The upper row (A–C) represents the microsurgical anatomical view through the operating microscope; the lower series (D–F) the endoscopic anatomical aspects using a 0-degree optic. A Microsurgical view of the outer arachnoid cover of the cerebellum. The arachnoid membrane of bridging the petrosal fissure was opened (black arrowhead). B Microscopic image of upper portion of the cerebellopontine angle. After release of CSF, the cerebellum became relaxed. The outer arachnoid cover is fixed on the cerebellar surface as well as at the level of the penetrating structures of the posterior fossa cisterns (black arrowheads at the level of the internal auditory meatus). This results in a stretching of the outer arachnoid at the lateral border of the cerebellopontine fissure. The arachnoid cover around the superior petrosal vein became detached from the junction at the superior petrosal sinus due to the gravity related retraction of the cerebellum (black double arrowheads). C Microsurgical view of the lower portion of the cerebellopontine angle. The outer arachnoid membrane follows the caudal cranial nerves during their penetration through the jugular foramen (black arrowheads). D 0-degree endoscopic overview of the mid-lower portion of the cerebellopontine angle with intact outer arachnoid cover. Note the several microvessels embedded into the arachnoid (black arrowheads) stretching between the cerebellum and the inner surface of the dura on the lateral wall of the cerebellopontine cistern (black double arrowhead). E Mid-upper portion of the cerebellopontine angle through a 30-degree angled endoscope. The outer arachnoid was opened (black arrowheads) to allow a panoramic view of the cerebellopontine cistern. Note the continuity of the outer arachnoid cover within the internal auditory meatus as well as the jugular foramen (black double arrowheads). F Panoramic endoscopic view of the most upper portion of the cerebellopontine angle with a 30-degree endoscope. The outer arachnoid cover of the superior cerebellar cistern was opened (black arrowhead). Note the embedded trochlear nerve along the tentorial edge (black arrow). The subdural space is widely opened, and the outer arachnoid is detached from the inner surface of the dura (black double arrowhead) due to the release of CSF. List of abbreviations: cereb., cerebellum; CN, caudal cranial nerves; IAM, internal auditory meatus; JF, jugular foramen; nIII, oculomotor nerve; nIV, trochlear nerve; nV, trigeminal nerve; nVIII, vestibulocochlear nerve; OA, outer arachnoid; CPF, petrosal fissure; SCA, superior cerebellar artery; SPV, superior petrosal vein
Pathoanatomical observations
The retrospective analysis of 35 video recordings of cerebellopontine angle tumors verified our topographic and surgical anatomical observations in cadavers. The goal of the intraoperative analysis was to describe the behavior of the outer arachnoid membranes in case of space-occupying lesions as well as to verify the anatomical findings of the cadaveric examinations. The typical pattern of the displacements of the outer arachnoid was characterized by the origin of the lesion observed through the retrosigmoid craniotomy.
Meningiomas
The description of the pathoanatomical relations of the outer arachnoid cover in case of CPA meningiomas is based on the intraoperative observations of 7 petroclival meningiomas (small, moderate, and extended size) and 2 jugular- and 2 suprameatal tubercle meningiomas. A representative case of mid-size petroclival meningioma was selected to depict our intraoperative findings (Figs. 3A and 4A, D).
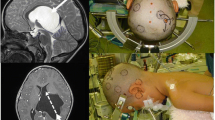
Axial sections of constructive interference in steady state (CISS)—MRI of four representative operative cases of cerebellopontine angle space-occupying lesions. A Moderate-size petroclival meningioma on the right side (red arrowhead). B Vestibular schwannoma (T3a) on the right side (red arrowhead). C Extended vestibular schwannoma (T4a) on the right side (red arrowhead). Note the sign of CSF on the lateral surface of the tumor representing the folding of arachnoid layers (blue arrowhead). D Epidermoid cyst of the left cerebellopontine angle (red arrowhead) with significant space-occupying effect
Intraoperative photomicrographs of a mid-size right sided petroclival meningioma (see Fig. 3A) operated through a retrosigmoid craniotomy in supine position. A After release of CSF and gravity-related self-retraction of the cerebellum, the tumor became visible at the petrotentorial junction. Note the dural attachment of the tumor and the epiarachnoidal growth. The outer arachnoid cover of the posterior fossa cisterns is detached from the inner surface of the dura and folded on the surface of the tumor (black arrowheads). B Inspection along the tentorial surface of the cerebellum. The trochlear nerve is embedded into the outer arachnoid cover (black arrowheads). The lateral portion of the tumor is herniated into the outer arachnoid cover (black double arrowheads). C During resection, the outer arachnoid cover serves as a protecting layer for the sensitive neurovascular structures (black arrowheads). D In a later stage of resection, the outer arachnoid (white arrowhead) could be used as a protecting layer for the ventral surface of the underlying brainstem. List of abbreviations: cereb., cerebellum; nIII, oculomotor nerve; nIV, trochlear nerve; nV, trigeminal nerve; nVIII, vestibulocochlear nerve; OA, outer arachnoid; SCA, superior cerebellar artery; SPV, superior petrosal vein; tent., tentorium; Tu, tumor
All of the observed meningiomas arose from microsurgical point of view in the subdural space on the inner surface of the dura. Depending on the extent and biology of the lesion, the outer arachnoid became primarily displaced from the inner surface of the dura rather than perforated. Following the craniotomy, dural opening, and release of CSF via the cerebellomedullary cistern, the level of the neuroforamina could be approached along the petrosal surface of the cerebellum. Smaller petroclival meningiomas did not cause displacement of the cerebellar surface. Maximal protection of the cisternal structures as well as the surface of the brainstem could be achieved if the outer arachnoid has not been opened during the dissections (Fig. 4A). The outer arachnoid at the level of the internal auditory meatus and the superior petrosal vein complex remained fixed, but the arachnoid base of the cerebellopontine cistern became displaced dorsally from the petrous and clival dura. If the tumor displaced the vestibulocochlear, facial, and trigeminal nerves, the outer arachnoid base of the cerebellopontine cistern was pressed against and stretched along the cisternal segments of the nerves (Fig. 4C). Their outer arachnoid cover at the level of the neuroforamina remained fixed. The superior petrosal vein complex had also this kind of outer arachnoid protection similarly to the cranial nerves. The exact direction of displacement of the intracisternal structures varied individually and depended on the origin and size of the CPA meningioma such as petroclival meningiomas: clival, petroclival, or sphenopetroclival subtypes or petrous meningiomas: suprameatal or jugular tubercle subtypes. The displaced outer arachnoid layer along the cisternal segments of the cranial nerves served as a safe plane for dissection of the tumor surface from the sensitive neurovascular structures (Fig. 4D). The outer arachnoid membranes attached to the surface of the tumor were partially strong enough to directly mobilize them together with the underlying neurovascular structures. The dorsally displaced outer arachnoid sheet of the petrous apex, petroclival, and clival region served as a safe plane to dissect the surface structures of the brainstem from the tumor. If the continuity of the outer arachnoid cover was disrupted at certain locations by an invasive tumor, it was aimed to find the remaining intact edge of the outer arachnoid which led back to the most secure plane for further dissections. Around the tentorial edge, the trochlear nerve had special anatomical relations due to its long cisternal segment which is embedded into the wall of the outer arachnoid (Fig. 4B). Mobilizing and detaching the outer arachnoid membrane from the ventral surface of a petroclival meningioma at the level of the cerebellomesencephalic fissure could help to protect the trochlear nerve from direct manipulations and potential injury. Special care must be taken at the dural exit of the nerve into the cavernous sinus where the outer arachnoid layer is fixed by the transition to an arachnoid sheath around its cavernous segment.
Vestibular schwannomas
Twenty vestibular schwannomas (VS) were involved in our inspections. Progressive analysis of the arachnoid relations depending on the size of VS (class T2, T3, to T4b) showed the steps of the outer arachnoid displacements caused by the tumor growth. Two representative cases of VS were selected to depict our intraoperative finding: a class T3A VS (Figs. 3B and 5A–D) and a class T4A (Figs. 3C and 6A–F) vestibular schwannoma. Intra-extrameatal small vestibular schwannomas (class T2) are growing purely intracisternally within the outer arachnoid sheath of the intrameatal segment of the vestibulocochlear nerve and bulging slightly into the cerebellopontine cistern. The lateral outer arachnoid wall of the cistern was not displaced by class T2 vestibular schwannomas. Class T3 VSs behave similar to class T2 tumors but filled the entire cerebellopontine cistern. Slightly bulging of the outer arachnoid on the lateral wall of the cerebellopontine cistern could be observed but without formation of folding of the outer arachnoid cover (Fig. 5A). In contrast to this class T4, VS with compression of the brainstem displaced the lateral wall of the cerebellopontine cistern against the strongly fixed arachnoid cover on the petrosal surface of the cerebellum. This resulted in an invagination of the subdural space between the lateral surface of the VS and cerebellum until the level of the of the cerebellopontine fissure. This space provided a safe plane for dissections of the cerebellum and the structures of cerebellopontine and petrosal fissures, especially for the here located veins. To get into this plane, an outer arachnoid fold on the most ventral part of the displaced cerebellum has to be identified (Fig. 6A). If this outer arachnoid fold was mobilized dorsally along the outer arachnoid cover on the lateral surface of the tumor, it was possible to dissect between the two sheets of the outer arachnoid membranes until the level of the cerebellopontine fissure (Fig. 6C) and safely reach the root zone of the vestibulocochlear and facial nerves (Fig. 6F). Similar folding could be observed in class T4 VS at the cranial and caudal pole of the tumor. Cranially the foraminal portion of the outer arachnoid invaginates between the superior petrosal vein and the surface of the tumor serving as a safe plane to dissect and protect the vein (Fig. 6D). Similar relations could be observed at the caudal pole of class T4 tumors and caudal cranial nerves (Fig. 6B). This layer serves as the safest plane to dissect the displaced caudal cranial nerves from the surface of the tumor using the outer arachnoid membranes.
Intraoperative photomicrographs of a right sided vestibular schwannoma (class T3a, see Fig. 3B) operated through a retrosigmoid craniotomy in supine position. In this particular case, the facial nerve was displaced and stretched on the lateral surface of the tumor. This condition is a rare finding during vestibular schwannoma surgery. A After release of CSF and gravity-related retraction of the cerebellum, the junction between the cerebellar (black double-arrowhead) and foraminal (black arrowhead) outer arachnoid membranes became visible. Due to the moderate size of the tumor, a displacement of the outer arachnoid could not be observed. B Dissection of the outer arachnid at the caudal pole of the tumor. The foraminal portion of the outer arachnoid has to be widely dissected (double arrowheads) to approach the tumor. The subdural space (asterisk) is clearly distinguishable from the subarachnoid space (double asterisks). The black arrowhead points to the junction between the foraminal and cerebellar portion of the outer arachnoid. C Dissection at the middle portion of the tumor. The approach to the cerebellopontine fissure (black arrowhead) along the lateral surface of the tumor must be performed entirely subarachnoidal in contrast to class 4 tumors (compare Fig. 6A and C). D Dissection at the cranial edge of the tumor. The ventral edge of the widely opened foraminal portion of the outer arachnoid is visible on the lateral surface of the tumor (black arrowhead). The outer arachnoid follows this surface of the tumor within the internal auditory meatus. List of abbreviations: cereb., cerebellum; CN, caudal cranial nerves; IAM, internal auditory meatus; JF, jugular foramen; nV, trigeminal nerve; nVII, facial nerve; OA, outer arachnoid; PICA, posterior inferior cerebellar artery; SPV, superior petrosal vein; Tu, tumor
Intraoperative photomicrographs of a right sided vestibular schwannoma (class T4a, see Fig. 3C) operated through a retrosigmoid craniotomy in sitting position. A After release of CSF, the cerebellum became relaxed, and the outer arachnoid collapsed. The black arrowheads point to the fold of the cerebellar outer arachnoid membrane which deserves as the safe plane of dissection along the lateral surface of the tumor compared to Fig. 8D. B The outer arachnoid cover at the caudal edge of the tumor forms a fold between the outer arachnoid cover of the caudal cranial nerves. This plane (black arrowheads) can be used to safely dissect and detach the caudal cranial nerves from the tumor. C Dissection along the arachnoid fold at the lateral surface of the tumor until the level of the cerebellopontine fissure. The black arrowhead indicates the plane of the dissection. D At the cranial edge of the tumor, the outer arachnoid relations are similar to the caudal pole compared to Fig. 6B. The superior petrosal vein can be gently detached from the surface of the tumor using the outer arachnoid fold (black arrowheads). E After opening the outer arachnoid at the bottom of the arachnoid fold at the cranial edge of the tumor, the dissections have to be continued subarachnoidally within the basal cisterns. The black arrowhead points to the superior cerebellar artery. F The dissection along the arachnoid fold at the lateral edge of the tumor (Fig. 6C) leads to the root zone of the facial nerve. The arachnoid fold has to be opened to get entrance into to the cerebellopontine cistern. Note the detached outer arachnoid membrane from the surface of the clivus (black arrowhead). List of abbreviations: AICA, anterior inferior cerebellar artery; cereb., cerebellum; CN, caudal cranial nerves; nVI, abducent nerve; nVII, facial nerve; OA, outer arachnoid; Tu, tumor
Epidermoid cysts
Four CPA epidermoid cysts (CPA-EC) were analyzed. The operated cases caused significant compression of the brainstem and displacement of the intracisternal structures. We selected an extended size CPA-EC to represent our operative findings (Figs. 3D and 7A–D).
Intraoperative photomicrographs of a left sided epidermoid cyst of the CPA (see Fig. 3D) operated through a retrosigmoid craniotomy in supine position. A After release of CSF, the outer arachnoid is not deflated due to the filling effect of the keratin content of the cyst in the subarachnoid space. Gentle retraction of the cerebellum caused a detachment of the outer arachnoid perforation around the superior petrosal vein (black arrowhead). B The outer arachnoid membrane has to be widely opened to approach the content of the cyst. Note the subarachnoidal tissue visible through the intact outer arachnoid around the caudal cranial nerves (black arrowhead). C After opening the outer arachnoid membranes (black arrowheads), the content of the cerebellopontine became visible filled entirely with keratin. D The keratin fills also hidden corners of the subarachnoid space. The outer arachnoid layer cannot be used for safe dissections. List of abbreviations: AICA, anterior inferior cerebellar artery; cereb., cerebellum; CN, caudal cranial nerves; JF, jugular foramen; nVIII, vestibulocochlear nerve; OA, outer arachnoid; SPV, superior petrosal vein; tent., tentorium; Tu, tumor
These lesions grew and remained purely intracisternally independently from the extent of the lesion. Perforations on the outer arachnoid membranes could not be observed during the operations. Some CPA-ECs extended laterally along the petrosal surface of the cerebellum mainly along the petrosal fissure but remained in the subarachnoid space and invaded the cerebellar folia and sulci despite of the natural strong adhesions between the here located pia mater and outer arachnoid. Medially, the lateral wall of the superior cerebellar, cerebellopontine, and premedullary cisterns was slightly bulged by the solid content of the CPA-ECs, but penetration or folding of the outer arachnoid could not be observed (Fig. 7A). Opening the outer arachnoid along this level was necessary to get access into the cerebellomesencephalic and cerebellopontine fissures as well as the intracisternal portion of the here located cranial nerves and vascular structures (Fig. 7D). As a result of the arachnoid sheaths around the cranial nerves formed by the outer arachnoid base of the cisterns, the subarachnoid space extends into the cavernous sinus, Meckel’s cave, Dorello’s canal, internal auditory meatus, and jugular foramen. This anatomical continuity led to the often observed undisrupted invasion of the CPA-EC into the mentioned interdural spaces and neuroforamina (Fig. 7C). The outer arachnoid cover provides only safe access to the content of the CPA-EC but no protection for the intracisternal structures during dissections.
Discussion
Safe surgery of the cerebellopontine angle requires detailed knowledge of the surgical microanatomy. Several studies deal with the microsurgical anatomy of the here located neurovascular and bony structures [11, 30, 33]. The importance of the arachnoid membranes in microsurgical approaches is well studied since its introduction by Yasargil [50]. During intradural manipulations, only the arachnoid membranes can be dissected without severe traumatization of the delicate intracranial structures. These membranes allow the gentle approach to the deep regions of the skull base, relax the brain tissue, or protect the sensitive neurovascular content of the cisterns [50]. To fully use the potential of the arachnoid dissections during microsurgical operations, the knowledge of anatomy of these membranes is mandatory [1, 23]. Most publications dealing with the arachnoid membranes concentrate on the anatomy of the inner arachnoid membranes located between the outer arachnoid cover and the pia mater separating the basal cisterns [24-27, 46-49]. The complex architecture of these inner membranes is an important roadmap to explore the individual cisterns separated by these thin membranes and to reach the targeted deep cisternal or intraparenchymal lesions [23]. The first step to approach these inner membranes is the dissection of the outer arachnoid cover of the brain. However, in case of neoplastic lesions of the skull base, the inner arachnoid membranes play an inferior role compared to the outer arachnoid cover which has direct contact to the operated lesion.
The outer arachnoid layer is a well visible structure on en bloc removed brains, and it is in detail depicted in the classic anatomical textbooks and atlases. The modern microsurgical studies dealing with the deep-seated inner arachnoid membranes dedicate only sporadic attention to this apparently simple and well-known layer of the intracranial meninges [9, 26, 27, 29, 32, 34, 37, 53]. Inoue et al. gave detailed description of the microsurgical and endoscopic anatomy of the arachnoid membranes [12]. In this study, they examined also the microanatomy of the basal outer arachnoid membranes but concentrated only on the supratentorial spaces. A detailed description of the surgical anatomy of the outer arachnoid cover of the posterior fossa is still missing in the literature. Lu et al. summarized in his recent detailed review [23] the current knowledge about the arachnoid membranes and their surgical relevance. In this paper, they gave a short summary about the anatomy of the outer arachnoid membranes too. Several research papers are dealing with the outer arachnoid sleeves around the cranial nerves within the bony canals of the skull base and the cavernous sinus [10, 16, 17, 24, 26, 27, 29, 31]. Our study could also verify the existence of the outer arachnoid sheaths around the trochlear, trigeminal, abducent, vestibulocochlear, and caudal cranial nerves arising from the basal outer arachnoid layer at the level of their dural penetration. The topographical relations of the outer arachnoid cover appear more complex during surgical approaches due to the displacement of the normal anatomical relations. We described these microsurgically relevant relations in detail as well as the related anatomy of the surrounding neurovascular structures. Based on our cadaveric examinations, we distinguished three main portions of the outer arachnoid cover in the cerebellopontine angle (Fig. 8A):
-
Cerebellar outer arachnoid membrane, which is located on the petrosal and tentorial surface of the cerebellum where it is strongly adhered to the pia mater.
-
Foraminal outer arachnoid membrane, which is located at the level of the neuroforamina. The cranial nerves are protruding through the here located outer arachnoid layer and covered distally by an arachnoid sleeve.
-
Basal outer arachnoid membrane, which is located on the ventral surface of the brainstem and around the mesencephalon where it is detached from the pial surface and forms the base of the superior cerebellar, cerebellopontine, premedullary, and prepontine cisterns.
Schematic representation of the authors’ understanding of the outer arachnoid layer of the right CPA in normal and pathological conditions. It has to be noted that the pial cover of the intracisternal portion of the cranial nerves is a rarely studied topic in the modern literature. Lang et al. [19] confirm this fact based on his early publications in German language. Pial cover of the Vth, VIIth, and VIIIth cranial nerves was described in the mentioned book in detail. Our illustrations depict continuous pial cover of the cranial nerves based on the literature and our intraoperative microsurgical observations but without further histological confirmation. A Outer arachnoid cover of the CPA. Note the three main topographic relations: on the petrosal surface of the cerebellum, the outer arachnoid cover is attached to the pia mater (cerebellar outer arachnoid membrane). At the level of the cerebellopontine fissure, the outer arachnoid is detached from the pial surface and covers the cranial nerves within the neuroforamina (foraminal outer arachnoid membrane). Medially from it the outer arachnoid layer forms the base of the posterior fossa cisterns (basal outer arachnoid membrane). B In case of petroclival meningiomas, the tumor is macroscopically adhered to the inner surface of the dura. The foraminal and basal outer arachnoid membrane is detached from the dura and pressed against the brainstem. The insert represents the safe plane of dissections (black arrowhead) between the surface of the tumor and the outer arachnoid. C Small- and mid-size vestibular schwannomas (class T2-T3) are entirely subarachnoid lesions. To approach the tumor, it is necessary to open the outer arachnoid membranes at the junction between the foraminal and cerebellar outer arachnoid membranes (black arrowhead). D Extended vestibular schwannomas (class T4) are causing significant compression of the cerebellum. This results in an invagination of the cerebellar outer arachnoid membrane and elongation of the foraminal outer arachnoid. This results in a folding of the cerebellar outer arachnoid membrane seemingly attached to the lateral surface of the tumor (see the insert). The black arrowhead represents the safe plane of dissection between the foraminal and cerebellar outer arachnoid layers to approach the root zone of the vestibulocochlear and facial nerve. The outer arachnoid layers allow the protection of the cerebellar as well as brainstem surface. E Space-occupying epidermoid cysts of the CPA are entirely subarachnoid pathologies independent from the size of the lesion. Similarly to the mid-size vestibular schwannomas, the outer arachnoid has to be widely dissected between the junction of the cerebellar and foraminal outer arachnoid membranes (black arrowhead) to approach the lesions. List of abbreviations: AICA, anterior inferior cerebellar artery; BA, basilar artery; IAM, internal auditory meatus; nVI, abducent nerve; nVIII, vestibulocochlear nerve
The surgical importance of these topographic anatomical relations became relevant when we try to mobilize the outer arachnoid membranes during the stages of a surgical approach or became displaced by space-occupying lesions.
The retrosigmoid craniotomy is the workhorse approach to the cerebellopontine angle [33]. The surgical anatomy of the intradural relations and cisternal approaches is well described in the literature [18, 33, 39, 42, 45]. Due to the crowded anatomy of the here located cisterns as well as the angulated relations of the different compartments like the internal auditory meatus, Meckel’s cave or the region of the dorsum sellae makes the use of endoscopes effective in the CPA [6, 18, 39, 42, 45]. The keyhole retrosigmoid craniotomy reduces the approach-related traumatization, but it means further limitation for the straight optical axis of the operating microscope. The frequent use of endoscopes helps to overcome these limitations [39, 42]. Knowledge of the endoscopic applied microanatomy of the CPA is therefore critical for good surgical results. Due to these aspects, we aimed to translate the results of our topographical cadaveric examinations of the outer arachnoid cover to the surgical steps of the retrosigmoid craniotomy. The detailed microsurgical and endoscopic anatomy of the outer arachnoid cover inspected and didactically dissected through the retrosigmoid craniotomy was described in detail in the present study. Important anatomical landmarks were defined to orientate and find the targeted areas.
In clinical cases, the outer arachnoid relations are typically undisturbed in case of vascular lesions such as cavernous malformations of the brainstem, posterior fossa aneurysms, or microvascular compression syndromes. In these cases, the described normal anatomy of the outer arachnoid membranes can be applied to approach these lesions. In case of extra-axial space-occupying lesions, the displacement of the outer arachnoid membranes is obvious. Meningiomas originate from the arachnoid cap cells within the leptomeninges. Their arterial vascular supply arises from the dural arteries. Macroscopically, these tumors are attached to the inner surface of dura. During the slow growth of the tumor the surrounding leptomeningeal, vascular and neural structures became displaced (Fig. 8B). Generally, during surgical resection, the vascular supply of the tumor is detached which is followed by the debulking of the tumor and the final dissection of the remaining enucleated parts from the surrounding neurovascular structures [50]. This step is the most critical stage of the operation and deciding factor of the postoperative neurological outcome. However, the outer arachnoid layer could be infiltrated by invasive growing meningiomas and infiltrating the pia mater too; this layer serves in general as a secure plane for dissections. The surgical strategy in case of CPA meningiomas is challenging due to the crowded anatomical relations of the posterior fossa: the sensitive surface structures like the brainstem, vessels, and the fragile filaments of the cranial nerves which cross the leptomeningeal layers around the meningioma. Special attention should be paid for detaching the tumor from these displaced structures. However, it is well known that the outer arachnoid layer can serve as a safe plane during dissection of CPA meningiomas, and a dedicated anatomical study was not published to our knowledge until now. In our recent work, we analyzed and described the most typical pattern of displacements of the outer arachnoid in case of CPA meningiomas.
Vestibular schwannomas (acoustic neuromas) are Schwann cell-derived tumors arising from the vestibular nerve within the internal auditory canal. Several classifications have been described to predict the outcome of surgical resection of these benign lesions [15, 36, 38]. In our descriptions, we used the Hannover classification [36]. During microsurgical resection of these lesions, the surgical strategy is strongly dependent from the extent of the lesion [50]. The importance of protection of the surrounding neurovascular structures by the arachnoid membranes during resections of VS has been described by several authors [41, 51]. The exact leptomeningeal relations of the VS is a controversial subject [14]. Yasargil proposed that VS are arising epiarachnoidal causing rearrangement of the arachnoid membranes [51]. Later studies could not verify the existence of arachnoid duplications and suggested the intracisternal growth of these tumors [20, 21]. Ohata gave a more differentiated description of the outer arachnoid membranes in case of VS [31]. If the tumor adheres to the arachnoid membrane at the porus of the internal auditory meatus, arachnoid duplications could be formed. In our study based on tumors operated in different stages of growth, we suggest the concept of intrameatal-subarachnoidal arise of these tumors (Fig. 8C, D). Arachnoid duplications could be microsurgically observed only at the lateral surface of class T4 lesions with significant displacement of cerebellum where the outer arachnoid is strongly adhered to the pial surface.
Epidermoid cysts of the cerebellopontine angle (CPA-EC) are non-neoplastic cystic lesions made of squamous epithelium filled with keratin. Due to their development in the early embryonic phase with inclusion of ectodermal elements, these lesions are located most often within the subarachnoid space [7, 52]. In case of extensive growth, surgical resection could be necessary [52]. To achieve the best prognosis radical resection of the cystic wall, removal of the avascular keratin content of the lesion should be the goal. In the cerebellopontine angle, the wall of the cyst is in most of the cases strongly adhered to the pia mater of the brainstem and to the here located neurovascular structures which makes the radical removal difficult. Therefore, most of the microsurgical resection of CPA-ECs are concentrated mainly on the removal of the space-occupying keratin content. Due to its embryonic development, the outer arachnoid is whether displaced nor folded by these lesions (Fig. 8E). The release of CSF does not result in a collapse of the invaded cisterns because of the subarachnoidal filling effect of the keratin content. To access the content of the target cisterns, the outer arachnoid has to be widely dissected between the neuroforamina to achieve relaxations of the surrounding neurovascular structures. During resection, the outer arachnoid does not provide any safe plane for dissections in contrast to the examined neoplastic lesions.
Limitations
Two main limitations of the study should be discussed which are restricted to the results of our intraoperative observations. First, intraoperative observations regarding the overall microanatomical and especially leptomeningeal relations of a particular tumor are limited by the chosen surgical approach. This limitation was also highlighted by Lu et al. [23]. Second, however, it is possible to take general considerations about the pattern of developments of specific tumor types, and the individual localization, extent, and biological behavior of a particular lesion could differ. The described pathoanatomical relations of three different space-occupying extra-axial CPA lesions are suggestions for the more effective intraoperative management and decision-making. To overcome the mentioned limitations, histological studies of untreated lesions could help to clarify these precise leptomeningeal relations of the entire surface of the lesions. Advanced imaging techniques in the future may could help to depict the microanatomical and leptomeningeal relations of an individual case as a part of the preoperative planning.
Conclusions
The outer arachnoid membrane covers the entire surface of the posterior fossa dura. In the cerebellopontine angle, three main types of topographic relations could be observed. The cerebellar outer arachnoid membrane on the petrosal and tentorial surface of the cerebellum is strongly adhered to the pia mater. The foraminal outer arachnoid membrane is located at the level of the neuroforamina and fixed to the dural exits of the cranial nerves. This portion of the outer arachnoid membrane forms arachnoid sleeves around the intraforaminal segments of the cranial nerves. The basal outer arachnoid membrane was located medially from the foraminal portion of the outer arachnoid membranes where it became displaced from the pial surface of the cerebellum and forms the base of the superior cerebellar, cerebellopontine, premedullary, and prepontine cisterns. Inspected through the retrosigmoid craniotomy, these topographical relations are crucial to perform safe microsurgical or endoscopic approaches. CPA meningiomas, vestibular schwannomas, and epidermoid cysts displace the outer arachnoid on different ways. Three typical patterns of displacements of the outer arachnoid could be observed depending on the origin of the lesions. In cases of CPA meningiomas, the foraminal and basal outer arachnoid layer is completely detached from the dural surface and serves as a safe plane for dissections around the entire surface of the lesion. In cases of vestibular schwannomas of class T2-T3 lesions, the outer arachnoid is not displaced. In class T4 tumors, the cerebellar and foraminal outer arachnoid is displaced at the lateral surface. Arachnoid folds could be here observed and used as a protecting layer for the dislocated structures. In cases of epidermoid cyst, the outer arachnoid is not displaced. Approaches to these lesions lead through the junction between the cerebellar and foraminal outer arachnoid membrane. They have to be widely dissected but do not provide protection for the neurovascular structures.
References
Adeeb N, Deep A, Griessenauer CJ, Mortazavi MM, Watanabe K, Loukas M, Tubbs RS, Cohen-Gadol AA (2013) The intracranial arachnoid mater : a comprehensive review of its history, anatomy, imaging, and pathology. Childs Nerv Syst 29:17–33. https://doi.org/10.1007/s00381-012-1910-x
Akiyama O, Kondo A, Arai H (2019) The rhomboid lip: anatomy, pathology, and clinical consideration in neurosurgery. World Neurosurg 123:e252–e258. https://doi.org/10.1016/j.wneu.2018.11.148
Axel Key GR (1875) Studien in der Anatomie des Nervensystems und des Bindegewebes, vol II. Samson & Wallin, Stockholm
Barany L, Baksa G, Patonay L, Ganslandt O, Buchfelder M, Kurucz P (2017) Morphometry and microsurgical anatomy of Bochdalek's flower basket and the related structures of the cerebellopontine angle. Acta Neurochir (Wien) 159:1539–1545. https://doi.org/10.1007/s00701-017-3234-9
Barany L, Baksa G, Patonay L, Racz G, Ganslandt O, Buchfelder M, Kurucz P (2018) Primary obstruction of the foramen of luschka: anatomy, histology, and clinical significance. World Neurosurg 112:e288–e297. https://doi.org/10.1016/j.wneu.2018.01.037
Belykh E, Onaka NR, Zhao X, Cavallo C, Yagmurlu K, Lei T, Byvaltsev VA, Preul MC, Nakaji P (2018) Endoscopically assisted targeted keyhole retrosigmoid approaches for microvascular decompression: quantitative anatomic study. World Neurosurg 119:e1–e15. https://doi.org/10.1016/j.wneu.2018.04.218
Berger MS, Wilson CB (1985) Epidermoid cysts of the posterior fossa. J Neurosurg 62:214–219. https://doi.org/10.3171/jns.1985.62.2.0214
Buss EJ, Wang TJC, Sisti MB (2021) Stereotactic radiosurgery for management of vestibular schwannoma: a short review. Neurosurg Rev 44:901–904. https://doi.org/10.1007/s10143-020-01279-2
Di Ieva A, Tschabitscher M, Matula C, Komatsu F, Komatsu M, Colombo G, Sherif C, Galzio RJ (2012) The subdiaphragmatic cistern: historic and radioanatomic findings. Acta Neurochir (Wien) 154:667–674. https://doi.org/10.1007/s00701-011-1220-1
Everton KL, Rassner UA, Osborn AG, Harnsberger HR (2008) The oculomotor cistern: anatomy and high-resolution imaging. AJNR Am J Neuroradiol 29:1344–1348. https://doi.org/10.3174/ajnr.A1089
Fujii K, Lenkey C, Rhoton AL Jr (1980) Microsurgical anatomy of the choroidal arteries. Fourth ventricle and cerebellopontine angles. J Neurosurg 52:504–524. https://doi.org/10.3171/jns.1980.52.4.0504
Inoue K, Seker A, Osawa S, Alencastro LF, Matsushima T, Rhoton AL Jr (2009) Microsurgical and endoscopic anatomy of the supratentorial arachnoidal membranes and cisterns. Neurosurgery 65:644–664. https://doi.org/10.1227/01.NEU.0000351774.81674.32
Jumah F, Narayan V, Samara A, Quinoa TR, Dossani RH, Gupta G, Nanda A (2020) Efficacy and safety of gamma knife radiosurgery for posterior cranial fossa meningioma: a systematic review. Neurosurg Rev 43:1089–1099. https://doi.org/10.1007/s10143-019-01144-x
Kohno M, Sato H, Sora S, Miwa H, Yokoyama M (2011) Is an acoustic neuroma an epiarachnoid or subarachnoid tumor? Neurosurgery 68:1006–1016. https://doi.org/10.1227/NEU.0b013e318208f37f
Koos WT, Day JD, Matula C, Levy DI (1998) Neurotopographic considerations in the microsurgical treatment of small acoustic neurinomas. J Neurosurg 88:506–512. https://doi.org/10.3171/jns.1998.88.3.0506
Kurucz P, Baksa G, Patonay L, Hopf NJ (2013) Endoscopic anatomical study of the arachnoid architecture on the base of the skull. Part I: the anterior and middle cranial fossa. Innovative Neurosurgery 1:55–67. https://doi.org/10.1515/ins-2012-0005
Kurucz P, Baksa G, Patonay L, Hopf NJ (2013) Endoscopic anatomical study of the arachnoid architecture on the base of the skull. Part II: level of the tentorium, posterior fossa and the craniovertebral junction. Innovative Neurosurgery 1(2):91–108. https://doi.org/10.1515/ins-2013-0008
Kurucz P, Baksa G, Patonay L, Thaher F, Buchfelder M, Ganslandt O (2017) Endoscopic approach-routes in the posterior fossa cisterns through the retrosigmoid keyhole craniotomy: an anatomical study. Neurosurg Rev 40:427–448. https://doi.org/10.1007/s10143-016-0800-1
Lang J (2001) Skull base and related structures: atlas of clinical anatomy. Schattauer Verlag, p 76
Lescanne E, Francois P, Bakhos D, Velut S, Robier A, Pollak A (2008) Vestibular schwannoma: dissection of the tumor and arachnoidal duplication. Otol Neurotol 29:989–994. https://doi.org/10.1097/MAO.0b013e3181845812
Lescanne E, Velut S, Lefrancq T, Destrieux C (2002) The internal acoustic meatus and its meningeal layers: a microanatomical study. J Neurosurg 97:1191–1197. https://doi.org/10.3171/jns.2002.97.5.1191
Liliequist B (1956) The anatomy of the subarachnoid cisterns. Acta radiol 46:61–71. https://doi.org/10.3109/00016925609170813
Lu J (2015) Arachnoid membrane: the first and probably the last piece of the roadmap. Surg Radiol Anat 37:127–138. https://doi.org/10.1007/s00276-014-1361-z
Lu J, Zhu X (2005) Microsurgical anatomy of the interpeduncular cistern and related arachnoid membranes. J Neurosurg 103:337–341. https://doi.org/10.3171/jns.2005.103.2.0337
Lu J, Zhu XI (2003) Microsurgical anatomy of Liliequist's membrane. Minim Invasive Neurosurg 46:149–154. https://doi.org/10.1055/s-2003-40743
Lu J, Zhu XL (2005) Characteristics of distribution and configuration of intracranial arachnoid membranes. Surg Radiol Anat 27:472–481. https://doi.org/10.1007/s00276-005-0025-4
Lu J, Zhu XL (2007) Cranial arachnoid membranes: some aspects of microsurgical anatomy. Clin Anat 20:502–511. https://doi.org/10.1002/ca.20448
Manduca Palmiero HO, Silva da Costa MD, Caramanti RL, Eduardo Aparecido Chaddad-Neto F (2018) Angular and metric analysis of the neural structures in the cerebellopontine angle. Br J Neurosurg 32:250–254. https://doi.org/10.1080/02688697.2018.1426722
Martins C, Yasuda A, Campero A, Rhoton AL Jr (2006) Microsurgical anatomy of the oculomotor cistern. Neurosurgery 58:ONS-220–ONS-227. https://doi.org/10.1227/01.NEU.0000204673.55834.BE
Matsushima K, Yagmurlu K, Kohno M, Rhoton AL Jr (2016) Anatomy and approaches along the cerebellar-brainstem fissures. J Neurosurg 124:248–263. https://doi.org/10.3171/2015.2.JNS142707
Ohata K, Tsuyuguchi N, Morino M, Takami T, Goto T, Hakuba A, Hara M (2002) A hypothesis of epiarachnoidal growth of vestibular schwannoma at the cerebello-pontine angle: surgical importance. J Postgrad Med 48:253–258
Qi ST, Zhang XA, Fan J, Huang GL, Pan J, Qiu BH (2011) Anatomical study of the arachnoid envelope over the pineal region. Neurosurgery 68:7–14. https://doi.org/10.1227/NEU.0b013e3182059e10
Rhoton AL (2000) The cerebellopontine angle and posterior fossa cranial nerves by the retrosigmoid approach. Neurosurgery 47:S93–S129. https://doi.org/10.1097/00006123-200009001-00013
Rhoton AL Jr (2000) The posterior fossa cisterns. Neurosurgery 47:S287–S297. https://doi.org/10.1097/00006123-200009001-00029
Salgado-Lopez L, Leonel LCP, Aydin SO, Peris-Celda M (2020) Surgical anatomy of the labyrinthine and subarcuate arteries and clinical implications. World Neurosurg 141:e880–e887. https://doi.org/10.1016/j.wneu.2020.06.083
Samii M, Matthies C (1997) Management of 1000 vestibular schwannomas (acoustic neuromas): the facial nerve--preservation and restitution of function. Neurosurgery 40:684–694. https://doi.org/10.1097/00006123-199704000-00006
Song-tao Q, Xi-an Z, Hao L, Jun F, Jun P, Yun-tao L (2010) The arachnoid sleeve enveloping the pituitary stalk: anatomical and histologic study. Neurosurgery 66:585–589. https://doi.org/10.1227/01.NEU.0000365371.50165.06
Sterkers JM, Morrison GA, Sterkers O, El-Dine MM (1994) Preservation of facial, cochlear, and other nerve functions in acoustic neuroma treatment. Otolaryngol Head Neck Surg 110:146–155. https://doi.org/10.1177/019459989411000202
Takemura Y, Inoue T, Morishita T, Rhoton AL Jr (2014) Comparison of microscopic and endoscopic approaches to the cerebellopontine angle. World Neurosurg 82:427–441. https://doi.org/10.1016/j.wneu.2013.07.013
Tanahashi K, Uda K, Araki Y, Takeuchi K, Choo J, Chalise L, Motomura K, Ohka F, Wakabayashi T, Natsume A (2020) Trautmann-focused mastoidectomy for a simple, safe presigmoid approach: technical note. J Neurosurg 134:843–847. https://doi.org/10.3171/2020.1.JNS193179
Tarlov E (1980) Total one-stage suboccipital microsurgical removal of acoustic neuromas of all sizes: with emphasis on arachnoid planes and on saving the facial nerve. Surg Clin North Am 60:565–591. https://doi.org/10.1016/s0039-6109(16)42136-5
Tatagiba MS, Roser F, Hirt B, Ebner FH (2014) The retrosigmoid endoscopic approach for cerebellopontine-angle tumors and microvascular decompression. World Neurosurg 82:S171–S176. https://doi.org/10.1016/j.wneu.2014.08.001
Thai NLB, Mai NY, Vuong NL, Tin NM, Karam D, Refaey MA, Shahin KM, Soliman AL, Al Khudari R, Thuan TM, Sabbah GM, El-Qushayri AE, Karimzadeh S, Hirayama K, Huy NT (2022) Treatment for vestibular schwannoma: Systematic review and single arm meta-analysis. Am J Otolaryngol 43:103337. https://doi.org/10.1016/j.amjoto.2021.103337
Tu YK, Yang SH, Liu HM (1999) The transpetrosal approach for cerebellopontine angle, petroclival and ventral brain stem lesions. J Clin Neurosci 6:336–340. https://doi.org/10.1054/jocn.1997.0065
Van Rompaey J, Bush C, McKinnon B, Solares AC (2013) Minimally invasive access to the posterior cranial fossa: an anatomical study comparing a retrosigmoidal endoscopic approach to a microscopic approach. J Neurol Surg A Cent Eur Neurosurg 74:1–6. https://doi.org/10.1055/s-0032-1330119
Vinas FC, Dujovny M, Fandino R, Chavez V (1996) Microsurgical anatomy of the infratentorial trabecular membranes and subarachnoid cisterns. Neurol Res 18:117–125. https://doi.org/10.1080/01616412.1996.11740389
Vinas FC, Dujovny M, Fandino R, Chavez V (1996) Microsurgical anatomy of the arachnoidal trabecular membranes and cisterns at the level of the tentorium. Neurol Res 18:305–312. https://doi.org/10.1080/01616412.1996.11740426
Vinas FC, Fandino R, Dujovny M, Chavez V (1994) Microsurgical anatomy of the supratentorial arachnoidal trabecular membranes and cisterns. Neurol Res 16:417–424. https://doi.org/10.1080/01616412.1994.11740266
Vinas FC, Panigrahi M (2001) Microsurgical anatomy of the Liliequist's membrane and surrounding neurovascular territories. Minim Invasive Neurosurg 44:104–109. https://doi.org/10.1055/s-2001-15999
Yasargil MG (1995) Microneurosurgery, vol IV B: CNS Tumors. Thieme
Yasargil MG (2002) The internal acoustic meatus. J Neurosurg 97:1014–1015. https://doi.org/10.3171/jns.2002.97.5.1014
Yasargil MG, Abernathey CD, Sarioglu AC (1989) Microneurosurgical treatment of intracranial dermoid and epidermoid tumors. Neurosurgery 24:561–567. https://doi.org/10.1227/00006123-198904000-00012
Zhang XA, Qi ST, Huang GL, Long H, Fan J, Peng JX (2012) Anatomical and histological study of Liliequist's membrane: with emphasis on its nature and lateral attachments. Childs Nerv Syst 28:65–72. https://doi.org/10.1007/s00381-011-1599-2
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kurucz, P., Ganslandt, O., Buchfelder, M. et al. Microsurgical anatomy and pathoanatomy of the outer arachnoid membranes in the cerebellopontine angle: cadaveric and intraoperative observations. Acta Neurochir 165, 1791–1805 (2023). https://doi.org/10.1007/s00701-023-05601-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05601-x