Abstract
Background
High-sensitivity C-reactive protein (hs-CRP) is a well-recognized biomarker of neurologic complications and clinical outcome of stroke patients. However, whether hs-CRP can predict the occurrence of acute kidney injury (AKI) in aneurysmal subarachnoid hemorrhage (aSAH) patients is still unclear. The objective of this study was to assess the feasibility of using serum hs-CRP level to predict the occurrence of AKI in aSAH patients.
Methods
One hundred sixty-four aSAH patients were enrolled into a prospective observational study. AKI was diagnosed using the modified Kidney Disease Improving Global Outcomes (KDIGO) standard. The relationship between serum hs-CRP level at admission and occurrence of AKI was analyzed.
Results
AKI occurred in 17 patients (10.4%) in this cohort. Patients with AKI had significantly higher hs-CRP levels than those without. The mortality of the AKI group tends to be higher than that of the non-AKI group, but the difference was not statistically significant (4/17 (23.5%) vs. 13/147 (8.8%), P = 0.081). After adjusting for possible confounding factors including World Federation of Neurosurgical Societies grade, diabetes, and serum creatinine, multivariate analysis revealed that serum hs-CRP level and antibiotic therapy were both significant factors independently associated with AKI following aSAH (serum hs-CRP: OR = 1.2, 95% confidence interval (CI) = 1.1–1.3, P = 0.003; antibiotic therapy: OR = 5.8, 95%CI = 1.6–20.7, P = 0.007). Receiver operating characteristic curve analysis showed that hs-CRP had a sensitivity of 76.5% and a specificity of 64.6% for predicting the development of AKI on the basis of the best thresholds. The post hoc log-rank test revealed that patients having serum hs-CRP level > 6.6 mg/L had a significantly higher AKI rate than patients having serum hs-CRP level ≤ 6.6 mg/L (P = 0.001).
Conclusions
Serum hs-CRP level might be helpful as a predictor for the development of AKI in aSAH patients. Delayed cerebral ischemia occurrence rate and mortality of patients with AKI tend to be higher than those of patients without in this cohort; however, they were not significantly different.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is one of the acute and critical neurosurgical conditions. In recent years, with advanced neurovascular imaging, development and popularization of endovascular coiling, and significantly increased availability of neurologic intensive care unit over time, prognosis of aSAH has significantly improved compared with that of 20 years ago. However, the death rate still remains high at ~ 30% within 3 months of aSAH onset [25]. In addition to neurologic complications, care of these patients is frequently associated with non-neurologic complications including the acute kidney injury (AKI), which occurs in 4–25% of patients after SAH [5, 21, 23, 33, 42]. AKI is detrimental to aSAH patients, resulting in increased healthcare costs and increased risk of poor neurological outcome [33]. However, the underlying mechanism for worse outcomes in AKI patients is not fully understood. Early prognosis of the risk of AKI may allow improved long-term outcome by changing perioperative management and disease prevention methods.
In this study, we hypothesized that the C-reactive protein (CRP) level might be helpful in predicting the occurrence of extracerebral organ dysfunction, including AKI, for aSAH patients. It has been reported that within the first 3 days of aSAH, most of patients could develop systemic inflammatory response, which has been correlated with the development and progression of extracerebral organ dysfunction [3, 12, 16, 41, 42]. CRP, as an acute-phase protein, could reflect the degree of inflammatory response, thus predicting the occurrence of several neurologic and cardiorespiratory complications and the clinical outcome of aSAH patients [4, 11, 20, 32, 40]. However, whether CRP level can predict the development of AKI following bleeding in aSAH patient is still unclear due to the lack of relevant research. Furthermore, because high-sensitivity CRP (hs-CRP) can represent CRP even at low concentrations, the aim of this study was to investigate the feasibility of using hs-CRP level to predict the occurrence of AKI following aSAH.
Materials and methods
Patients
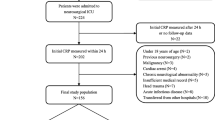
The aSAH patients admitted to the Department of Neurosurgery of the First Affiliated Hospital of Fujian Medical University between June 2017 and May 2018 were prospectively enrolled in this study, and of these aSAH patients, 164 patients who met the inclusion and did not meet any exclusion criteria were included in our analysis.
The inclusion criteria were as follows: (1) the patient was admitted to the hospital within 24 h of SAH onset, (2) serum hs-CRP level at admission was obtained, (3) SAH was caused by intracranial aneurysm and was confirmed via computed tomography angiography (CTA) or digital subtraction angiography (DSA), and (4) clipping or interventional treatment of aneurysm was performed within 48 h of aSAH onset. Exclusion criteria were as follows: (1) patients who did not survive the first 24 h after admission; (2) patient who was discharged within 14 days and was not able to re-visit the hospital for the completion of follow-up serum creatinine examination; (3) patient who had surgery or severe infection within the past month before aSAH; (4) prior onset of SAH or other neurological diseases such as ischemic stroke, hemorrhagic stroke, or severe head trauma; (5) previous use of antiplatelet and anticoagulant drugs or immunosuppressants; and (6) other systemic diseases, such as autoimmune disease, uremia, cirrhosis, cancer, chronic heart diseases, and chronic lung diseases.
The study protocol was designed in accordance with guidelines outlined in the Declaration of Helsinki and approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University (Fujian, China). Informed consent form was obtained from the patients or their authorized legal representative (if the patient did not have the ability to sign the form) in accordance with the Chinese law.
Patient management
Clinical management followed the guidelines of the American Heart Association and American Stroke Association [10]. Critical care management followed the guideline of the Neurocritical Care Society [13]. Nimodipine was applied to all patients, either orally (6 × 60 mg/day) or intravenously (2 mg/h) since the day of admission. Generally, the treatment of AKI is based on optimizing the fluid administrations, antibiotics with least nephrotoxic potential, and adequate nutritional support [38].
Clinical variable collection
Clinical information was collected for each patient. The location and size of the aneurysm was determined via CTA or DSA. A modified Fisher grade was used to evaluate the amount of subarachnoid blood presented on the admission CT image. Severity of aSAH was assessed using the admission World Federation of Neurosurgical Societies (WFNS) grade. Each patient was graded by two doctors and the average score was taken. If a large deviation was seen, the final score would be given by a third doctor.
The hs-CRP level at admission was obtained, using particle-enhanced immunonephelometry with the Nephstar protein analysis system (Goldsite Inc., Shenzhen, China). The hs-CRP testing assay has a detection limit of 0.15 mg/L and has the intra-assay coefficient of variation (COV) and inter-assay COV for concentrations of less than 0.35 and 0.5 mg/L, respectively.
Cerebral vasospasm and delayed cerebral ischemia were examined using CTA (Aquilion ONE, Toshiba Medical Systems, Nasu, Japan) or 3.0-T 3-dimensional time-of-flight magnetic resonance angiography (MRA) (MAGNETOM Verio Tim, Siemens, Erlangen, Germany, or another system: Skyra, Siemens, Erlangen, Germany) for all subjects. When vascular narrowing, with or without neurological symptoms, was observed on the images, it was defined as angiographic vasospasm. Delayed cerebral ischemia was diagnosed based on the following criteria [17]: “(1) clinical deterioration (i.e., a new focal deficit, decrease in level of consciousness, or both) and/or (2) a new infarct on the CT image that was not visible on the admission or immediate post-operative scan and cannot be attributed to other causes by means of clinical assessment, imaging of the brain, or appropriate laboratory studies.”
Pneumonia was defined as a new infiltrate observed on chest X-ray and/or computed tomography images, with purulent sputum and/or positive microbiological cultures [14]. Intracranial infection was defined as when two or more of the following changes were observed: (1) temperature higher than 38 °C or lower than 36 °C, positive signs of meningeal irritation (nuchal rigidity, Brudzinski sign, and Kernig sign), vomiting, and headache; (2) positive changes in cerebrospinal fluid (CSF) specimens: white blood cell count > 1000 × 106 cells/L, glucose < 2.2 mmol/L, or < 40% of serum glucose; and (3) positive CSF cultures [9].
Outcome assessment
The primary outcome was the development of AKI within the 14-day period post-aSAH. AKI was diagnosed using the modified Kidney Disease Improving Global Outcomes (KDIGO) standard [27]: 48-h absolute serum creatinine elevated for ≥ 26.5 μmol/L or serum creatinine elevated for ≥ 50%. Laboratory values assessed included daily serum creatinine level of each patient in the first 14 days post-aSAH. This time frame was used because it is considered the most risky period for clinically significant vasospasm and subsequent exposure to multiple interventions. The secondary outcomes were 28-day mortality and medical complications including angiographic vasospasm, delayed cerebral ischemia, pneumonia, intracranial infection, and sepsis.
Statistical analysis
Statistical analysis was performed with SPSS 17.0 (SPSS Inc., Chicago, Illinois). Continuous variables were expressed as mean ± standard deviation and analyzed by the 2-sample t test. Categorical variables were expressed as counts (percentage) and analyzed by either the Pearson χ2 test or Fisher exact test. Predictors of AKI were assessed using univariate logistic regression analysis for narrowing down the potential factors and then multivariate logistic regression model analysis. Candidate predictor variables for univariate logistic regression analysis included all available demographics and baseline variables that had univariate associations (P < 0.15) with the occurrence of AKI. All variables having P < 0.05 from univariate logistic regression analyses were included in multivariate analysis, in which the backward stepwise multivariate regression was performed to create the final model whereby the least non-significant variables were removed from the model one by one, until all remaining variables had P < 0.05. The corresponding sensitivities and specificities of these variables were also calculated, using the best thresholds for WFNS grades, serum creatinine level on admission, and serum hs-CRP level on admission, which were derived from the receiver operating characteristic (ROC) curve analyses. Area under the curve (AUC) model performance was tested using the Z test. The percentage of patients surviving AKI for 14 days was calculated using Kaplan–Meier method, and the survival curves were drawn, which were compared using the log-rank test. P ≤ 0.05 was considered significant.
Results
Patient characteristics
One hundred sixty-four patients were included in this study and categorized into AKI and non-AKI groups. Demographics and baseline characteristics were compared between the two groups and results are shown in Table 1. Patients with AKI had significantly higher average hs-CRP level than those without (Table 1). The angiographic vasospasm and pneumonia occurrence rates of patients with AKI were significantly higher than those of patients without AKI (P = 0.030 and 0.044), respectively. Delayed cerebral ischemia occurrence rate and mortality of patients with AKI tend to be higher than those of patients without, although they were not significantly different.
AKI and hs-CRP level at admission
There were 17 patients (10.4%) who experienced AKI within 2 weeks of aSAH onset. Five variables, including the WFNS grade, diabetes, serum hs-CRP level, serum creatinine level, and antibiotic therapy, had univariate associations (P < 0.15) with the development of AKI (Table 1) and were put into univariate and multivariate model analyses (Tables 2 and 3).
After adjusting for possible confounding factors including the WFNS grade, diabetes, and serum creatinine level, the multivariate analysis showed that serum hs-CRP level and antibiotic therapy were both predictive factors (serum hs-CRP level: OR = 1.2 (1.1–1.3), P = 0.003; antibiotic therapy: 5.8 (1.6–20.7), P = 0.007) of AKI following aSAH (Table 3).
The sensitivity, specificity, and Youden index of serum hs-CRP level in predicting the development of AKI were derived by ROC curve analysis, and they were calculated to be 76.5% (13/17), 64.6% (95/147), and 0.411, respectively, based on the best threshold of 6.6 mg/L (Fig. 1). The AUC of serum hs-CRP level, WFNS grade, serum creatinine at admission, and antibiotic therapy was 0.72 (95%CI = 0.61–0.81), 0.61 (95%CI = 0.47–0.76), 0.65 (95%CI = 0.52–0.78), and 0.64 (95%CI = 0.51–0.78), respectively. Z tests revealed that there was no significant difference between the AUC of these parameters (serum hs-CRP level vs. WFNS grade: Z = 1.128, P = 0.259; serum hs-CRP level vs. serum creatinine: Z = 0.809, P = 0.418; serum hs-CRP level vs. antibiotic therapy: Z = 0.845, P = 0.398).
Although Z tests revealed that the AUCs of serum hs-CRP level, WFNS grade, serum creatinine level, and antibiotic therapy were still not significantly different (serum hs-CRP level vs. WFNS grade: Z = 1.128, P = 0.259; serum hs-CRP level vs. serum creatinine level: Z = 0.809, P = 0.418; serum hs-CRP level vs. antibiotic therapy: Z = 0.845, P = 0.398), it seemed that serum hs-CRP level had better performance in predicting the AKI following aSAH than all other three predictors with a higher AUC (Fig. 1).
AKI-free survival of patients with and without higher hs-CRP level
Among these patients in this cohort (n = 164), there were 65 patients (39.6%) who had serum hs-CRP level > 6.6 mg/L. Post hoc log-rank testing revealed that patients having serum hs-CRP > 6.6 mg/L had a significantly higher rate of AKI than those having hs-CRP ≤ 6.6 mg/L (20% (13/65) vs. 4.0% (4/99), P = 0.001) (Fig. 2).
Discussion
AKI is a severe non-neurologic complication of aSAH and usually occurs within 14 days of aSAH [21, 42]. The incidence of AKI in aSAH patients has increased in the past decade; furthermore, it is detrimental to patient outcomes and increases healthcare costs [33]. Therefore, identification of an easily measurable biomarker for predicting AKI might be helpful for risk mitigation. Our study found that the serum hs-CRP level at admission was significantly associated with AKI, and the most suitable threshold was 6.6 mg/L. Serum hs-CRP level had a sensitivity of 76.5% and a specificity of 64.6% for predicting the development of AKI on the basis of the best thresholds. The serum level of hs-CRP, an easily measurable biomarker, could reflect the degree of inflammatory response and predict neurologic and non-neurologic complications of aSAH, which might be able to indicate the risk of AKI occurrence and might be helpful in optimizing medical care.
In our analysis, the overall incidence of AKI was 10.4%. After adjusting for possible confounding factors including WFNS grade, diabetes, and serum creatinine level, the multivariate analysis revealed that serum hs-CRP and antibiotic therapy were the most significant factors associated with AKI following aSAH. Serum hs-CRP level might be a helpful predictive factor of AKI following aSAH, but considering its sensitivity of 76.5% and specificity of 64.6%, it might not be enough to use just serum hs-CRP for predicting AKI. Nevertheless, serum hs-CRP is still an important parameter to evaluate the occurrence of AKI, especially in several critical conditions where clinicians have to perform liberal glucose management, use corticosteroids, and use nephrotoxic antibiotics or dehydrants that could lead to kidney dysfunctions of patients. For those aSAH patients having serum hs-CRP > 6.6 mg/L, special attention should be paid during the treatment to prevent the occurrence of AKI. For example, liberal glucose management (> 220 mg/dL) is associated with increased risk of infection, which may lead to renal injury or use of antibiotics. A target glucose range of 80–140 mg/dL is recommended [13], but administration of high-dose corticosteroid is not recommended in acute SAH patients, as it may induce an electrolyte disorder and aggravate renal function [13].
Several research studies reported that abuse of nephrotoxic antibiotics or dehydrants could lead to kidney dysfunctions of patients in critical condition [24, 31]. Our results revealed that with all the antibiotics used for this cohort, vancomycin might result in a significant impact on AKI occurrence (P = 0.007, between the AKI group and non-AKI group). Treatment with vancomycin has been reported to induce acute interstitial nephritis and/or acute tubular necrosis [36]. The mechanism of serum hs-CRP level to predict the occurrence of AKI might be the following. (1) According to literature, systemic inflammatory response syndrome would occur in 29–87% aSAH patients, which has been correlated with the development and progression of extracerebral organ dysfunction [8], and the risk of the development of extracerebral organ dysfunction could be reflected by CRP as it indicates the degree of inflammatory response. (2) The CRP level at an early stage of aSAH can predict many kinds of neurologic and cardiorespiratory complications, such as cerebral vasospasm [20, 32], which is in a certain degree related to AKI. In this cohort, the occurrence rate of angiographic vasospasm of patients with AKI was significantly higher than that of patients without AKI (P = 0.030). For patients with cerebral angiospasm, there is a need for a more aggressive cerebrospinal fluid management to increase cerebral blood flow, osmotic agents are used to treat elevated intracranial pressure, and contrast media is used to confirm diagnosis, all of which could increase the burden of the kidney, thus explaining the association of cerebral angiospasm with AKI [2, 22]. Furthermore, patients who develop cerebral vasospasm may have altered endothelial functions, including the release of vasoregulatory mediators such as nitric oxide and endothelin, which may lead to changed regional perfusion regulation and potentially increase the risk of renal vasoconstriction [1, 6, 28, 34].
In this aSAH cohort, having diabetes, aging, and serum creatinine level at admission, which are often considered risk factors for the development of AKI, were not significantly associated with AKI following aSAH. Serum creatinine is the most frequently used parameter in the diagnosis of AKI. However, since it lags behind in the decline and recovery in glomerular filtration rate by days, serum creatinine is insensitive and considered an unreliable biomarker during short-term changes in kidney function. Moreover, serum creatinine is affected by multiple factors, including the age, race, muscle weight, volume of distribution, medications, and protein intake of the patient. Therefore, there is a need for more sensitive and specific biomarkers for earlier AKI diagnosis and rapid measurement of response to the therapy. Neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 are considered novel biomarkers for AKI. They may play a role in the future to help identify patients at risk or allow early detection of AKI, thus providing a window of opportunity for early therapeutic interventions [15, 26, 30]. However, due to insufficient clinical evidence, as well as lack of standard clinical testing methods, in the near future, actual application of these two factors will not be realized in a clinical setting [29]. On the other hand, serum hs-CRP level is a routine parameter measured in a clinical setting, widely available in hospitals of all tiers, has a standard clinical measurement protocol, and has become a regular admission test item for several diseases, which might be more clinically relevant. Compared with “standard sensitivity” CRP, the detection range of hs-CRP is 0.1–10 mg/L. CRP is limited in its sensitivity when the value is lower than 5 mg/L. According to the results, the best threshold is 6.6 mg/L for hs-CRP in predicting the development of AKI. The level of 6.6 mg/L is high enough for “standard sensitivity” CRP to be used. Thus, the detection of CRP can also be suitable for assessing AKI following SAH, because measurement of CRP is cheaper and more acceptable to become a routine item clinically compared with that of hs-CRP.
Significant improvements have been made in the neurologic management of aSAH in the past decade. Although the prognosis of aSAH has been significantly improved compared with what was 20 years ago, its 3-month death rate could still be as high as 30% [25]. To further improve the treatment results of aSAH patients, minimizing the non-neurologic complications associated with the high morbidity and mortality has become the focus of research [18, 37]. Our data showed that although not statistically significant, the 28-day mortality rate of patients with AKI tends to be higher than that of patients without AKI. Zacharia et al [43] reported that aSAH patients with renal failure had a 2-fold higher risk of 3-month poor outcome and significant increased risk of death. Therefore, if early prognosis of the risk of AKI could be achieved, optimized care and improved allocation of healthcare resources might be facilitated.
In the management of intracranial hypertension, hyperosmolar therapy with mannitol and hypertonic saline has been the cornerstone [19]. Research has shown that hyperosmolar therapy is a dangerous trigger of AKI [35], but in this study, mannitol had been used for almost all patients. Although it has been reported that contrast-induced nephropathy could be found in 3–8% of aSAH patients [5, 7, 39], the rate of exposure to intravenous contrast agents (CTA, MRA, and DSA) was also not different between the AKI and non-AKI groups in our patient population. Almost all patients had CTA, MRA, or DSA examination on the day of admission for diagnosis of the aneurysm and a comprehensive pre-surgery assessment. Moreover, the overall increased awareness of the risks of contrast exposures may be a contributing factor for clinical management.
There were still a number of limitations of this study. First, as a single-center observational study, the inherent limitations of analyzing it apply here. Future studies are expected to shed more light on the potential biomarker AKI. Second, the number of consecutive patients with SAH included in this study was not large enough, which makes statistical comparison difficult. Thus, data collected and results summarized should be analyzed carefully. Additional studies are needed to investigate the pathophysiologic processes and interventions to prevent the development of AKI following SAH. We anticipate future studies about this important non-neurologic complication following SAH.
Conclusions
The incidence rate of AKI was nearly 10% in aSAH patients included in this study. Delayed cerebral ischemia occurrence rate and mortality of patients with AKI tend to be higher than those of patients without in this cohort; however, they were not significantly different. Both elevated baseline serum hs-CRP level and antibiotic therapy used were significant factors independently associated with AKI following aSAH. Serum hs-CRP level at admission might be helpful as a predictor for the development of AKI. Further research is needed to determine whether changes in serum hs-CRP levels over time are related to the onset of AKI.
Change history
19 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00701-021-04734-1
Abbreviations
- hs-CRP:
-
High-sensitivity C-reactive protein
- AKI:
-
Acute kidney injury
- aSAH:
-
Aneurysmal subarachnoid hemorrhage
- CTA:
-
Computed tomography angiography
- DSA:
-
Digital subtraction angiography
- WFNS:
-
World Federation of Neurosurgical Societies
- MRA:
-
Magnetic resonance angiography
- CSF:
-
Cerebrospinal fluid
- KDIGO:
-
Kidney Disease Improving Global Outcomes
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
References
Ahmad A, Dempsey SK, Daneva Z, Azam M, Li N, Li P-L, Ritter JK et al (2018) Role of nitric oxide in the cardiovascular and renal systems. Int J Mol Sci 19:E2605
Al-Mufti F, Amuluru K, Damodara N, El-Ghanem M, Nuoman R et al (2018) Novel management strategies for medically-refractory vasospasm following aneurysmal subarachnoid hemorrhage. J Neurol Sci 390:44–51
Al-Mufti F, Misiolek KA, Roh D, Alawi A, Bauerschmidt A, Park S et al (2019) White blood cell count improves prediction of delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. Neurosurgery 84:397–403
Badjatia N, Cremers S, Claassen J, Connolly ES, Mayer SA, Karmally W, Seres D (2018) Serum glutamine and hospital-acquired infections after aneurysmal subarachnoid hemorrhage. Neurology 91:e421–e426
Bercker S, Winkelmann T, Busch T, Laudi S, Lindner D et al (2018) Hydroxyethyl starch for volume expansion after subarachnoid haemorrhage and renal function: results of a retrospective analysis. PLoS One 13:e0192832
Chang CZ, Wu SC, Kwan AL, Hwang SL, Howng SL (2011) Magnesium lithospermate B alleviates the production of endothelin-1 through an NO-dependent mechanism and reduces experimental vasospasm in rats. Acta Neurochir 153:2211–2217
Chavakula V, Gross BA, Frerichs KU, Du R (2013) Contrast-induced nephropathy in patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care 19:157–160
Chen S, Li Q, Wu H, Krafft PR, Wang Z, Zhang JH (2014) The harmful effects of subarachnoid hemorrhage on extracerebral organs. Biomed Res Int 2014:858496
Chen K, Wu Y, Wang Q, Wang J, Li X, Zhao Z, Zhou J (2015) The methodology and pharmacokinetics study of intraventricular administration of vancomycin in patients with intracranial infections after craniotomy. J Crit Care 30(218):e211–e215
Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J et al (2012) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 43:1711–1737
Csajbok LZ, Nylen K, Ost M, Sonander H, Nellgard B (2015) In-hospital C-reactive protein predicts outcome after aneurysmal subarachnoid haemorrhage treated by endovascular coiling. Acta Anaesthesiol Scand 59:255–264
Dhar R, Diringer MN (2008) The burden of the systemic inflammatory response predicts vasospasm and outcome after subarachnoid hemorrhage. Neurocrit Care 8:404–412
Diringer MN, Bleck TP, Claude Hemphill J, Menon D, Shutter L et al (2011) Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. J Neurocrit Care 15:211–240
Drenckhahn C, Brabetz C, Major S, Wiesenthal D, Woitzik J, Dreier JP et al (2013) Criteria for the diagnosis of noninfectious and infectious complications after aneurysmal subarachnoid hemorrhage in DISCHARGE-1. Acta Neurochir Suppl 115:153–159
Flechet M, Guiza F, Schetz M, Wouters P, Vanhorebeek I et al (2017) AKIpredictor, an online prognostic calculator for acute kidney injury in adult critically ill patients: development, validation and comparison to serum neutrophil gelatinase-associated lipocalin. Intensive Care Med 43:764–773
Frijns CJ, Fijnheer R, Algra A, van Mourik JA, van Gijn J, Rinkel GJ (2006) Early circulating levels of endothelial cell activation markers in aneurysmal subarachnoid haemorrhage: associations with cerebral ischaemic events and outcome. J Neurol Neurosurg Psychiatry 77:77–83
Frontera JA, Fernandez A, Schmidt JM, Claassen J, Wartenberg KE et al (2009) Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke 40:1963–1968
Hall A, O'Kane R (2018) The extracranial consequences of subarachnoid hemorrhage. World Neurosurg 109:381–392
Hauer EM, Stark D, Staykov D, Steigleder T, Schwab S, Bardutzky J (2011) Early continuous hypertonic saline infusion in patients with severe cerebrovascular disease. Crit Care Med 39:1766–1772
Jeon YT, Lee JH, Lee H, Lee HK, Hwang JW, Lim YJ, Park HP et al (2012) The postoperative C-reactive protein level can be a useful prognostic factor for poor outcome and symptomatic vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol 24:317–324
Kumar AB, Shi Y, Shotwell MS, Richards J, Ehrenfeld JM (2015) Hypernatremia is a significant risk factor for acute kidney injury after subarachnoid hemorrhage: a retrospective analysis. Neurocrit Care 22:184–191
Lazaridis C, Naval N (2010) Risk factors and medical management of vasospasm after subarachnoid hemorrhage. Neurosurg Clin N Am 21:353–364
Lee HG, Kim WK, Yeon JY, Kim JS, Kim KH, Jeon P, Hong SC (2018) Contrast-induced acute kidney injury after coil embolization for aneurysmal subarachnoid hemorrhage. Yonsei Med J 59:107–112
Luther MK, Timbrook TT, Caffrey AR, Dosa D, Lodise TP, LaPlante KL (2018) Vancomycin plus piperacillin-tazobactam and acute kidney injury in adults: a systematic review and meta-analysis. Crit Care Med 46:12–20
Mackey J, Khoury JC, Alwell K, Moomaw CJ, Kissela BM, Flaherty ML et al (2016) Stable incidence but declining case-fatality rates of subarachnoid hemorrhage in a population. Neurology 87:2192–2197
McWilliam SJ, Antoine DJ, Jorgensen AL, Smyth RL, Pirmohamed M (2018) Urinary biomarkers of aminoglycoside-induced nephrotoxicity in cystic fibrosis: kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin. Sci Rep 8:5094
Mizuno T, Sato W, Ishikawa K, Shinjo H, Miyagawa Y, Noda Y et al (2012) KDIGO (Kidney Disease: Improving Global Outcomes) criteria could be a useful outcome predictor of cisplatin-induced acute kidney injury. Oncology 82:354–359
Mukerji SS, Buchbinder BR, Singhal AB (2015) Reversible cerebral vasoconstriction syndrome with reversible renal artery stenosis. Neurology 85:201–202
Prowle JR (2015) Measurement of AKI biomarkers in the ICU: still striving for appropriate clinical indications. Intensive Care Med 41:541–543
Rampoldi B, Tessarolo S, Giubbilini P, Gaia P, Corino SD et al (2018) Neutrophil gelatinase-associated lipocalin and acute kidney injury in endovascular aneurysm repair or open aortic repair: a pilot study. Biochem Med (Zagreb) 28:010904
Rennie TJW, De Souza N, Donnan PT et al (2018) Risk of acute kidney injury following community prescription of antibiotics: self-controlled case series. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfy187
Romero FR, Cataneo DC, Cataneo AJ (2014) C-reactive protein and vasospasm after aneurysmal subarachnoid hemorrhage. Acta Cir Bras 29:340–345
Rumalla K, Mittal MK (2016) Acute renal failure in aneurysmal subarachnoid hemorrhage: nationwide analysis of hospitalizations in the United States. World Neurosurg 91:542–547
Salem S, Jankowski V, Asare Y, Liehn E, Welker P, Raya-Bermudez A et al (2015) Identification of the vasoconstriction-inhibiting factor (VIF), a potent endogenous cofactor of angiotensin II acting on the angiotensin II type 2 receptor. Circulation 131:1426–1434
Sam R, Hart P, Haghighat R, Ing TS (2012) Hypervolemic hypernatremia in patients recovering from acute kidney injury in the intensive care unit. Clin Exp Nephrol 16:136–146
Sawada A, Kawanishi K, Morikawa SA, Davey P, Dreischulte T, Bell S (2018) Biopsy-proven vancomycin-induced acute kidney injury: a case report and literature review. BMC Nephrol 19:72
Schuiling WJ, Dennesen PJ, Rinkel GJ (2005) Extracerebral organ dysfunction in the acute stage after aneurysmal subarachnoid hemorrhage. Neurocrit Care 3:1–10
Shah SR, Tunio SA, Arshad MH, Moazzam Z, Noorani K, Feroze AM et al (2015) Acute kidney injury recognition and management: a review of the literature and current evidence. Glob J Health Sci 8:120–124
Shen J, Karki M, Jiang T, Zhao B (2018) Complications associated with diagnostic cerebral angiography: a retrospective analysis of 644 consecutive cerebral angiographic cases. Neurol India 66:1154–1158
Srinivasan A, Aggarwal A, Gaudihalli S, Mohanty M, Dhandapani M, Singh H et al (2016) Impact of early leukocytosis and elevated high-sensitivity C-reactive protein on delayed cerebral ischemia and neurologic outcome after subarachnoid hemorrhage. World Neurosurg 90:91–95
Tam AK, Ilodigwe D, Mocco J, Mayer S, Kassell N, Ruefenacht D, Schmiedek P et al (2010) Impact of systemic inflammatory response syndrome on vasospasm, cerebral infarction, and outcome after subarachnoid hemorrhage: exploratory analysis of CONSCIOUS-1 database. Neurocrit Care 13:182–189
Tujjar O, Belloni I, Hougardy JM, Scolletta S, Vincent JL, Creteur J et al (2017) Acute kidney injury after subarachnoid hemorrhage. J Neurosurg Anesthesiol 29:140–149
Zacharia BE, Ducruet AF, Hickman ZL, Grobelny B, Fernandez L et al (2009) Renal dysfunction as an independent predictor of outcome after aneurysmal subarachnoid hemorrhage: a single-center cohort study. Stroke 40:2375–2381
Acknowledgments
We are grateful to all colleagues who participated in providing cases (Dr. Jiawei Cai and Dr. Fuxin Lin).
Funding
This study was supported by grants from the Key Clinical Specialty Discipline Construction Program of Fujian, P.R.C., and the major project of Fujian Provincial Department of Science and Technology (No. 2014YZ0003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
De-Zhi Kang received grants from the Key Clinical Specialty Discipline Construction Program of Fujian, P.R.C., and the major project of Fujian Provincial Department of Science and Technology (No. 2014YZ0003). All other authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All procedures in the study were approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University (Fujian, China).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Comments
This paper reports an association between admission plasma levels of high-sensitivity C-reactive protein and development within 14 days of acute kidney injury in patients with aneurysmal subarachnoid haemorrhage. This is an interesting observation, which warrants consideration regarding the mechanism of association. However, as a clinician I probably would not add this relatively expensive test to my prognostic toolbox at this time.
Kirsten Moeller
Copenhagen, Denmark
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Vascular Neurosurgery - Aneurysm
Rights and permissions
About this article
Cite this article
Yang, BH., He, Q., Ding, CY. et al. High-sensitivity C-reactive protein as a predictive factor of acute kidney injury following aneurysmal subarachnoid hemorrhage: a prospective observational study. Acta Neurochir 161, 1783–1791 (2019). https://doi.org/10.1007/s00701-019-04006-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-019-04006-z