Abstract
Background
Surgery in the semi-sitting position is susceptible to changes in motor (MEP) and somatosensory evoked potentials (SEPs), which are not related to neurological impairment. These changes have been suggested to be caused by the insulating effect of subdural air collection. This study sought to investigate the correlation of MEP and SEP final-to-baseline amplitude ratios to postoperative volumetry of frontoparietal subdural air collection.
Methods
Median nerve SEP and hand MEP findings of 47 patients operated on in the semi-sitting position were compared with 7 patients operated on in the supine position. Computed tomography was routinely performed on the 1st postoperative day in all patients, and subdural air volumetry was calculated. Final-to-baseline MEP and SEP amplitude ratios were calculated and correlated to subdural air volumetry.
Findings
SEP changed in 12 patients, and MEP changed in 7 patients. Postoperative subdural air collections were significantly different between the groups (semi-sitting group, mean 31.2 cm3; supine group, mean 2 cm3; p = 0.000). For the SEP ratios, a moderate negative correlation with subdural volumetry was found in the semi-sitting group (p = 0.044). Conversely, there was no correlation in the subset of patients with SEP attenuation (p = 0.846). As concerns the MEP ratios, no correlation was demonstrated in any group (semi-sitting, p = 0.967; supine, p = 0.193).
Conclusions
Although SEP amplitude reductions were associated with large subdural air collections, this was not observed in the subset of patients with SEP attenuation and for the MEP monitoring, suggesting other pathophysiological mechanisms, such as brain shift, for the artificial amplitude reduction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since its introduction, intraoperative evoked potential monitoring (IOM) has become a routine measurement during several neurosurgical procedures because of improvements in surgical results and reduction in surgical-related neurological morbidity [13, 19, 30]. Motor (MEP) and somatosensory evoked potentials (SEPs) are now accepted techniques for monitoring both motor and sensory functional integrity, in particular when used concurrently [13]. The basic principle of IOM is the identification of an imminent neurological injury in a timely fashion to reverse the damaging process [30]. Thus, false-positive alarms are of concern because of unnecessary surgical prolongation [23, 24, 30]. While false-negative findings (no IOM changes and postoperative neurological deficit) have been well studied and mostly attributed to lesions outside the monitored pathways [30, 31], false-positive findings (IOM changes and no postoperative neurological deficit) have been related to the use of the patient’s semi-sitting position [30] or possibly to evoked potential fading [10].
Some authors have advocated that surgery in the semi-sitting position provides particular advantages, such as a reduction of venous pressure, a clean surgical field due to facilitated drainage of blood and cerebrospinal fluid (CSF) by gravity, as well as easier hemostasis [7, 17, 25]. Disadvantages have also been discussed, namely hemodynamic instability, air embolism, and pneumocephalus [17].
Previous studies have shown that intraoperative MEP and SEP monitoring during surgery in the semi-sitting position is susceptible to changes that are not related to neurological impairment [7, 8, 11, 12, 16, 21, 22, 27–29].These changes have been suggested to be most likely caused by the insulating effect of subdural air collection [7, 8, 11, 12, 16, 20–22, 27–29]. We hypothesised that, this being the case, a negative correlation is expected so that the larger the supratentorial pneumocephalus is, the lower the final MEP and SEP amplitudes. This study sought to investigate the correlation of MEP and SEP final-to-baseline amplitude ratios to postoperative volumetry of frontoparietal subdural air collection in neurologically intact patients.
Methods
Fifty-four patients (23 men and 31 women) undergoing posterior fossa surgery were prospectively enrolled and retrospectively evaluated between August 2006 and August 2007 at the Department of Neurosurgery, Eberhard Karls University Hospital, Tübingen, Germany. Patients gave informed consent for intraoperative monitoring. This study was approved by the local ethics committee.
Median nerve SEP and hand MEP recordings of 47 patients operated on in the semi-sitting position (group 1) were compared with 7 patients operated in the supine position (group 2) (Table 1). Patients were placed in the semi-sitting position with the head hyper-extended, turned 30° toward the affected side, and flexed. The legs were raised to or above the level of the heart, and the knees were slightly flexed, whereas patients operated on in the ventral or dorsal position were generally regarded as being in the supine position.
Some of these data were partially reported in a previous publication in which we investigated the success rates of orbicularis oculi and orbicularis oris facial MEP (FMEP) for facial nerve monitoring and their usefulness in predicting the immediate postoperative facial function using only final-to-baseline FMEP ratios during cerebellopontine angle surgeries [1].
Anaesthetic protocol and intraoperative monitoring
Anaesthesia was induced with thiopental and subsequent infusion of sufentanil and rocuronium, and was maintained with a continuous infusion of remifentanil and propofol. In the operating theatre, patients underwent routine and continuous monitoring, which included electrocardiography, body temperature, a central venous catheter in the right atrium for air embolus aspiration, radial artery catheter to permit invasive blood pressure monitoring, haemodynamic parameters (systolic and diastolic pressure, heart rate), mean arterial blood pressure, pulse oximetry, and capnography with end-tidal CO2. A precordial Doppler ultrasonography device was attached to recognise air embolus. Recently, air emboli have been monitored by using transesophageal echocardiography.
Multimodal monitoring [SEP, MEP, brainstem auditory evoked potential (BAEP), and electromyography (EMG)] were continuously performed by an experienced electrophysiological team (Endeavor, Viasys Healthcare, Madison, WI). For large tumours that reached the lower cranial nerves, EMGs of the glossopharyngeal, vagus, accessorius, and hypoglossal nerves were also monitored. These data were not considered simultaneously for this study.
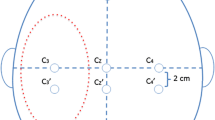
Bilateral SEPs were always used for patient positioning. SEPs were measured after median nerve stimulation by using square-wave electrical pulses with 200-μs duration and 16–25-mA intensity delivered at a 5.1-Hz stimulation rate by placing surface electrodes on the wrist. Recording electrodes were placed in the parietal area at C3′, C4′, and FZ according to the International 10–20 EEG System. SEP waveforms were obtained by using a 10–250-Hz bandpass filter, 50-ms analysis time, and 300 to 500 sweeps. After positioning, unilateral SEPs were obtained in accordance with the surgical side.
Hemispheric transcranial electrocortical stimulation (TES) was performed using corkscrew-like electrodes, which were inserted into the scalp and positioned at CZ and C3 or C4, according to the International 10-20 EEG system for left or right side stimulation, respectively. Constant-voltage stimulation was always applied contralaterally to the affected side using three or five rectangular pulses, ranging from 160 to 600 V (mean 385 V) with a 50-μs pulse duration and an interstimulus interval (ISI) of 2 ms. Bandpass filters (150–3,000 Hz) were used to attain waveforms. The impedance of all electrodes was maintained below 5 kOhm. Mild changes in voltage intensity (20–160 V, mean 50 V) were used in some patients to achieve better MEP responses because of progressive anaesthesia-induced suppression of motor neuronal activity (evoked potential fading) [10, 11]. Hand MEPs were recorded from paired needles inserted in the contralateral abductor pollicis brevis (APB) muscle. TES was intermittently performed with BAEP and SEP recordings.
MEP and SEP latencies were defined as the time from stimulus onset to the first wave deflection. For this study, latencies were not evaluated because of previous observations that IOM changes in the semi-sitting position affect amplitude exclusively [27]. MEP and SEP amplitudes were defined as the voltage between the maximum positive peak and the maximum negative peak of the waveforms. SEP baseline measures were recorded during positioning and the best amplitude response before dural opening was considered as the MEP baseline value. MEP and SEP final measures were recorded at dural closure. Final-to-baseline MEP and SEP amplitude ratios were calculated and correlated to postoperative subdural air volumetry. MEP and SEP amplitude ratio reduction of more than 50% was considered abnormal.
Postoperative imaging and volumetric measurements
Cranial computed tomography (CT) was performed routinely on the 1st postoperative day in all patients, and volumetry of frontoparietal subdural air collection was calculated. Intracranial air volumes were determined by a semi-automatic 3D region growing approach implemented in a previously described and validated computer program [4, 5]. Shortly, CT scans were converted into 3D volumes and saved in Analyze format. Using a manually defined seed point, a 3D region-growing algorithm with an upper density limit of -800 HU was used to obtain the number of voxels containing air, which was then multiplied by the voxel volume to calculate intracranial air volumes. The radiologist (TKH) was blinded to the surgical positioning and intraoperative patient data.
Postoperatively, all patients underwent neurological examination by a staff member who was not directly involved in IOM recordings. Only postoperative neurologically intact patients at discharge were included in this study. Collected data included patient age, gender, surgical positioning, baseline MEP amplitude, baseline SEP amplitude, final-to-baseline MEP amplitude ratio, final-to-baseline SEP amplitude ratio, operation time, and postoperative volumetry of frontoparietal subdural air collection.
Statistical analysis
Statistical analyses were performed with SPSS 13.0 (SPSS, Inc., Chicago, IL). Categorical variables were compared by chi-square analysis. Non-categorical variables were compared by Mann-Whitney U test. Nonparametric Spearman correlation coefficients were calculated to evaluate the correlation between MEP and SEP final-to-baseline amplitude ratios with postoperative frontoparietal subdural air volume. All comparisons were two-tailed, and the level of significance was set at p < 0.05.
Results
Both groups were matched in age, gender, MEP and SEP amplitude ratio, as well as operation time (Table 1). Supratentorial pneumocephalus was encountered in the postoperative radiological evaluation in 44 patients (93.6%) in group 1 and 4 patients (57.1%) in group 2. Postoperative frontoparietal subdural air collections were significantly different between groups (Table 1). Three patients in group 1 were affected by tension pneumocephalus and underwent needle trephination for evacuation of the subdural air (patients 5, 8 and 25) (Table 2) (Fig. 1). SEP amplitude reduction was observed in only one of the three patients (no. 8) (Table 2).
Intraoperative SEP (a) and MEP (b) recordings in patient 8 (Table 2) obtained before dural opening (baseline, left) and at the end of the surgery (final, right). This patient underwent surgery while in the semi-sitting position (group 1) for brainstem cavernoma resection. SEP and MEP final-to-baseline amplitude ratios were 42% and 87%, respectively. Postoperatively, a tension pneumocephalus was diagnosed clinically and radiologically (c). The patient was then submitted to needle trephination for air evacuation. Bone window cranial CT (c) revealed an extensive pneumocephalus of 145 cm3 in the air volumetry. Note that air in the ventricle was not included in the volumetric measurement. The absolute amplitude values are represented in parentheses (microvolts for SEP and millivolts for MEP)
MEP and SEP amplitude ratio reduction (greater than 50%) unrelated to postoperative neurological impairment was, almost exclusively, observed in patients operated on in the semi-sitting position. MEP amplitude attenuation occurred in 6 patients (12.8%) in group 1 and 1 patient (14.3%) in group 2, while a decrease in SEP amplitude was observed in 12 patients (25.5%) in group 1 and none in group 2. MEP loss was documented in only one patient (no. 22, group 1) (Table 2).
For the SEP amplitude ratios, a moderate negative correlation with subdural volumetry was found in group 1 (r = - 0.299, p = 0.044), whereas in group 2, the correlation was positive (r = 0.852, p = 0.015). Conversely, there was no correlation between MEP amplitude ratios and subdural volumetry in any of the groups (group 1, r = - 0.006, p = 0.967; group 2, r = 0.616, p = 0.193) (Fig. 2). Although the SEP amplitude ratio was correlated with subdural air volume when group 1 was analysed as a whole, there was no correlation in the subset of patients who developed amplitude changes as demonstrated in Fig. 3. Finally, duration of the surgical procedure had no correlation with postoperative pneumocephalus in either group (group 1, r = 0.171, p = 0.251; group 2, r = - 0.037, p = 0.937).
Correlation between final-to-baseline SEP (a) and MEP (b) amplitude ratios and postoperative subdural air volume in the semi-sitting group. A moderate negative correlation was found only for the SEP monitoring, in which the higher the amplitude ratio was, the lower the frontoparietal subdural air volumetry (r = - 0.299, p = 0.044)
Correlation between final-to-baseline SEP (a) and MEP (b) amplitude ratios and postoperative subdural air volume in the patients who developed amplitude attenuation at the end of the surgical procedure (amplitude ratio <50%). No correlation between the SEP and MEP amplitude ratio and size of the pneumocephalus was found, indicating random distribution in such patients (A, r = 0.063, p = 0.846; B, r = 0.000, p = 1.000)
Discussion
Surgeries in the sitting position are prone to the occurrence of IOM false-positive alarms resulting in limited application of MEP and SEP monitoring. This phenomenon was first described by McPherson et al. [12] and Schubert et al. [21]. Several case reports [16, 27] followed, including one study of MEP observations [7]. The largest report in the field was provided by Wiedemayer et al. in 2002 [28], in which the authors confirmed previous observations of the occurrence of this phenomenon, specifically SEP amplitude attenuation; however, no postoperative imaging was performed.
Initially, a decrease in evoked potential amplitude was attributed to mechanical disturbances caused by surgical manoeuvres, or to a temperature decrease in the brainstem or upper cervical cord [7, 27]. The absence of IOM changes in the lateral position and of neurological deterioration postoperatively together with a temporal relationship to dural or cisternal opening practically excluded these theories [7, 21, 27, 28].
The overall finding of subdural air in the intraoperative or postoperative imaging of such patients directed the pathogenesis of IOM changes to the insulating effect of air [7, 12, 16, 21, 27, 28]. Watanabe et al. [27] suggested that air collection displaced the N20 generator of SEP waveforms. In addition, the observation of a decrease in exclusive amplitude with unchanged latency indicated disturbances in the electrostatic conduction property [27]. Immediate recovery of MEP and SEP waveforms following adoption of the supine position gave further strength to attributing these changes to subdural air [7, 27, 28].
Based on the results of our study, we observed that these considerations are not consistent.
-
First, if subdural air is responsible for this phenomenon, one would not expect only a subgroup of patients harbouring pneumocephalus to develop such IOM changes.
-
Second, MEP changes had no correlation to the volume of frontoparietal subdural air collection.
-
Third, SEP changes had a moderate negative correlation to postoperative air volume when analysing group 1 as a whole; however, there was no correlation in the subset of patients who developed SEP amplitude changes, indicating a random distribution of subdural air volumetry and evoked potential attenuation.
-
Fourth, IOM changes were rarely observed in patients affected by tension pneumocephalus.
-
Finally, recovery of waveforms after placing patients in the supine position may be due to the return of the displaced motor and somatosensory cortex to the proximity of the scalp electrodes.
Pneumocephalus is an invariable result of intracranial surgery [17]. As surgery proceeds, CSF is drained progressively, giving rise to the potential subdural space, which may become filled with air [9, 17]. This phenomenon was described by Lunsford et al. [9] as analogous to an “inverted pop bottle” in which air bubbles to the top of the subdural space, especially in patients operated on in the sitting position, because of the increased gravitational effect on CSF drainage [17, 26]. Supratentorial pneumocephalus is then practically a sine qua non condition in the sitting position [26], as the present study has confirmed.
On the other hand, brain deformation, also known as brain shift, is a well-recognised phenomenon that occurs during intracranial surgeries as a consequence of surgical manipulation [14]. Brain shift may suffer influences from tissue characteristics, size of the tumour, extent of the tumour resection, brain swelling, patient positioning, use of brain retractors, and administration of diuretic medication, amongst other influences [3, 14, 18, 24]. It has been extensively studied in the context of neuronavigation equipment in order to improve application accuracy, especially by using intraoperative magnetic resonance imaging (MRI) [3, 6, 14, 18].
Brain deformation has at least two major components, namely surface shift and subsurface shift [2, 14, 18]. Cortical or surface shift is detected mostly by inward cortical movement of more than 7 mm in patients undergoing surgery in the supine position [14] and seems to be associated with CSF drainage from the subarachnoid space and general brain sinking [2]. Nimsky et al. [14] observed that the direction of brain shift is mainly influenced by the direction of gravity. By placing the patient in the sitting position, a sudden fall in the supratentorial epidural pressure can be observed [15]. A further fall in pressure can be demonstrated after the opening of the posterior fossa dura or of the cisterna magna [15]. Thus, patients operated on in the sitting position are also susceptible to inward cortical movement as a result of gravity and CSF drainage.
The amount of brain shift has a temporal relationship with the opening of the dura so that cortical displacement is already observed immediately after dural splitting but is greater at the end of the surgical procedure [3, 6, 7, 18]. This temporal relationship may be correlated to the time course observed for the development of MEP and SEP attenuation that occurs after a variable period of time following dural opening and remains until the end of surgery [7, 12, 16, 21, 22, 27, 29].
The causes, extent, and biomechanical processes underlying brain shift are still poorly understood as brain deformation is a dynamic process that is difficult to predict because of its great variability [3, 6, 7]. As pneumocephalus and surface shift share the same mechanisms, we suggest that gravity promotes cortical displacement after the opening of the dura and the “potential” subdural space is occupied by air, giving the false impression that subdural air is playing a role in the artefactual intraoperative evoked potential changes. As soon as the patient is placed in the supine position, the cortical surface comes into close contact with the scalp electrodes, increasing the amplitude rapidly to the baseline level. This assumption is further supported by the observation that brain shift can also affect other IOM techniques by moving the cortical surface away from the recording electrodes, thus leading to inaccurate recordings not directly caused by motor pathway injury [23, 24].
Interestingly, subdural air volumetry exerted different effects on intraoperative MEP and SEP recordings. SEP ratios showed a significant correlation with the amount of frontoparietal subdural air, even though it was not observed for the SEP attenuation subgroup and for the MEP monitoring. Distinct electrophysiological principles may contribute to such a discrepancy as SEP reflects localised cortical potentials after peripheral electrical stimulation (mean amplitude, 2 μV), whereas MEP reflects peripheral muscle potentials after central electrical stimulation (mean amplitude, 1000 μV). A slight SEP amplitude reduction therefore has a greater impact on SEP ratios than MEP amplitude reduction such that an absolute deterioration of 1 μV acts as a 50% SEP ratio reduction.
Moreover, we hypothesise that subdural air volumetry may indirectly represent a brain shift, thereby justifying the significant correlation of SEP, but to a certain level that is not sufficient to reach clinically relevant thresholds. This finding further restricts the role of pneumocephalus in artificial amplitude reduction since brain deformation is rather unpredictable.
It therefore remains unclear whether this phenomenon is caused by subdural air collection or brain deformation. Our study cannot solve the issue completely. However, we have confirmed that an air collection is not the main factor influencing intraoperative evoked potential changes. It is even possible that current methods of intraoperative electrophysiological monitoring and intraoperative imaging do not yet allow us to answer this question definitely. In obvious respects, only studies documenting intraoperative images, either CT scans or MR imaging, performed while in the semi-sitting position and ultimately after the adoption of the supine position would be able to detect brain movements as a result of brain shift, thereby confirming or excluding our hypothesis.
Conclusions
Our results demonstrate that SEP and MEP recordings may have limitations to their interpretation during surgery in the semi-sitting position according to previous studies. It has not been completely clarified why only a subgroup of patients operated on in the semi-sitting position develops such IOM changes. Although SEP amplitude reductions were associated with large subdural air collections, this was not observed for the subset of patients with SEP attenuation and for the MEP monitoring, suggesting other pathophysiological mechanisms, such as brain shift, for the artificial amplitude reduction. Further studies with intraoperative imaging in both positions are necessary to elucidate this issue definitely.
References
Acioly MA, Liebsch M, Carvalho CH, Gharabaghi A, Tatagiba M (2010) Transcranial electrocortical stimulation to monitor the facial nerve motor function during cerebellopontine angle surgery. Neurosurgery 66:ONS354–ONS362
Black PM (2000) Quantification of, visualization of, and compensation for brain shift using intraoperative magnetic resonance imaging. Neurosurgery 47:1079
Dorward NL, Alberti O, Velani B, Gerritsen FA, Harkness WF, Kitchen ND, Thomas DG (1998) Postimaging brain distortion: magnitude, correlates, and impact on neuronavigation. J Neurosurg 88:656–662
Gröschel K, Hauser TK, Luft A, Patronas N, Dichgans J, Litvan I, Schulz JB (2004) Magnetic resonance imaging-based volumetry differentiates progressive supranuclear palsy from corticobasal degeneration. Neuroimage 21:714–724
Hauser TK, Luft A, Skalej M, Nägele T, Kircher TT, Leube DT, Schulz JB (2006) Visualization and quantification of disease progression in multiple system atrophy. Mov Disord 21:1674–1681
Hill DL, Maurer CR Jr, Maciunas RJ, Barwise JA, Fitzpatrick JM, Wang MY (1998) Measurement of intraoperative brain surface deformation under a craniotomy. Neurosurgery 43:514–528
Kombos T, Suess O, Pietilä T, Brock M (2000) Subdural air limits the elicitation of compound muscle action potentials by high-frequency transcranial electrical stimulation. Br J Neurosurg 14:240–243
Levy WJ Jr (1987) Clinical experience with motor and cerebellar evoked potential monitoring. Neurosurgery 20:169–182
Lunsford LD, Maroon JC, Sheptak PE, Albin MS (1979) Subdural tension pneumocephalus. Report of two cases. J Neurosurg 50:525–527
Lyon R, Feiner J, Lieberman JA (2005) Progressive suppression of motor evoked potentials during general anesthesia: the phenomenon of “anesthetic fade”. J Neurosurg Anesthesiol 17:13–19
MacDonald DB (2006) Intraoperative motor evoked potential monitoring: overview and update. J Clin Monit Comput 20:347–377
McPherson RW, Toung TJ, Johnson RM, Rosenbaum AE, Wang H (1985) Intracranial subdural gas: a cause of false-positive change of intraoperative somatosensory evoked potential. Anesthesiology 62:816–819
Neuloh G, Schramm J (2002) Intraoperative neurophysiological mapping and monitoring for supratentorial procedures. In: Deletis V, Shils JL (eds) Neurophysiology in neurosurgery: a modern intraoperative approach. Academic Press, San Diego, pp 339–401
Nimsky C, Ganslandt O, Cerny S, Hastreiter P, Greiner G, Fahlbusch R (2000) Quantification of, visualization of, and compensation for brain shift using intraoperative magnetic resonance imaging. Neurosurgery 47:1070–1080
Nornes H, Magnaes B (1971) Supratentorial epidural pressure recorded during posterior fossa surgery. J Neurosurg 35:541–549
Paisansathan C, Koenig HM, Wheeler PJ, Baughman VL, Hoffman WE (2003) Loss of SSEP during sitting craniotomy. J Neurosurg Anesthesiol 15:327–329
Porter JM, Pidgeon C, Cunningham AJ (1999) The sitting position in neurosurgery: a critical appraisal. Br J Anaesth 82:117–128
Reinges MH, Nguyen HH, Krings T, Hütter BO, Rohde V, Gilsbach JM (2004) Course of brain shift during microsurgical resection of supratentorial cerebral lesions: limits of conventional neuronavigation. Acta Neurochir (Wien) 146:369–377
Sala F, Manganotti P, Tramontano V, Bricolo A, Gerosa M (2007) Monitoring of motor pathways during brain stem surgery: what we have achieved and what we still miss? Neurophysiol Clin 37:399–406
Schramm J, Watanabe E, Strauss C, Fahlbusch R (1989) Neurophysiologic monitoring in posterior fossa surgery. I. Technical principles, applicability and limitations. Acta Neurochir (Wien) 98:9–18
Schubert A, Zornow MH, Drummond JC, Rosenbaum AE, Wang H (1986) Loss of cortical evoked responses due to intracranial gas during posterior fossa craniectomy in the seated position. Anesth Analg 65:203–206
Sloan T (2010) The incidence, volume, absorption, and timing of supratentorial pneumocephalus during posterior fossa neurosurgery conducted in the sitting position. J Neurosurg Anesthesiol 22:59–66
Suess O, Kombos T, Suess S, Stendel R, Pietilae T, Brock M (2001) The influence of intra-operative brain shift on continuous cortical stimulation during surgery in the motor cortex—an illustrative case report. Acta Neurochir (Wien) 143:621–623
Suess O, Kombos T, Ciklatekerlio O, Stendel R, Suess S, Brock M (2002) Impact of brain shift on intraoperative neurophysiological monitoring with cortical strip electrodes. Acta Neurochir (Wien) 144:1279–1289
Tatagiba M, Acioly MA (2008) Retrosigmoid approach to the posterior and middle fossae. In: Ramina R, Pires Aguiar PH, Tatagiba M (eds) Samii’s Essentials in Neurosurgery. Springer, Stuttgart, pp 137–154
Toung TJ, McPherson RW, Ahn H, Donham RT, Alano J, Long D (1986) Pneumocephalus: effects of patient position on the incidence and location of aerocele after posterior fossa and upper cervical cord surgery. Anesth Analg 65:65–70
Watanabe E, Schramm J, Schneider W (1989) Effect of a subdural air collection on the sensory evoked potential during surgery in the sitting position. Electroencephalogr Clin Neurophysiol 74:194–201
Wiedemayer H, Schaefer H, Armbruster W, Miller M, Stolke D (2002) Observations on intraoperative somatosensory evoked potential (SEP) monitoring in the semi-sitting position. Clin Neurophysiol 113:1993–1997
Wiedemayer H, Sandalcioglu IE, Regel J, Armbruster W, Schaefer H, Stolke D (2003) Enhanced stability of somatosensory evoked potentials attained in the median nerve by using temporal electrodes for intraoperative recording in patients in the semisitting position. J Neurosurg 99:986–990
Wiedemayer H, Sandalcioglu IE, Armbruster W, Regel J, Schaefer H, Stolke D (2004) False negative findings in intraoperative SEP monitoring: analysis of 658 consecutive neurosurgical cases and review of published reports. J Neurol Neurosurg Psychiatry 75:280–286
Zhou HH, Kelly PJ (2001) Transcranial electrical motor evoked potential monitoring for brain tumour resection. Neurosurgery 48:1075–1081
Acknowledgments
We gratefully acknowledge Ronir Raggio Luiz, MD, for his assistance with statistical analyses. None of the authors have received financial support in the generation of this article. The authors have no personal or institutional interest in any of the drugs, materials, or devices described in this article.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Acioly, M.A., Ebner, F.H., Hauser, T.K. et al. The impact of subdural air collection on intraoperative motor and somatosensory evoked potentials: fact or myth?. Acta Neurochir 153, 1077–1085 (2011). https://doi.org/10.1007/s00701-011-0960-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-011-0960-2