Abstract
Purpose
To elucidate the question whether expansion of pituitary adenomas into the cavernous sinus (CS) has to be regarded as focal penetration rather than invasion, a microanatomical study of the medial wall (MW) of the CS was performed.
Method
Fourteen sellar hemiblocks underwent microsurgical dissection from lateral and medial approach. The thickness of the MW of the CS was examined by diaphanoscopy.
Findings
The internal carotid artery (ICA) was adherent to the MW in five cases. In five specimens the lateral wall of the sella turcica consisted of a single layer without perforations. In nine cases this wall had two layers. There was no perforation of both layers in any case. Diaphanoscopy revealed thin MW in the lateral border of the sella (n = 13), below the horizontal segment of the ICA (n = 10), and antero-inferiorly to the carotid syphon (n = 9).
Conclusions
Expansion into the CS may be facilitated by low anatomical resistance against chronic tumor growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently it has been stated that the medial wall of the cavernous sinus (CS) is significantly thinner than its superior and lateral wall, and small defects can be detected histologically. Expansion of pituitary adenomas into the cavernous sinus could therefore be regarded as focal penetration of weak anatomical structures rather than invasion by aggressive tumors [32]. This still is a matter of debate, since several biological markers of pituitary adenomas correlating with an aggressive growth pattern have been described, such as overexpression of metalloproteinases [9, 10, 19] and increased expression of Ki-67 [21]. These data, however, were not confirmed by others [8, 15, 29, 32]. On the other hand, three weak areas of the covering of the CS, which could serve as gates for tumor entry, have been described: the venous channels along the superior orbital fissure, the meningeal coverings of the entry point of the oculomotor and trigeminal nerves, and the histologically loose lateral wall of the sellar compartment [11]. In case of intracavernous extension of nonmeningeal tumors, those of the medial wall have the worst surgical results [22]. In non-functioning pituitary adenoma, tumor invasion of the CS is associated with an unfavorable surgical outcome [20].
Against this background a microanatomical study of the lateral wall of the pituitary fossa was performed.
Materials and methods
Fourteen formaline-fixed sellar hemiblocks (eight right-sided, six left-sided) were obtained from eight whole-body donors (six females, two males, age 60–96 years, mean 79 years, fixation by pressure perfusion with formaldehyde 4%, storage in formaldehyde 4%): the medial edge of the samples was at the midline, the anterior edge anterior to the optic canal, the lower edge parallel to the palate, the lateral edge through the foramen spinosum, and the posterior edge posterior to the petrous apex.
The specimens were pinned to a two-level mounting with an individual, wooden template for each sample. To prevent unintended translucency during diaphanoscopy, black plasticine was used to cover the margins of the samples. A halogen light source (3 × 20 W) in the lower plane enabled alternating microscopic views either with coaxial light or with diaphanoscopy [video documentation, inserted ruler (mm)]. First, the bone of the sphenoid was rarefied by drilling and removed in an eggshell fashion without damaging the connective tissue (Fig. 1). Then the specimens underwent stepwise microsurgical dissection alternating from a lateral to medial approach, including resection of the cavernous part of the internal carotid artery (ICA, Fig. 2). Thickness of the medial wall (MW) of the cavernous sinus (CS) was evaluated by examination of translucency of the connective tissue layers by diaphanoscopy. In case of bright translucency, water tightness of the sample was examined by application of water to its upper side. For interpretation, color printouts of videoscreen shots were used both in episcopic and diaphanoscopic mode. Areas of translucency were traced to transparent foils. According to their individual relationship to the floor and anterior wall of the sella as well as to the intracavernous course of the ICA, the areas were extrapolated and then superimposed, if necessary, in the fashion of a mirror image (Fig. 6).
(a) Setting for microsurgical dissections and diaphanoscopic evaluation of sellar specimens including video documentation. (b) Two-level mounting allowing microsurgical episcopic dissection (white arrow) and diaphanoscopic view with halogen light source (blue arrow). (c) Right-sided sellar hemiblock fixed to two-level mounting, caulking with black plasticine. Medial episcopic view through operation microscope. The sellar floor is identified (green arrow). The sphenoid bone is rarefied by drilling. (d) The bone (red arrow) then is removed in an eggshell fashion without damaging the underlying connective tissue
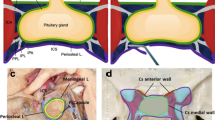
Left-sided sellar hemiblock. (a) Lateral episcopic and (b) diaphanoscopic view through operation microscope after resection of the lateral wall of the cavernous sinus. The cutting edge of the latter (red arrow) defines the area of translucency as do the ascending (green arrow) and horizontal (blue arrow) part of the intracavernous ICA . (c) Episcopic and (d) diaphanoscopic view after resection of the intracavernous part of the ICA. The distal (yellow arrow) and proximal (white arrow) stumps are indicated. In this case the medial wall of the cavernous sinus was not adherent to the artery
Left-sided sellar hemiblock. Same specimen as in Fig. 2. (a) Medial episcopic and (b) diaphanoscopic microscopic view after resection of the lateral wall of the cavernous sinus. The sellar floor (green arrow) is identified only in episcopic view. The ascending part of the ICA (red arrow) is indicated. (c) Episcopic and (d) diaphanoscopic view after resection of the intracavernous part of the ICA, now showing additional translucent areas in the lateral wall of the sella (yellow arrow), close to the former course of the ascending (blue arrow) and horizontal part of the intracavernous ICA. In this case, the lateral wall of the sellar compartment was double layered. In the outer layer of this specimen no perforation was found by microscopic evaluation, diaphanoscopy, or testing for water-tightness
Left-sided sellar hemiblock. (a) Medial episcopic and (b) diaphanoscopic view after resection of ICA. The sellar floor (red arrow) and the area of translucency in the lateral wall of the sella (blue arrow) are indicated. After resection of the inner layer in a double-layered lateral wall of the sellar compartment, a very thin outer layer is seen in C episcopic view with bright translucency (green arrow) in (d) diaphanoscopic mode. However, a perforation was not present (no fluid leak)
Synopsis of diaphanoscopic findings in 14 sellar hemiblocks, extrapolated to the sellar floor (blue dotted line) and the intracavernous course of the ICA (red dotted lines) as a blueprint on a right sided hemiblock, view from medial. Numbers of green and blue areas of translucency indicate the absolute frequency. Three frequently occurring areas of thin medial wall of the cavernous sinus (yellow) are resulting: the lateral wall of the sellar compartment (13 out of 14 samples), below the horizontal part of the ICA (10 out of 14 specimens), and anteroinferiorly to the carotid knee (9 out of 14 hemiblocks)
T1-weighted coronal MRI of a silent ACTH adenoma with bilateral parasellar extension. The lateral wall of the former sellar compartment is penetrated by tumor on both sides (white arrows). With ballooning of the sella the weak areas of the medial wall of the cavernous sinus inferior to the intracavernous part of the ICA are also reached (black arrows on the left side)
Results
There was no evidence of pituitary pathology in any of the cases. In eight donors, one presellar type and seven sellar type sphenoid sinuses [7] were seen. The mean anterior-posterior diameter of the sellar compartment was 9.1 mm (range 7.8 mm to 10.2 mm), its mean height was 5.75 mm (range 4.8 mm to 6.8 mm). The following venous intercavernous connections could be identified in the midline: basal sinus (behind the clivus) in seven out of eight donors, posterior intercavernous sinus (at the posterior inferior border of the sella) in five out of eight cases. An anterior intercavernous sinus was not found in any of the cases. The carotid siphon was omega-shaped or double siphon-shaped in all specimens [18]. The horizontal part of the intracavernous segment of the ICA was adherent to the medial wall of the cavernous sinus in five cases; a compartment of the cavernous sinus medial to this part of the internal carotid artery was present in nine cases (Fig. 2).
In 5 (of 14) specimens, the lateral wall of the sella turcica consisted of a single layer only (36%). In these cases no perforations of the membrane could be observed. In 9 (out of 14) cases, this wall had two layers, i.e., a pituitary capsule was dissectable separately (64%). In two of these nine cases a perforation was present in the inner layer (22%), in three cases in the outer layer (33%). There was no perforation of both layers in any of the cases (Fig. 4). In one of six donors with bilateral investigation, a difference between both sides was observed: on the right side two layers were present, on the left only one. In one case with two layers of the medial wall of the cavernous sinus, an extension of pituitary tissue through the inner layer was observed. The outer layer, however, was not penetrated by pituitary tissue.
Diaphanoscopy revealed a thin medial wall of the cavernous sinus in the area of the lateral border of the sella in 13 out of 14 cases (93%), below the horizontal segment of the internal carotid artery in 10 out of 14 hemiblocks (71%), and antero-inferiorly to the anterior knee of the carotid syphon in 9 cases (64%, Figs. 2, 3, 4 and 5). No leak through the lateral wall of the sellar compartment was observed in any of the cases by testing with water.
Discussion
In the majority of our specimens a sellar type of the sphenoid sinus was observed, reflecting the age of the whole-body donors. With aging, the pneumatization of the sphenoid sinus progresses, and its bony walls become thinner [5, 7]. In 66% of cases the bone between the sphenoid mucosa and dura is less than 1 mm thick [25, 28]. In the present series, the sphenoid bone was particularly thin over the intracavernous carotid knee, as seen by others [16, 27]. Fitting in well with other observations, in this area the bone was thinner than 0.5 mm in 80% of cases and absent in 8% of cases [5].
The venous compartment medial to the horizontal part of the intracavernous ICA may be obliterated by progressing elongation and bending of the artery with aging [16, 25]. A direct contact between the artery and the medial wall of CS was found in 12 out of 30 cases [24], and in 23 out of 44 specimens, respectively [30]. We found firm adherence between both structures in 5 out of 14 of our formalin-fixed specimens. This and the potential lack of overlying bone have to be taken into account in any case of performance of an extended transsphenoidal approach to the parasellar compartment [13] to avoid vascular damage.
In 24% of 17 decalcified sellar specimens, an invagination of pituitary tissue into the medial wall of the sellar compartment was observed, but no penetration [3]. In our series, in 1 out of 14 cases (7%) such an extension of pituitary tissue into the wall of the cavernous sinus was found. Others observed a lateral extension towards the CS in 45% and 28% of their specimens, respectively [26, 30], but again no signs of penetration through the lateral wall of the sellar compartment [30]. Since the age of the donors in this series is not stated, it remains unclear whether this observation could be due to elongation of the ICA in the medial direction rather than extension of the gland laterally. Early observations of large unselected autopsy series report a lateral compression of the pituitary gland by an elongated ICA in 22% of cases [1]. Due to the relatively high age of the donors in our series, in all specimens marked curving of the intracavernous ICA was evident [18].
Contrary to others [16, 27, 30], in our specimens no anterior inferior intercavernous sinus could be identified. However, since the wall of the cavernous sinus rather than the venous cavity was the primary point of interest in our study, we did not inject the veins with colored latex. This would have hindered the diaphanoscopic investigation.
We found a double-layered lateral wall of the sellar compartment in 9 out of 14 cases (64%), whereas others describe a double layer (dura plus pituitary capsule) in all their specimens either by microsurgical dissection [17, 30] or histological evaluation [23]. On the other hand, only one layer has been identified microsurgically by others [4]. It is noteworthy that in 5 of our 14 specimens there was only one dissectable layer of connective tissue separating the CS from pituitary tissue, with the pituitary capsule included. Others found a dural wall between the gland and CS in 36 cadaveric specimens, but they did not comment on whether an additional pituitary capsule was present or not [27]. In ten formalin-fixed skull base specimens, a dense glandular capsule as the inner layer was described, surrounded by a loose circumferential fibrous bed, thus resulting in a double layer of the lateral sellar wall [12].
In summary, the data concerning the number of layers of the lateral wall of the sellar compartment are inconsistent, to some extent due to different definitions and methodologies. Our study is the first to describe different numbers of layers with the same technique, indicating interindividual and also intraindividual anatomical variations in this area.
In 3 out of 30 coronal sections of 10 decalcified sellar specimens, small histologically proven gaps in the otherwise mono-layered lateral wall of the sellar compartment have been observed [32]. Others did not find defects in the MW of the CS macro- or microscopically [31], avoiding decalcification artifacts by plastination technique [16], or by microsurgical bone removal as in our study. Our diaphanoscopic data, however, reveal a marked thinning of the medial wall of the cavernous sinus at the sellar segment compared to other sites. The lateral wall of the sellar compartment was significantly thinner than its inferior wall [23] and than the diaphragma sellae [17]. Like in our cohort, in both series no fenestration of the medial wall of the CS was observed. Morphometric data revealed the thinnest areas of the lateral wall of the sellar compartment to be in its posterior [31] or inferior part [17]. In 13 out of our 14 specimens, superimposed diaphanoscopic data suggested the area anterior to its center to be the weakest (Fig. 5).
Expansion of pituitary adenomas into CS may be facilitated by low anatomical resistance against chronic tumor growth. Without sellar enlargement, only the lateral wall of the sella turcica with its potentially weak areas could be penetrated by tumors. Since this is the case in a relatively high percentage of small tumors in Cushing's disease [14], this may be due to specific expression patterns of proteases in different types of pituitary adenomas [15]. With sellar enlargement due to expansive macroadenomas, all three areas of rarefication in the medial wall of the cavernous sinus described in our report could be reached (Fig. 6). In these cases, expansive tumor growth could rarefy the wall of the CS by chronic pressure alone, thus enabling penetration of tumor tissue into the CS. This corresponds to observations that tumor size is related to tumor extension into different compartments [6] and that large non-functioning pituitary adenomas extending into the CS did not reveal signs of biological aggressiveness [8]. It has also been speculated that anatomical variations like a strong diaphragma sellae with small openings for the pituitary stalk may promote the growth pattern of pituitary macroadenomas caudally and laterally [2, 17].
Conclusions
Anatomical rarefication of the medial wall of the CS is identifiable in distinct areas even though fenestrations are absent. With sellar enlargement due to expansive macroadenomas, all three areas of rarefication in the medial wall of the cavernous sinus described here could be reached and penetrated by the tumors. In microadenomas, only the lateral wall of the sella turcica with its potentially weak areas can be reached by tumors. Expansion into the CS may be facilitated by low anatomical resistance against chronic tumor growth. Additional aggressive properties of the tumors may contribute to CS infiltration.
References
Bergland RM, Ray BS, Torack RM (1968) Anatomical variations in the pituitary gland and adjacent structures in 225 human autopsy cases. J Neurosurg 28:93–99
Campero A, Martins C, Yasuda A, Rhoton AL Jr (2008) Microsurgical anatomy of the diaphragma sellae and its role in directing the pattern of growth of pituitary adenomas. Neurosurgery 62:717–723. doi:10.1227/01.neu.0000317321.79106.37
Destrieux C, Kakou MK, Velut S, Lefrancq T, Jan M (1998) Microanatomy of the hypophyseal fossa boundaries. J Neurosurg 88:743–752
Dietemann JL, Kehrli P, Maillot C, Diniz R, Reis M Jr, Neugroschl C, Vinclair L (1998) Is there a dural wall between the cavernous sinus and the pituitary fossa? Anatomical and MRI findings. Neuroradiology 40:627–630. doi:10.1007/s002340050653
Fujii K, Chambers SM, Rhoton AL Jr (1979) Neurovascular relationship of the sphenoid sinus. J Neurosurg 50:31–39
Garibi J, Pomposo I, Villar G, Gaztambide S (2002) Giant pituitary adenomas: clinical characteristics and surgical results. Br J Neurosurg 16:133–139. doi:10.1080/02688690220131723
Hamberger CA, Hammer G, Norlén G (1961) Transsphenoidal hypophysectomy. Arch Otolaryngol 74:2–8
Honegger J, Prettin C, Feuerhake F, Petrick M, Schulte-Mönting J, Reincke M (2003) Expression of Ki-67 antigen in non-functioning pituitary adenomas: correlation with growth velocity and invasiveness. J Neurosurg 99:674–679
Hussaini IM, Trotter C, Zhao Y, Abdel-Fattah R, Amos S, Xiao A, Agi CU, Redpath GT, Fang Z, Leung GK, Lopes MB, Laws ER (2007) Matrix metalloproteinase-9 is differentially expressed in nonfuntioning invasive and non-invasive pituitary adenomas and increases invasion in human pituitary adenoma cell line. Am J Pathol 170:356–365. doi:10.2353/ajpath.2007.060736
Kawamoto H, Uozumi T, Kawamoto K, Arita K, Yano Y, Hirohata T (1996) Type IV collagenase activity and cavernous sinus invasion in human pituitary adenomas. Acta Neurochir (Wien) 138:390–395. doi:10.1007/BF01420300
Kawase T, van Loveren H, Keller JT, Tew JM (1996) Meningeal architecture of the cavernous sinus. Clinical and surgical implications. Neurosurgery 39:527–536. doi:10.1097/00006123-199609000-00019
Kehrli P, Ali M, Redis M, Maillot C, Dietemann JL, Dujovny M, Ausman JI (1998) Anatomy and embryology of the lateral sellar compartment (cavernous sinus) medial wall. Neurol Res 20:585–592
Kitano M, Taneda M, Shimono T, Nakao Y (2008) Extended transsphenoidal approach for surgical management of pituitary adenomas invading the cavernous sinus. J Neurosurg 108:26–36. doi:10.3171/JNS/2008/108/01/0026
Knappe UJ, Luedecke DK (1996) Persistent and recurrent hypercortisolism after transsphenoidal surgery for Cushing‘s disease. Acta Neurochir (Wien) 65(Suppl):31–34.
Knappe UJ, Hagel C, Lisboa BW, Wilczak W, Luedecke DK, Saeger W (2003) Expression of serine proteases and metalloproteinases in human pituitary adenomas and anterior pituitary lobe tissue. Acta Neuropathol 106:471–478. doi:10.1007/s00401-003-0747-5
Knopp U, Kleedehn M, Kuhnel W, Sepehrnia A (2005) Mikroanatomie des Sinus cavernosus. Ann Anat 187:127–134. doi:10.1016/j.aanat.2004.06.004
Kursat E, Yilmazlar S, Aker S, Aksoy K, Oygucu H (2008) Comparison of lateral and superior walls of the pituitary fossa with clinical emphasis on pituitary adenoma extension: cadaveric-anatomic study. Neurosurg Rev 31:91–99. doi:10.1007/s10143-007-0112-6
Lazorthes G, Bastide G, Gomès FA (1961) Les variations du trajet de la carotide interne d’après une étude artèriographique. Arch Anat 9:129–133
Liu W, Kunishio K, Matsumoto Y, Okada M, Nagao S (2005) Matrix metalloproteinase-2 expression correlates with cavernous sinus invasion in pituitary adenomas. J Clin Neurosci 12:791–794. doi:10.1016/j.jocn.2005.03.010
Losa M, Mortini P, Barzaghi R, Ribotto P, Terreni MR, Marzoli SB, Pieralli S, Giovanelli M (2008) Early results of surgery in patients with non-functioning pituitary adenomas and analysis of the risk of tumor recurrence. J Neurosurg 108:525–532. doi:10.3171/JNS/2008/108/3/0525
Mastronardi L, Guidicci A, Spera C, Puzzilli F, Liberati F, Maira G (1999) Ki-67 labelling index and invasiveness among pituitary adenomas: analysis of 103 cases using the MIB-1 monoclonal antibody. J Clin Pathol 52:107–111. doi:10.1136/jcp. 52.2.107
Pamir MN, Kilic T, Ozek MM, Ozduman K, Ture U (2006) Non-meningeal tumours of the cavernous sinus: a surgical analysis. J Clin Neurosci 13:626–635. doi:10.1016/j.jocn.2006.04.004
Peker S, Kurtkaya-Yapicier O, Kilic T, Pamir MN (2005) Microsurgical anatomy of the lateral walls of the pituitary fossa. Acta Neurochir (Wien) 147:641–649. doi:10.1007/s00701-005-0513-7
Ramina R, Samii M, Baumann H, Warnke PC (1985) The surgical anatomy of the cavernous sinus. In: Dietz H, Brock M, Klinger M (eds) Advances in Neurosurgery 13. Heidelberg, Springer-Verlag, Berlin, pp 207–211
Rhoton AL Jr, Harris FS, Renn W (1976) Microsurgical anatomy of the sellar region and cavernous sinus. Clin Neurosurg 24:54–85
Rhoton AL Jr (2002) The sellar region. Neurosurgery 51:S335–S374. doi:10.1097/00006123-200208000-00007
Romano A, Zuccarello M, van Loveren HR, Keller JT (2001) Expanding the boundaries of the transsphenoidal approach: a microanatomic study. Clin Anat 14:1–9. doi:10.1002/1098-2353(200101)14:1<1::AID-CA1000>3.0.CO;2–3
Umansky F, Nathan H (1982) The lateral wall of the cavernous sinus. With special reference to the nerves related to it. J Neurosurg 56:228–234
Yamada S, Ohyama K, Taguchi M, Takeshita A, Morita K, Takano K, Sano T (2007) A study of the correlation between morphological findings and biological activities in clinically non-functioning adenomas. Neurosurgery 61:580–584. doi:10.1227/01.NEU.0000279970.10309.B5
Yasuda A, Campero A, Martins C, Rhoton AL Jr, Ribas GC (2004) The medial wall of the cavernous sinus: microsurgical anatomy. Neurosurgery 55:179–190. doi:10.1227/01.NEU.0000126953.59406.77
Yilmazlar S, Kocaeli H, Aydiner F, Korfali E (2005) Medial portion of the cavernous sinus: quantitative analysis of the medial wall. Clin Anat 18:416–422. doi:10.1002/ca.20160
Yokoyama S, Hirano H, Moroki K, Goto M, Imamura S, Kuratsu J-I (2001) Are nonfuntioning adenomas extending into the cavernous sinus aggressive and/or invasive? Neurosurgery 49:857–863. doi:10.1097/00006123-200110000-00014
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
The authors present an interesting paper that addresses the clinical problem of invasion of the cavernous sinus by pathological processes, specifically pituitary tumors. They elegantly demonstrate the microanatomy of the medial wall of the cavernous sinus and, in so doing, help readers to better understand the anatomical reasons that explain how these tumors can grow into the cavernous sinus.
Mario Zuccarello
Mayfield Clinic, Cincinnati, OH
Rights and permissions
About this article
Cite this article
Knappe, U.J., Konerding, M.A. & Schoenmayr, R. Medial wall of the cavernous sinus: microanatomical diaphanoscopic and episcopic investigation. Acta Neurochir 151, 961–967 (2009). https://doi.org/10.1007/s00701-009-0340-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-009-0340-3