Abstract
Myrtaceae is a family with high taxonomic complexity, especially the very rich genera such as Myrcia. For this genus, there is not a complete and recent taxonomic revision, and it has some species complexes that are poorly delimited. One of these complexes includes M. laruotteana, M. selloi, M. lajeana and M. tomentosa. In order to better understand the species boundaries in this complex, we analyzed the genetic variability and structure of multiple populations of these four species using inter simple sequence repeat markers, checking if there is any relationship between genetic, morphological and geographical patterns. The amount of genetic diversity found was similar (H E = 0.215) compared to species with similar biological characteristics. No groupings that were previously tested (morphological, geographical and the ones used in the traditional taxonomy) showed significant genetic structure. The groups suggested by Bayesian analysis showed a higher genetic structure through analyses of molecular variance: 12 % of the variation between populations within groups and 27 % of the variation between groups. The Bayesian analysis and the neighbor-joining dendrogram showed two major groups, the first with all populations of M. tomentosa, and the second containing populations from the other species. According to the unified species concept, there is enough evidence for the recognition of M. tomentosa, M. laruotteana and M. selloi, but not for M. lajeana. These data will be used as the basis of a new taxonomic classification of the species in the M. laruotteana complex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species identification is one of the aims of general biological classification and can be troublesome in some plant groups with taxa that are closely related (Rieseberg et al. 2006). Single-locus molecular markers have been successfully used as a biosystematic tool, in order to define interspecific limits within species complexes (Crawford and Mort 2004). Among these, the ISSR (inter simple sequence repeat, Zietkiewicz et al. 1994) is very useful for detecting genetic polymorphisms at species level (Gaiero et al. 2011; Amirmoradi et al. 2012; Rana et al. 2012), since it produces a large number of polymorphic fragments that can be interpreted as multiloci genetic profiles (Zietkiewicz et al. 1994).
Myrtaceae is one of these plant families where identification problems are very common (Biffin et al. 2010). Molecular studies have been conducted successfully in the family, mostly on the phylogeny and delimitation of higher taxonomic levels, such as subfamilies, tribes and subtribes (Wilson et al. 2001, 2005; Lucas et al. 2005, 2007, 2011). Nevertheless, studies at generic and specific levels are still scarce. In the family, ISSR has been used to access the relationships among species of Eucalyptus (Balasaravanan et al. 2005) and among populations of Metrosideros polymorpha (Wright 2007). ISSR has also been used to investigate genetic diversity in E. grandis (Okun et al. 2008) and Rhodomyrtus tomentosa (Xiaoling 2010), and the genetic structure of Myrcia splendens in a limited geographical area (Brandão et al. 2011).
Myrcia is one of the richest genera in tribe Myrteae, with about 400 species (Govaerts et al. 2013), all of them occurring in the Neotropics. Besides its large species number, there is not a recent taxonomic revision for the genus [the last one made by Berg (1857-59)], and the delimitation between some species is difficult. In this genus, there is a species complex, named here as the “M. laruotteana complex” that includes M. laruotteana Cambess., M. selloi (Spreng.) N.Silveira, M. lajeana D.Legrand and M. tomentosa (Aubl.) DC. (Lima 2013). The species in this complex occur from Panama to southern Brazil, Paraguay and Argentina: M. laruotteana occurs in Brazil, from the state of Maranhão to Santa Catarina, and also in Argentina and Paraguay (Legrand and Klein 1969, Sobral et al. 2013); M. tomentosa occurs from Panama to southern Brazil, in the state of Paraná (Kawasaki 1989, Sobral et al. 2013); M. lajeana occurs only in Brazil, from Paraná to Rio Grande do Sul (Sobral et al. 2013); and M. selloi occurs in Argentina and Brazil, from Paraná to Rio Grande do Sul (Rotman 1994, Sobral et al. 2013), but there are herbarium specimens from Minas Gerais and Bahia that seem to belong to this species. The species ranges overlap mainly in central and southern Brazil, but the four species co-occur only in the state of Paraná, in the south (Sobral et al. 2013).
All these species but M. lajeana were included in a phylogenetic analysis of Myrcia (Lucas et al. 2011), and they all belong to a single clade with a high support. This clade can be distinguished by flower buds with a constriction below the ovary; panicles that are triangular but with an abrupt apex and asymmetrical branches; free calyx lobes that are reflexed and acute; and acute bracteoles that are usually persistent after fruit abscission (Lucas et al. 2011). The distinction between M. laruotteana, M. lajeana and M. selloi is not very clear (Legrand and Klein 1969; M. selloi cited as a synonym, M. ramulosa DC.), but they could be recognized by leaf morphology and size, general pubescence and persistence of the hypanthium tube and calyx in the fruits: In general, M. laruotteana has longer and wider leaves than M. selloi and M. lajeana; M. selloi has caducous hypanthium and calyx, leaving the fruit with a circular apical scar. Myrcia tomentosa usually has denser pubescence than other three species, but is morphologically similar to some forms of M. laruotteana (Lucas et al. 2011). The same authors mentioned that there are specimens with intermediate character states, which they suspected to be hybrids. Overall, the characters that are traditionally used to distinguish these species are highly variable, casting doubts about the validity of these entities as distinct taxa. The species in this complex are pollinated by bees (Gressler et al. 2006), and herbarium records show that the species flower in the same period, between September and December. Studies on reproductive biology in Myrteae are still scarce, and among the species in the complex, only M. tomentosa (cited as a synonym, M. rhodeosepala Kiaersk.) has been studied (Proença and Gibbs 1994), being self-compatible.
Since the M. laruotteana complex shows a lot of morphological variation and overlapping character states that make it difficult to distinguish its putative taxa, we propose an investigation of the genetic variability patterns in several populations belonging to this complex. This analysis may indicate whether this complex is made up of several distinct species or of a single widespread and polymorphic species. Our goals are (1) to understand genetic variation and structure among populations of M. laruotteana, M. selloi, M. lajeana and M. tomentosa through ISSR markers; (2) evaluate genetic divergence and relationships between these populations and the taxa that have been traditionally included in this complex; and (3) evaluate the correlation between genetic and morphological patterns among the taxa.
Methods
Population sampling
We sampled 17 populations, from which five belong to M. laruotteana, four to M. tomentosa, four to M. lajeana and four to M. selloi (Table 1). The locations were chosen mainly in central and southern Brazil, where geographical ranges of the four species overlap (Fig. 1). Each population had four to fifteen individuals, totaling 200 individuals in the whole study, from which we collected leaves and one voucher per population, now in the herbaria UEC and UPCB (acronyms following Thiers 2013).
DNA extraction and amplification
The DNA was extracted from leaves stored in silica gel, following Doyle and Doyle (1987). A total of 25 ISSR primers were tested for amplification reactions, from which six were chosen due to better resolution and reproducibility (Table 2). The amplification reactions were carried out in 8.2 μL reaction volumes, using the kit TopTaq Master Mix (Qiagen Biotechnology) with 0.3 units of Taq polymerase, 1.5 mM of MgCl2, 0.2 mM of dNTP, 0.8 μM of primers and 10–100 ng of genomic DNA. The amplification conditions were premelting at 94 °C for 2 min, 37 cycles with denaturing at 94 °C for 15 s, annealing at 42 °C for 30 s and extension at 72 °C for 1 min, followed by a final extension at 72 °C for 7 min. PCR products were analyzed by electrophoresis on 1.5 % agarose gels with 0.5 × TAE buffer, under 100 V for approximately 3.5 h. The gels were stained with ethidium bromide at 0.5 µg/ml and photo-documented. The molecular size of the fragments was estimated using a 100-pb ladder (Ludwig Biotechnology).
Population genetic variability analysis
From the electrophoretic profiles, we built a binary matrix, where each individual was genotyped for presence (1) or absence (0) of fragments. Loci with low resolution were excluded from the analysis. All bands from the same primer with similar molecular size were considered homologous (Thormann et al. 1994). Genetic variability estimates were based on the number of polymorphic (N) and exclusive (N ex) loci, percentage of polymorphic loci (P), Shannon diversity index (I) and average heterozygosity expected for the populations (H E), assessed using the software GenAlEx 6.5 (Peakall and Smouse 2012).
Analysis of molecular variance (AMOVA) was also performed through software GenAlEx 6.5. The AMOVA provides the proportion of intraspecific genetic diversity explained by interpopulational differentiation (Φ ST, analogous to F statistics from Wright 1921). The populations were tested in different grouping scenarios: (1) the four taxonomic groups (i.e., the traditional circumscription of the four species in the complex: M. laruotteana × M. tomentosa × M. lajeana × M. selloi); (2) six groups according to leaf morphology (Table 3); and (3) ten groups based on geographical distances (populations distant less than 200 km from each other and/or less than 500 m altitude from each other).
In order to infer a probability for the genotyped dataset assigned to a given number of clusters (K) and an estimation of the individual’s ancestry (q), we performed a Bayesian analysis through the software STRUCTURE 2.3.4 (Pritchard et al. 2000). The values of K (genetic groups) were estimated between 1 and 17. Ten independent runs for each K were performed, with 1,000,000 MCMC (Markov Chain Monte Carlo) iterations and burn-in period of 100,000, admitting the admixture model of ancestry and correlated allele frequencies. Evannos’s index (Evanno et al. 2005) was used to detect the number of clusters (K) in the dataset. The individual’s ancestry (q) values of 0.9 were used to assign individuals to pure or hybrid classes (Vähä and Primmer 2006). An AMOVA was performed for the groups indicated by this Bayesian analysis through GenAlEx 6.5.
Another Bayesian analysis was performed through the package GENELAND 4.0.3 (Guillot and Santos 2010) available for the software R (http://www.cran.r-project.org/). In this approach, the geographical structure of populations was considered. The values for K were estimated between 1 and 18, with 10 independent runs and 200,000 MCMC iterations (admitting the admixture model, with alleles correlated between populations). In this analysis, we were able to access posterior probabilities for clusters of structured populations and the probability, for each individual, to belong to the respective population or to come from an external source, and also inferring putative migration events and hybrid zones between these populations.
Another AMOVA was performed with the groups indicated by the Bayesian analyses from STRUCTURE 2.3.4 and GENELAND 4.0.3, also through GenAlEx 6.5. A matrix with the Φ ST values from all group pairs indicated by this Bayesian analysis was performed through software AFLP-SURV 1.0 (Vekemans 2002). This matrix was used to build a neighbor-joining dendrogram through the “Neighbor” package, available in the software PHYLIP (Felsenstein 1993). Statistical support of the branches was calculated through the package CONSENSE, from the same software. The resulting tree was visualized with software TREEVIEW (Page 1996).
Results
Intrapopulational genetic diversity
The six primers provided 59 polymorphic loci. Each primer produced from seven to 11 different fragments, these ranging from 300 to 1,300 bp (Table 2). No exclusive fragment was found for any population. The proportion of polymorphic loci per population varied between 81.36 % (CXC) and 30.51 % (RBS).
As for the average heterozygosity expected within the populations, CXC showed the highest value (0.306), while NIT showed the lowest (0.109). The same populations CXC and NIT also showed, respectively, the highest (0.452) and lowest (0.165) values for the Shannon diversity index (Table 4).
Populational relationships and genetic structure
The highest genetic similarity was found between the populations LON and CAM (0.954), and the lowest between RBS and SFP (0.612). The value of genetic structure (Φ ST) of the 17 populations was 0.377. As for the three different grouping scenarios, we found 10–17 % of variation between groups, 21–29 % of variation between the populations within the groups and 61–62 % variation inside the populations. The genetic structure of these groups (Φ ST) varied from 0.385 and 0.392 (Table 5).
The populations were ordered in two clusters in the Bayesian analysis through STRUCTURE 2.3.4 (Fig. 2a). One group (K #1) was made of populations of M. tomentosa (except by one individual of ANA) and the population NIT (except by one individual too). The second (K #2) had all other populations, mixing M. laruotteana, M. lajeana and M. selloi (except by one individual of CXC). The coefficient of ancestry showed that 14 samples presented q value lower than 0.9, ranging from 0.534 to 0.885, indicating that these individuals might be interpreted as hybrids. A second round of the analysis was performed only with K #2 (Fig. 2b), showing the populations clustered again in two groups, one with BRA-2 and CXC (K #3) and other with LON, CAM, CMOU, QUI-1, QUI-2, JOIN, SFS, NOP, SFP and PIRA (K #4). The genetic variation (AMOVA) found between K #1, K #3 and K #4 (14 %) is lower than between the populations in each group (27 %; Table 5).
Clusters obtained from the analysis through STRUCTURE 2.3.4 for individuals from the Myrcia laruotteana complex. a. 200 individuals from the whole complex; b. 129 individuals from a subgroup (K #2, see “Results” section for its description). Each column represents an individual, and colors are used to represent their clusters
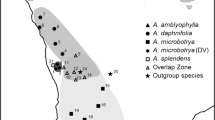
In the other Bayesian analysis, using GENELAND 4.0.3, the populations were clustered in 11 genetic groups (Fig. 3; Table 6), with no indication that either migration or hybridization happened in these populations. No individual showed a posterior probability to belong to any other group than its original group. Populations from different species did not group together. Group #1 has the populations NOP and SFP, which are morphologically similar and are distant only about 70 km from each other. Populations QUI-1 and QUI-2 form group #2 and are also morphologically similar and share the same habitat type. Group #3 has populations SFS and JOIN, both morphologically similar, but showing a posterior probability to belong in group #2. Groups #2 and #3 are in the same geographical region, but SFS and JOIN are at sea level, while QUI-1 and QUI-2 are located at more than 1,000-m altitude. Group #4 has three populations from the same region (LON, CAM and CMOU). Group #5 has ANA and BRA-1, but these populations showed posterior probability to belong in group #6, which in turn has only BRA-2. Groups #7 and #8 have only one population each (respectively PRA and CXC), but CXC showed posterior probability to join PRA in group #7. The last three groups also have only one population each (NIT in group #9, PIRA in group #10 and RBS in group #11). The genetic variation (AMOVA) found between these groups (27 %) is higher than between the populations in each group (12 %) (Table 5).
a Distribution of the posterior probability of a number of K genetic clusters for 17 populations of Myrcia laruotteana complex. b Map of the populations of M. laruotteana complex in genetic domains inferred by GENELAND 4.0.3. See Table 1 for locations of populations
The consensus tree with the Φ ST values showed only two branches with support higher than 50 %, and only one of these higher than 80 % (Fig. 4). The tree shows two main groups, the first one with the populations recognized as M. tomentosa and the second one with populations recognized as M. laruotteana, M. selloi and M. lajeana all mixed together.
Unrooted dendrogram showing the relationships between the groups formed in the Bayesian analysis, based on the values of pairwise Φ ST and neighbor-joining algorithm. Bootstrap percentage greater than 40 % are shown beside branches. Open triangle Myrcia laruotteana; open circle Myrcia tomentosa; closed circle Myrcia selloi; open square Mrycia lajeana
Discussion
Intrapopulational genetic diversity
All primers that were tested showed a few amplified loci (ranging from seven to eleven), the same as found for other species in tribe Myrteae, either using ISSR (3–11 loci per primer in Myrcia splendens; Brandão et al. 2011), and RAPD (5–9 loci per primer in Eugenia dysenterica DC.; Trindade and Chaves 2005). On the other hand, species from other tribes in Myrtaceae seem to provide a larger number of loci per primer for ISSR, like in Eucalyptus sp. (44–102 loci; Balasaravanan et al. 2005) and Metrosideros polymorpha (31–41 loci; Wright 2007). The small number of fragments amplified by ISSR may be characteristic of the species in tribe Myrteae. Despite the small amount of loci, the analyses were able to answer the initial questions proposed by these works.
As for other tropical Myrtaceae, the genetic diversity in the M. laruotteana complex (0.215) is similar to the one found in the polymorphic Metrosideros polymorpha (0.2436; Wright 2007). The species in the M. laruotteana complex also have a genetic diversity similar to the species with similar characters, such as perennial (0.25), cross-pollinated (0.27) or wide-distributed (0.22; Nybom 2004, using RAPD).
Populational relationships and genetic structure
The populations studied here are more structured (Φ ST = 0.377) than species with similar characters that are perennial (0.25) or cross-pollinated (0.27). However, this value is similar to the one found in widely distributed species (0.34; Nybom 2004). The proportion of genetic variability within populations (62 %) larger than between populations (38 %) agrees with other works on tropical tree species (Nybom and Bartish 2000; Nybom 2004). The weak genetic divergence found between populations suggests that some genetic flow may have been kept by pollen and seed transfers (Hamrick and Godt 1990). Myrcia is largely entomophyllous and zoochoric (Gressler et al. 2006), which may ensure moderate- to long-distance genetic flow (Levin 1981). The genetic structure is consequently reduced, due to allelic homogeneity (Loveless and Hamrick 1984). This also explains why populations that are close to each other are morphologically similar, since they exchange alleles more frequently than populations that are distant from each other.
The genetic variation in the grouping scenarios (morphological, geographical and taxonomic) was larger between populations inside the groups (21–35 %) than between the groups (3–17 %). This means that these groups are not genetically homogeneous, nor significantly structured. The lack of correlation between genetic structure and predetermined morphological patterns has been already found in the family (Eucalyptus sp.; Balasaravanan et al. 2005).
The differences between the two Bayesian analyses, through STRUCTURE and GENELAND can be explained by different approaches adopted by each method. While STRUCTURE recovers homogeneous genetic groups, indicating lineages that can be recognized (Pritchard et al. 2000), GENELAND considers the geographical structure in addition to genetic structure, “forcing” the recognition of the lineages under a geographical context (Guillot and Santos 2010).
The Bayesian analysis performed by STRUCTURE 2.3.4 initially separated populations of M. tomentosa and NIT (K #1) from the others (K#2). We did not expect NIT grouping with populations of M. tomentosa, because this population is morphologically similar to the ones in cluster K#2, with plants regarded as M. laruotteana, M. selloi and M. lajeana. This analysis showed 14 possible hybrid individuals between these groups, distributed through all the sampled area, with 11 of these distributed from Goiás to São Paulo, indicating a possible hybrid zone. It suggests that populations can be exchanging alleles between them, and it also can explain the morphological diversity found in the complex. The AMOVA performed for this analysis showed lower divergence between the clusters (14 %) than between the populations inside the clusters (27 %), showing that these groups are not significantly genetically structured. The analysis restricted to K#2 showed two groups, respectively, with populations from northern areas (BRA-2 and CXC) and populations from southern areas (LON, CAM, CMOU, QUI-1, QUI-2, JOIN, SFS, NOP, SFP and PIRA).
The groups determined by Bayesian analysis trough GENELAND 4.0.3. showed a stronger genetic structure than the ones described above. In this case, the genetic variation between the groups (27 %) is larger than the variation between the populations inside each group (12 %). All the populations coming from a same geographical region were grouped in this analysis, except for the populations considered here as from different species. Again, populations that are located close to each other are more genetically and morphologically similar than populations that are geographically distant. Within the species in this complex, plants regarded as M. laruotteana are widely distributed, showing a morphological gradient that follows a geographical pattern: The populations from northern areas are morphologically more similar to plants regarded as M. tomentosa, while the ones from southern areas are more similar to plants regarded as M. selloi and/or M. lajeana. Clusters of M. tomentosa and M. laruotteana have a high posterior probability to cluster among themselves (ANA/BRA-1 plus PRA, with BRA-2 plus CXC, respectively), corroborating the morphological gradient.
Despite low bootstrap values found for the relationships inferred by the Bayesian analysis, patterns shown by the dendrogram are still useful to explain the morphological gradient in the M. laruotteana complex. Actually, the low bootstrap values found here reflect the nature of the data gathered in this study. The bootstrap method consists of resampling the characters to infer how variable the branches are in a tree (Felsenstein 1985). Therefore, whenever there are few characters in an analysis, bootstrap values are expected to be low (Soltis and Soltis 2003). Another explanation would be that these low values may reflect the absence of exclusive loci in the populations studied here.
The genetic differences between populations from northern and southern areas can also be seen in the group with the populations of M. tomentosa, where there is a well-supported (82.8 %) distinction between the northern populations (PRA, BRA-1 e ANA) from the one collected southwards (RBS). There is another gradient in the group formed by the populations of M. laruotteana, M. selloi and M. lajeana, with another well-supported (68.9 %) distinction between the populations collected to the north of the State of São Paulo (NIT, BRA-2 and CXC) and the ones to the south of it (QUI-1, QUI-2, NOP, SFP, LON, CAM, CMOU, PIRA, JOIN and SFS). In general, this structure is the same to the one provided by STRUCTURE 2.3.4.
According to the same geographic-morphological gradient, it would be expected that the populations CXC and BRA-2 (the northernmost populations of M. laruotteana) would be closer to the group with the populations belonging to M. tomentosa. Nevertheless, this did not occur, mostly because of the population NIT. This population is remarkable in the sense that it clearly belongs to M. selloi, and it could absolutely not be mistaken for M. tomentosa or M. laruotteana. Moreover, NIT was also the population with lower genetic diversity, which in turn could be explained by the distance between it and the other ones (Nybom 2004).
The groups shown in the tree followed the leaf morphological patterns too, as shown in Table 3. Pattern #1 was found in all populations regarded as M. tomentosa, which also were grouped in the dendrogram. Populations CXC and BRA-2 (the northernmost M. laruotteana) formed another group, and both represent pattern #2. Patterns #3, #4, #5, and #6 belong to one group, with populations from three species (M. lajeana, M. laruotteana and M. selloi). The population NIT did not follow the general pattern: It would be expected to be in this last big group, since it is one of the two species with leaves following pattern #5 (the other is PIRA).
The population NIT shares the caducous calyx and hypanthium with JOIN, SFS and PIRA. This character makes M. selloi distinct from the other species and therefore, it would be expected that all these populations should be grouped together. Again, this was not observed.
Remarks on the taxonomy of the M. laruotteana complex
The tests that we performed based on leaf morphological patterns and with the species that have been recognized in the traditional taxonomic circumscription did not show significant genetic structure between groups, nor a genetic discontinuity between the populations that were sampled here. This means that both morphological differences between the species and also the recognition of the four species within the complex are not supported by the genetics presented here. In general, both Bayesian analyses tend to separate populations of M. tomentosa from the others. Secondarily, these other populations were separated according to their geographical regions. Despite the low supports, our work presents some patterns that may be useful.
Taxonomic implications based on our results may follow two lines. One interpretation would be that all populations belong to a single species, because (1) there is a morphological continuum that agrees, at least in part, with geographical distribution, and therefore, there are no clear limits between these four putative species; (2) genetic variation within the populations is stronger than the variation among populations, and the overall diversity index is low, which means that the populations are genetically similar and moreover, there may be some genetic flow between them, irrespectively from which putative species they belong. Following this line, all species studied here should be synonymized under the older name, M. tomentosa. In this case, M. tomentosa “sensu latu” could be regarded as an ochlospecies (Cronk 1998), which means a species that is highly polymorphic but weakly polytypic. Morphological characters vary independently in an ochlospecies, and may follow complex patterns, which in turn may be related to geographical and ecological traits presented by the populations. Ochlospecies are frequently widespread, occur in a wide range of habitats, and usually have a large number of synonyms. Thus, they do not easily fit formal taxonomic treatments (White 1998).
Another interpretation for our data would be to accept that classical taxonomy may rely on molecular and statistical data, but also may deal with overall morphology of the plants. Looking at that, even accepting that there is no discontinuity between M. laruotteana and M. tomentosa, we can see that there is a clear distinction between M. lajeana/M. selloi and M. tomentosa. According to the unified species concept (de Queiroz 2007), species are segments of lines in a metapopulation that is constantly evolving. In this case, the traditional species concepts should be regarded as secondary expressions within this wider concept. Following this approach, there is evidence for the recognition of two separate lines, the first with M. tomentosa and the second with M. laruotteana. The limits of these two species may be explained by the two clusters shown in Fig. 4, considering that M. lajeana and M. selloi belong to M. laruotteana’s line. These species are subjected to evolution processes and may be because their divergence is too recent, they still share morphological and ecological characters. This character sharing occurs mainly in central and eastern Brazil, right in the places where the species co-occur. On the other hand, populations from the two species are more distinct to the north or to the south. Both facts may indicate that the divergence process between the two species may have occurred in central and eastern Brazil, followed by posterior colonization to the north and to the south, but this hypothesis can only be tested with phylogeographical studies.
Myrcia lajeana cannot be regarded as a separate line, since there is no disjunction and no genetic evidence to support it as a different species. Moreover, the characters based on which this species was described are not clearly distinct from M. laruotteana, as noted by Legrand and Klein (1969). In the case of M. selloi, despite the morphological (more precisely regarding vegetative characters) and genetic similarity toward M. laruotteana, there is a reproductive character that makes it distinct. Thus, M. selloi could be recognized as a line distinct from M. laruotteana or an infraspecific entity under M. laruotteana, due to morphological evidence—its caducous hypanthium and calyx. The lack of genetic variation between species by itself is not necessarily a complete evidence that these represent a single species, since there may be more variable loci that were not selected for these analyses (Barrett and Freudenstein 2011), and this undetected variability could show that these species are actually distinct.
Following this scenario, taxonomic adjustments must be made for this complex. Taking into account, both our data on genetic markers and morphological and ecological aspects presented by these populations, we propose to keep the names M. tomentosa, M. laruotteana and M. selloi, and the synonymization of M. lajeana under M. laruotteana. As for the status of M. selloi, either as another species or as an infraspecific entity under M. laruotteana, more studies are needed to clear its taxonomic status.
References
Amirmoradi B, Talebi R, Karami E (2012) Comparison of genetic variation and differentiation among annual Cicer species using start codon targeted (SCoT) polymorphism, DAMD-PCR, and ISSR markers. Plant Syst Evol 298:1679–1688
Balasaravanan T, Chezhian P, Kamalakannan R, Ghosh M, Yashoda R, Varghese M, Gurumurth K (2005) Determination of inter- and intra-species genetic relationships among six Eucalyptus species based on inter-simple sequence repeats (ISSR). Tree Physiol 25:1295–1305
Barrett CF, Freudenstein JV (2011) An integrative approach to delimiting species in a rare but widespread mycoheterotrophic orchid. Mol Ecol 20:2771–2786
Berg O (1857–59) Myrtaceae. In: Martius CFP (ed) Flora Brasiliensis 14(1):1–656
Biffin E, Lucas EJ, Craven LA, Costa IR, Harrington MG, Crisp MD (2010) Evolution of exceptional species richness among lineages of flesh-fruited Myrtaceae. Ann Bot London 106:79–93
Brandão MM, Vieira FA, Carvalho D (2011) Estrutura genética em microescala espacial de Myrcia splendens (Myrtaceae). Revista Árvore 35(5):957–964
Crawford DJ, Mort ME (2004) Single-locus molecular markers for inferring relationships at lower taxonomic levels: observations and comments. Taxon 53(3):631–635
Cronk QCB (1998) The ochlospecies concept. In: Huxley CR, Lock JM, Cutler DF (eds) Chorology, taxonomy and ecology of the floras of Africa and Madagascar. Royal Botanic Gardens, Kew, pp 155–170
de Queiroz K (2007) Species concepts and species delimitation. Syst Biol 56(6):879–886
Doyle JJ, Doyle JL (1987) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39(4):783–791
Felsenstein J (1993) PHYLIP (Phylogeny inference package). Version 3.5c. Department of Genetics, University of Washington, Seattle
Gaiero P, Mazzella C, Agostini G, Bertolazzi S, Rossato M (2011) Genetic diversity among endangered Uruguayan populations of Buttia Becc. species based on ISSR. Plant Syst Evol 292:105–116
Govaerts R, Sobral M, Ashton P, Barrie F, Holst BK, Landrum LL, Matsumoto K, Mazine FF, Nic Lughadha E, Proença C, Soares-Silva LH, Wilson PG, Lucas E (2013) World Checklist of Myrtaceae. Royal Botanical Gardens, Kew. http://apps.kew.org/wcsp/. Accessed 10 June 2013
Gressler E, Pizo MA, Morellato PC (2006) Polinização e dispersão de sementes em Myrtaceae do Brasil. Rev Bras Bot 4:509–530
Guillot G, Santos F (2010) Using AFLP markers and the Geneland program for the inference of population genetic structure. Mol Ecol Resour 10(6):1082–1084
Hamrick JL, Godt MJW (1990) Allozyme diversity in plant species. In: Brown AHD, Clegg MT, Kahler AL, Weir BS (eds) Plant population genetics. Breeding and Genetic Resources, Sinauer, pp 43–63
Kawasaki ML (1989) Flora da Serra do Cipó: Myrtaceae. Bol Bot Univ São Paulo 11:121–170
Legrand CD, Klein RM (1969) Mirtáceas: Myrcia. In: Reitz PR (ed) Flora ilustrada Catarinense. Herbário Barbosa Rodrigues, Itajaí, pp 218–330
Levin DA (1981) Dispersal versus gene flow in plants. Ann Missouri Bot Gard 68(2):233–253
Lima DF (2013) Estudos biossistemáticos e taxonômicos sobre o complexo Myrcia laruotteana Cambess. (Myrtaceae). Dissertation, Universidade Estadual de Campinas
Loveless MD, Hamrick JL (1984) Ecological determinants of genetic structure in plant populations. Annu Rev Ecol Syst 15:65–95
Lucas E, Belsham SR, Nic Lughadha EM, Orlovich DA, Sakuragui CM, Chase MW, Wilson PG (2005) Phylogenetic patterns in the fleshy-fruited Myrtaceae: preliminary molecular evidence. Plant Syst Evol 251:35–51
Lucas EJ, Harris SA, Mazine FF, Belsham SR, Nic Lughadha EM, Telford A, Gasson PE, Chase MW (2007) Suprageneric phylogenetics of Myrteae, the generically richest tribe in Myrtaceae (Myrtales). Taxon 56(4):1105–1128
Lucas EJ, Matsumoto K, Harris SA, Nic Lughadha EM, Benardini B, Chase MW (2011) Phylogenetics, morphology, and evolution of the large genus Myrcia s. l. (Myrtaceae). Int J Plant Sci 172(7):915–934
Nybom H (2004) Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol Ecol 13:1143–1155
Nybom H, Bartish IV (2000) Effects of life history traits and sampling strategies on genetic diversity estimates obtained with RAPD markers in plants. Perspect Plant Ecol Evol Syst 3:93–114
Okun DO, Kenya EU, Oballa PO, Odee DW, Muluvi GM (2008) Analysis of genetic diversity in Eucalyptus grandis (Hill ex Maiden) seed sources using inter simple sequence repeat (ISSR) molecular markers. Afr J Biotechnol 7(13):2119–2123
Page RDM (1996) Treeview: an application to display phylogenetic trees on personal computers. Computat Appl Biosci 12:357–358
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 28(19):2537–2539
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Proença CEB, Gibbs PE (1994) Reproductive biology of eight sympatric Myrtaceae from Central Brazil. New Phytol 126:343–354
Rana TS, Narzary D, Ohri D (2012) Molecular differentiation of Chenopodium album complex and some related species using ISSR profiles and ITS sequences. Gene 495:29–35
Rieseberg LH, Wood TE, Baack EJ (2006) The nature of plant species. Nature 440:524–527
Rotman AD (1994) Las species argentinas del genero Myrcia (Myrtaceae). Bol Soc Argent Bot 30(1–2):81–93
Sobral M, Proença C, Souza M, Mazine F, Lucas E (2013) Myrtaceae in Lista de espécies da flora do Brasil. http://reflora.jbrj.gov.br/jabot/listaBrasil/. Accessed 10 June 2013
Soltis OS, Soltis DE (2003) Applying the bootstrap in phylogeny reconstruction. Stat Sci 18(2):256–267
Thiers B (2013) Index Herbarium: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih. Accessed 10 June 2013
Thormann CE, Ferreira ME, Camargo LEA, Tivang JG, Osborn TC (1994) Comparision of RFLP and RAPD markers to estimating genetic relationships within and among cruciferous species. Theor Appl Genet 88:973–980
Trindade MG, Chaves LJ (2005) Genetic structure of natural Eugenia dysenterica DC. (Myrtaceae) populations in northeastern Goiás, Brazil, accessed by morphogical traits and RAPD markers. Genet Mol Biol 28(3):407–413
Vähä JP, Primmer CR (2006) Efficiency of model-based Bayesian methods for detecting hybrids individuals under different hybridization scenarios and with different numbers of loci. Mol Ecol 15:63–72
Vekemans X (2002) AFLP-SURV version 1.0. Laboratoire de Génétique et Ecologie Végétale, Université Libre de Bruxelles, Belgium
White F (1998) The vegetative structure of African Ebenaceae and the evolution of rheophytes and ring species. In: Hopkins HCF, Huxley CR, Pannell CM, Prance GT, White F (eds) The biological monograph: the importance of field studies and functional syndromes for taxonomy and evolution of tropical plants. Royal Botanic Gardens, Kew, pp 95–113
Wilson PG, O’brien MM, Gadek PA, Quinn CJ (2001) Myrtaceae revisited: a reassessment of infrafamilial groups. Am J Bot 88:2013–2025
Wilson PG, O’brien MM, Heslewood MM, Quinn CJ (2005) Relationships within Myrtaceae sensu lato based on a matK phylogeny. Plant Syst Evol 251:3–19
Wright S (1921) Systems of mating. Genetics 6:111–178
Wright ME (2007) A genetic comparison of bog vs. forest populations of Metrosideros polymorpha (Myrtaceae) in Hawaii. Dissertation, University of Colorado
Xiaoling Y (2010) Mating system and genetic diversity of Rhodomyrtus tomentosa (Myrtaceae) detected by ISSR markers. The University of Hong Kong, Dissetation
Zietkiewicz E, Rafalski A, Labuda D (1994) Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics 20:176–183
Acknowledgments
We thank Jair E.Q. Faria, Mariana O. Bünger, Fábio S. Vieira, Cláudio N. Fraga, Martin Molz, Julia Meirelles, Carlos R. Boelter and André Soller for the assistance in the field; Mayara K. Caddah for help with the analyses; Marcos Sobral for confirming the identifications of the plants; André O. Simões for suggestions on early versions of this manuscript. Field and lab work were funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq/Brazil (“Edital Universal” #475099/2011-7). DFL, AVM and RG received grants from CNPq. We thank Instituto Ambiental do Paraná (IAP) for the collecting permits in the state of Paraná.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lima, D.F., Mauad, A.V.S., da Silva-Pereira, V. et al. Species boundaries inferred from ISSR markers in the Myrcia laruotteana complex (Myrtaceae). Plant Syst Evol 301, 353–363 (2015). https://doi.org/10.1007/s00606-014-1078-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-014-1078-9