Abstract
Larger flowers increase pollinator visit rates and reproductive success, so selection on flower size is usually mediated by pollinators. However, larger flowers involve costs imposed by resource limitation so environmental conditions may also modulate flower size. “Male function” hypothesis entails that the intensity of selection is sex-dependent, being greater through male fitness, whereas female fitness is more limited by resources. In this study we analyse pollinator-mediated phenotypic selection on flower size through both sexes in a large-flowered Mediterranean species, Cistus ladanifer. We experimentally manipulated flower size in two populations, measured its effect on male and female fitness and estimated the strength and direction of phenotypic selection through both sexes and populations. Unmanipulated control flowers received higher pollinator visit rates and dispersed a higher pollen amount than reduced flowers. This translated into selection towards larger flowers through male fitness in both populations. Nevertheless, flower size had little effect on female fitness. Fruit set was high but selection through this component of female function was not significant. Seed number increased in control flowers, especially in one population, where we detected positive selection on flower size. Our results suggest that pollinator-mediated phenotypic selection on flower size in this large-flowered Mediterranean species is especially modulated by male fitness, but flower size adjustment may also be a result of a simultaneous selection through both sexes that, in turn, is dependent of ecological context.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attractiveness to pollinators plays a key role in the reproductive ecology of entomophilous plants. Larger flowers increase visits rates, favoring pollen dispersal and deposition and, consequently, increasing both male and female fitness (Bell 1985; Conner and Rush 1996; Aigner 2005; Nattero et al. 2011). Hence, pollinators are considered as one of the main factors causing evolution on flower size (Fenster et al. 2004; Willmer 2011). Phenotypic selection studies in natural populations have also confirmed significant selection towards larger flowers (reviewed in Harder and Johnson 2009). However, small flowers and spatial–temporal variation in flower size still persist in populations so, from an evolutionary perspective, a unilateral view of the role of pollinators is probably oversimplistic (Galen 1999). For example, larger flowers are associated with more visits of floral enemies (Shykoff et al. 1996; Galen 1999; McCall and Irwin 2006) and greater requirements of biomass, carbon and water for production and maintenance of floral structures (Galen et al. 1999; Halpern et al. 2010; Teixido and Valladares 2013). Consequently, flower size is not only influenced by pollen limitation but also by resource limitation due to the combined effect of biotic and abiotic environmental factors. Since this trait is genetically controlled and is heritable, its variability generates potential to evolve in natural plant populations (Weiss et al. 2005; Ashman and Majestic 2006).
Bateman (1948) proposed that the intensity of selection is sex-dependent being higher through male fitness, whereas female fitness is more resource-limited. Therefore, male function could benefit more from pollinator attraction and thus flower size would mainly evolve through selection on male reproductive success (“male function” hypothesis, see also Wade 1979; Burd and Callahan 2000; Jones 2008). This implies that female function is less dependent on pollinator attraction, since few visits could be enough to fertilize all the ovules, and more dependent on resource availability for fruit and seed production, whereas more visits would be required to disperse high amounts of pollen. In fact, this assumption is supported in dioecious zoophilous plants where male individuals have larger flowers than conspecific females, but it is a more complex process in hermaphroditic species since there could be differential and conflicting sexual selection where its strength and direction are usually context-dependent (reviewed in Ashman and Morgan 2004).
Reproductive success analyses and estimates of selection in hermaphroditic plants require simultaneous studies through both sexes together with studies of spatial variation in pollinator assemblage. Most studies on reproductive success and phenotypic selection on flower size in hermaphroditic plant species have exclusively focused on female function, mainly due to the complexity of measuring male function in natural populations (Herrera et al. 2006). Molecular analyses with genetic markers, including DNA extraction and genotyping are needed to record direct estimates of male reproductive success (reviewed in Conner 2006). Though indirect methods such as the amount of dispersed pollen do not necessarily imply paternity success, i.e., that a particular dispersed pollen grain from a certain plant’s flower confers paternity to one seed, pollen dispersal is a representative component of male success and is a useful measure to disentangle the mechanisms, the strength and the direction of phenotypic selection through this sex (Snow and Lewis 1993; Maad and Alexandersson 2004; Arista and Ortiz 2007). Many works have analysed the spatial variation in phenotypic selection on flower size and/or its variation through both sexes (e.g. Caruso et al. 2003; Maad and Alexandersson 2004; van Kleunen and Ritland 2004; Arista and Ortiz 2007; Hodgins and Barrett 2008; Nattero et al. 2010a), but less have considered the effects of spatial variation in pollinator environment and patterns of visit rates on the strength and direction of phenotypic selection (Aigner 2005; Sletvold and Ågren 2010; Sahli and Conner 2011; Sletvold et al. 2012). However, as far as we know, there is a lack of studies combining pollinator assemblage in different populations with selection analyses on flower size through male and female success, but they are essential to identify reliable estimates of phenotypic selection (Conner 2006; see also Herrera et al. 2006).
The extent to which current selection on flower size is mediated by pollinators has rarely been determined experimentally. Overall, reliable estimates of pollinator-mediated phenotypic selection on flower size require assessment of a relationship between trait and relative fitness and, ultimately, if this relationship is at least partly the result of interaction with pollinators. For female fitness, pollinator-mediated phenotypic selection on flower size can be detected by comparing the strength and direction of selection between open-pollinated and hand-pollinated flowers receiving supplemental pollination (reviewed in Ashman and Morgan 2004). Following this methodology, a growing body of studies has documented pollinator-mediated positive and directional phenotypic selection on flower size through female function (Galen 1996; Totland 2001; Fishman and Willis 2008; Parachnowitsch and Kessler 2010; Sletvold and Ågren 2010; Bartkowska and Johnston 2012). Other studies have compared selection with and without the selective agent by experimentally manipulating the presence of the most abundant or effective pollinators (e.g. Galen 1989; Sahli and Conner 2011). For male fitness, a relationship between pollinator visit rates and flower size variation can be used to test whether larger flowers are differentially visited by more pollinators. Then, analyzing pollen removal rates in relation to flower size variation and differential visitation rates can be used to estimate pollinator-mediated phenotypic selection on flower size through male function, at least in an indirect way (Snow and Lewis 1993; Herrera et al. 2006).
In this paper, we estimated phenotypic selection on flower size and analysed whether this process differs through both sexes by means of an experimental approach in two populations of Cistus ladanifer L. (Cistaceae), a pollinator generalist, self-incompatible, hermaphroditic and large-flowered Mediterranean shrub (Herrera 1992; Talavera et al. 1993; Guzmán et al. 2013). Though we did not compare selection between open- and hand-pollinated flowers, we know that C. ladanifer in our study populations may suffer some pollen limitation and, consequently, may be sensitive to pollinator-mediated phenotypic selection on flower size through female function (Teixido and Valladares, submitted elsewhere). We here experimentally assessed the relationship between flower size and relative fitness and, at least partly, its dependence on pollinators by conducting flower size manipulation and pollinator visit rates. This implies that flower size may be potentially mediated by pollinators and other selective agents, such as environmental factors. In this regard, high temperatures and water shortage of Mediterranean environment may affect flowering and limit plant reproduction (Larcher 2000; Thompson 2005; Aragón et al. 2008). Larger-flowered individuals of C. ladanifer involve higher indirect costs in terms of fruit and seed production (Teixido and Valladares 2013). As a consequence, Mediterranean stressful conditions may limit flower size, potentially favoring small-flowered plants and thus indicating that resource limitation may be greater than pollen limitation (Galen 2005; Teixido and Valladares 2013).
Studying a large-flowered species inhabiting a Mediterranean ecosystem represents a good model system to determine current phenotypic selection on flower size through male and female success, thus evaluating whether flower size is more resource-than pollen-limited through female function and it is male fitness the one differentially responding to flower size variation. Specifically, we hypothesized that (1) larger flowers receive more pollinator visits; (2) this relationship increases male reproductive success and, to a lesser extent, the female one; and (3) this pattern translates into sex- and context-dependent phenotypic selection on flower size, with differential selection towards larger flowers through male function.
Materials and methods
Species and study area
Cistus ladanifer (Cistaceae) is a shrub 100–250 cm tall that inhabits open, hot and dry areas with acid soils of the western Mediterranean. The flowering period spans March to June and each plant produces white flowers of ~7–10 cm in diameter, often exhibiting dark-coloured spots at their bases (Muñoz-Garmendía and Navarro 1993; Teixido et al. 2011). The flowers are the largest in the family with an average of more than 150 anthers and 1,000 ovules, are self-incompatible and hermaphroditic and secrete some nectar (Herrera 1992). Flower opening occurs synchronously each day within populations and flowers last only several hours when pollinated and/or under warm temperatures (Teixido and Valladares unpublished data). Fruits are globular woody capsules with a variable number of valves (5–12) and seeds (approx. range 300–1,200) 0.8 × 0.6 mm in size (Talavera et al. 1993; Narbona et al. 2010).
The study was conducted from April to July of 2013 in two populations in Madrid province, central Spain (39°53′–41°09′N, 3°03′–4°34′W). The two populations were merely chosen as replicates. Both populations had similar orientation (south), slope (0°–10°) and tree canopy cover (0–10 %). One population was located in Tres Cantos [732 m above sea level (a.s.l.); 40°34′N, 3°42′W], where individuals bloom between April and May. Substrate is predominantly clay and sand and vegetation is dehesa-like with scattered Quercus ilex L. (Fagaceae) and Pinus pinea L. (Pinaceae) interspersed in a shrub matrix. Mean annual temperature is 14 °C and mean annual precipitation is 544 mm (Ninyerola et al. 2005; N = 20 years). The other population was located in El Escorial (1,156 m a.s.l.; 40°35′N, 4°09′W) where plants bloom in June. Substrate is granite and shrubby vegetation is interspersed with scattered Pinus pinaster Aiton (Pinaceae) and Juniperus oxycedrus Sibth. and Sm. (Cupressaceae) trees. Mean annual temperature is 11 °C and mean annual precipitation is 899 mm (Ninyerola et al. 2005; N = 20 years).
Experimental design
Flower size manipulation
During the flowering peak (when all the individuals bloomed more than 20 flowers per day). 30 plants per population without spots on their corollas were randomly selected and tagged. We selected this phenotype to avoid possible effects of these spots on pollinator visit rates. In other species, dark petal spots have been shown to act as visual signals for insect pollinators (Johnson and Midgley 1997; Thomas et al. 2009). Additionally, flowers would turn into dark flowers when reduced. Flower size was experimentally manipulated to evaluate its effect on pollinator visit rates and three reproductive success components, one for the male function (pollen dispersal) and two for the female one (fruit and seed production). We conducted the experiment under sunny conditions, suitable for pollinator activity. At each population, for 10–15 days, we cut petals at sepal height of the half of the flowers of each plant on 2–5 plants per day at predawn before the opening of the flowers. Thus, we artificially reduced flower diameter and we also divided each plant in two different treatments having a similar number of flowers to avoid the possible effect of flower number on pollinator attraction: (1) unmanipulated control flowers and (2) flowers with reduced size by cutting petals (hereafter “reduced flowers”) (Fig. 1).
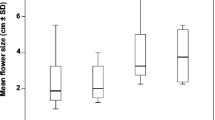
In each treatment, flowers (except those collected to estimate male success; see “Male and female reproductive success”) were tagged with threads differing in colour and left to natural pollination until afternoon. Reduced flowers were randomly chosen to avoid possible differences in the number of flowers at each treatment with a particular orientation. Petals were cut with large kitchen scissors which allowed a simple and single cut without mechanical damage to corollas. Reduced flowers also maintained the original floral shape of C. ladanifer, resembling natural small flowers (Fig. 1). Corolla diameter (cm) of five flowers per treatment and plant was recorded using a caliper (to the nearest mm) and then averaged per treatment and plant as a proxy for flower size. The diameter of reduced flowers varied between 3.0 and 6.1 cm (mean ± SD 4.77 ± 0.54 cm), about the half of the natural diameter (mean ± SD 8.24 ± 0.88 cm).
Pollinators
We evaluated the relationship between the number and identity of pollinators with flower size and subsequent effects on reproductive male and female success. The insect observations were conducted on sunny days with little wind during the flowering peak at each population. Between 2 and 3 plants used in the experimental design were daily observed. Overall, pollinator visit rates were recorded in 20 plants per population and, on each plant, we observed during four 10-min periods on five flowers per treatment, covering up to ~27 h of sampling per population. In Tres Cantos, observations were conducted between 10:00 a.m. and 14:00 p.m., and in El Escorial between 9:00 a.m. and 13:00 p.m., corresponding to the peak of pollinator activity, respectively.
During each observation period we noted the number and identity of visitors to flowers and number of visits per each visitor. A visit was defined to have occurred when the visitor’s body contacted stigma and/or the anthers. At each treatment and plant, we calculated visit rate as total number of visits per 40 min. We categorised each visitor into seven pollinator functional groups or clusters of pollinator species that behave in a similar way in the flowers (Fenster et al. 2004). The functional groups were bumblebees (Bombus spp.), solitary bees (Andrenidae, Colletidae and Halictidae), honeybees (Apis mellifera), wasps (Ichneumonidae), hover flies (Syrphidae), muscoid flies (Muscidae and Anthomyiidae) and beetles (Coleoptera). Then we recorded the frequency of visits of each pollinator functional group to each plant. In the absence of data on a visitor’s efficiency, the frequency of visits can be used as a surrogate of their relative potential importance for the plant species (Fenster et al. 2004).
Male and female reproductive success
To evaluate the male fitness based on differences in flower size we carried out an indirect estimate by means of the amount of dispersed pollen in the flowers of each treatment and plant after natural pollination occurred. Hence, we collected four flowers on each plant at predawn, before the anthesis, and four flowers per treatment and plant between 14:00 a.m. and 15:00 p.m., after the peak of pollinator activity. Though occasionally the flowers remained open after those hours, floral longevity in C. ladanifer is short and lasts only few hours (Teixido et al. 2011), so after noon petals drop off and calyxes close up. All flowers were individually frozen at −10 °C. In the laboratory, the anthers of each flower were collected and oven-dried for 24 h at 60 °C and then weighed to the nearest 0.1 mg with a microbalance (MX5; Mettler-Toledo International, Greifensee, Switzerland). Thus, we recorded the dry mass of each flower’s pollen (mg) and assessed the mean dry mass of both undispersed pollen per plant from those flowers collected at predawn before anthesis and undispersed pollen for control and reduced flowers. To obtain an estimate of pollen dispersal, the individual dry mass of undispersed pollen per treatment and plant was subtracted from the mean dry mass of undispersed pollen per plant at each population. This difference is a good estimate of pollen dispersal in C. ladanifer, where pollen dry mass is significantly correlated with number of anthers (r p = 0.91, p = 0.004, N = 40) and number of anthers with flower size (Herrera 1992; Talavera et al. 1993).
To determine the female fitness, all ripe fruits from previously tagged flowers per treatment and plant were picked before seed dispersal in July to evaluate fruit set and mean seed number per fruit, treatment and plant (hereafter “seed number”). Fruit set estimates pollination intensity as a proportion of pollinated flowers, whereas seed number estimates the quality of mating (Fenster et al. 2004). Fruit set per treatment and plant was obtained by dividing the number of mature fruits set by all flowers tagged per treatment and plant, respectively. To determine seed number, 5–10 mature fruits were randomly selected per treatment and plant and then the seeds per fruit were counted using a four digit manual hand tally counter. Subsequently, the number of seeds recorded per fruit was added up at each treatment and plant and then averaged (±SD) by dividing by the number of fruits utilized, thus recording mean seed number per fruit, treatment and plant (i.e., seed number).
Statistical analysis
To test differences between populations and years in the frequency of visits of each pollinator functional group to each plant we used PerMANOVA. PerMANOVA is a permutation-based version of the multivariate analysis of variance (Anderson 2001). It uses the distances between samples to partition variance and randomizations or permutations of the data to produce the p value for the hypothesis test. It is non-parametric (or semi-parametric for multi-factor models) and, therefore, robust to the assumption of multivariate normality making it less prone to Type I errors. Count data of visits of each pollinator functional group were square root transformed to improve normality. Bray–Curtis similarity index was calculated before performing the analysis (Anderson 2001). All PerMANOVA analyses were performed in Primer 6.0 (Clarke and Gorley 2006).
To determine whether pollinator visit rates increase with flower size and significantly differ between populations, we conducted an ANOVA including population and treatment (fixed factors), plant within population (random factor) and the interaction between population and treatment. A significant interaction indicates a differential effect of the treatment on visit rates depending on the population. Assumptions of normality and homogeneity of variance were tested using Shapiro–Wilk’s test and Levene’s test, respectively. ANOVA was performed using the lme4 statistical package in R v2.12.1 (R Development Core Team 2010).
To determine the effects of the treatment on reproductive success we fitted three Generalized Linear Mixed Models (GLMMs) with population and treatment (fixed factors), plant nested within population (random factor) and the interaction between population and treatment on every fitness component, i.e., pollen dispersal, fruit set and seed number. A significant interaction indicates a differential effect of the treatment on components of reproductive success between populations. For pollen dispersal and seed number we assumed a normal error distribution with an identity link function. For fruit set we assumed a binomial error distribution with a logit link function. For all models we used the restricted maximum likelihood (REML) and, because our data were unbalanced, we used Satterthwaite’s method to determine the approximate denominator degrees of freedom of residuals (Littell et al. 1996; Quinn and Keough 2002). Additionally, since the treatment affected both pollinator visit rates and components of reproductive success (see “Results”), we subsequently tested the effect of visit rates on pollen dispersal, fruit set and seed number in each population, respectively, by means of linear regression. All the GLMMs were performed using the GLIMMIX Macro of SAS (SAS Statistical Package 1990; SAS Institute, Cary, NC, USA) and the regressions were analysed with R (R Development Core Team 2010).
To estimate phenotypic selection on flower size through every component of reproductive success we assessed standardized selection differentials (s) using linear regression analyses with relative pollen dispersal, fruit set and seed number (individual fitness/population mean fitness, w) as the response variable, respectively, and standardized flower size (with a mean of 0 and a variance of 1) as explanatory variable (Lande and Arnold 1983). We used floral diameter of both reduced and control flowers as an estimate of flower size of each population. Likewise, we used pollen dispersal, fruit set and seed number of both reduced and control flowers as fitness components. Each fitness component was correlated with flower size in a linear regression where the slope was the estimate of the strength and direction of linear selection (Lande and Arnold 1983; Kingsolver et al. 2001). Additionally, we calculated nonlinear selection gradients (γ) to estimate stabilizing/disruptive selection by obtaining quadratic deviations from the mean for both single and quadratic terms of flower size (Lande and Arnold 1983). Therefore, we used flower size and its quadratic component in the regression model. Quadratic regression coefficients were doubled to estimate properly stabilizing/disruptive selection gradients (Lande and Arnold 1983; Stinchcombe et al. 2008). All the regression models were performed in R (R Development Core Team 2010).
Results
Pollinators
Five and seven functional groups were identified in Tres Cantos and in El Escorial, respectively (Fig. 2). In Tres Cantos, functional groups were similar for both treatments but, in control flowers, honeybees accounted for nearly 50 % of visits, whereas the variability of pollinators on reduced flowers was higher, dominated mostly by bees and flies (muscoid and hoverflies) (approx. 95 %). In El Escorial, beetles were the dominant functional group in both treatments, especially in reduced flowers (approx. 40 %), along with muscoid and hoverflies. Together, these three groups (beetles, muscoid flies and hoverflies) accounted for 80–90 % of visits in this population. Visits by bees were scarce and so were visits by bumblebees and wasps (these only in reduced flowers). The latter differences in type of pollinators were significant between populations and treatments (pseudo-F 1, 44 = 8.45, p < 0.001; pseudo-F 1, 44 = 23.54, p < 0.001, respectively), but no statistical differences were found between the interaction of treatment and population (pseudo-F 1, 44 = 0.98, p = 0.389).
Whereas flowers of C. ladanifer were significantly larger in El Escorial (F 1, 95 = 6.30, p = 0.013, see Table 1), visit rates were constant between populations (Tables 1, 2). However, visit rates differed significantly between treatments (Table 2). This entailed a positive effect of flower size since in control flowers there was a twofold increase in visit rates in both populations (Fig. 3, mean range 0.30 ± 0.22–0.61 ± 0.29; see also Table 2, Population × Treatment not significant). Pollinator visit rates also showed a high variation between plants and treatments (range 0.03–1.08), with minimum values corresponding to reduced flowers (≤0.78) and the highest ones to control flowers (≥0.23).
Male and female reproductive success
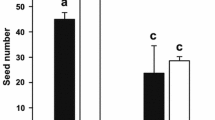
The amount of pollen dispersed per plant in C. ladanifer was highly variable (mean range 16.7–66.6 %), but the mean dry weight of dispersed pollen was similar between populations, despite differences in mean total pollen per flower (Tables 1, 3). Thus, overall, flowers of El Escorial dispersed the same amount of pollen as flowers of Tres Cantos, despite having more pollen available (Table 3). Control flowers dispersed significantly more pollen than the reduced ones (Table 3; Fig. 4a). Overall, control flowers dispersed about 8–48 mg of pollen, whereas reduced flowers dispersed between 0 and 40 mg. In Tres Cantos, control flowers dispersed up to 44 % of pollen, whereas reduced flowers dispersed up to 37 %. In El Escorial, these values were 38 and 29 %, respectively. Concerning the relationship between visit rates and male reproductive success, significant effects were detected only in El Escorial (Table 4). In fact, this relationship was also detected in this population when analyzing control flowers separately, i.e., under natural conditions of flower size variation (R 2 = 0.58, F 1, 19 = 12.33, p = 0.007).
Fruit set in C. ladanifer ranged between 31 and 100 % but was, on average, relatively high (mean range ± SD 77.05 ± 13.43 and 85.30 ± 12.25 % for reduced and control flowers, respectively, Fig. 4b). Although we did not find any significant difference in fruit set between populations, this component did significantly vary between treatments and plants (Table 3). However, the effect of treatment was due to differences found in El Escorial since in Tres Cantos fruit set was similar between control and reduced flowers (Population × Treatment marginally significant, Table 3; see also Fig. 4b). Visit rates did not affect fruit set in any population (Table 4).
Variability in seed number was very high (mean ± SD 127.51 ± 145.32 and 1533.33 ± 234.87) and all the analysed variables had a significant effect (Table 3). Overall, between treatments, seed number was 13 % higher in control flowers (mean range ± SD 796.56 ± 206.70 and 703.31 ± 108.51; control vs. reduced flowers, respectively). Between populations, seed number was 14 % higher in Tres Cantos (Table 1). The effect of the interaction between population and treatment was also significant, as evidenced by a larger decline in seed number in reduced flowers compared to control flowers in El Escorial compared to Tres Cantos (Fig. 4c). Although the number of seeds decreased a 4 % in reduced flowers in Tres Cantos, this decrease reached to a 25 % in El Escorial. Visit rates also significantly influenced seed number in El Escorial (Table 4).
Phenotypic selection
The results revealed sex-dependent effects in the phenotypic selection on flower size. Thus, phenotypic selection had a differential effect through male success. In both populations, a direct positive selection was detected on flower size through pollen dispersal (Table 5). Relative to female function, selection towards larger flowers was only detected in El Escorial through seed number, but the strength of selection was lower than for pollen dispersal (Table 5).
Discussion
This study provides insights into spatial variation in the patterns of pollinator visits as well as current phenotypic selection on flower size through both sexual functions in a hermaphroditic and large-flowered species in a Mediterranean environment. Overall, we verified that larger flowers in C. ladanifer attract more pollinators within populations and this relationship differentially affects sexual functions, especially favoring male fitness by significantly increasing pollen dispersal. The female function benefits to a lesser extent and may depend on the strength of pollen and/or resource limitation on fruit set and seed number. In Tres Cantos, where the flowers were smaller, pollinator assemblage might reduce pollen limitation. Otherwise, in El Escorial, pollination seems to be scarce to fertilize all the ovules of these larger flowers. In agreement with this, interestingly, we detected sex-dependent phenotypic selection on flower size, with stronger selective pressures towards larger flowers through male fitness.
Variation in pollinator visit rates
The interactions between C. ladanifer and pollinating agents recorded in our study supported the generalist character of this species. Pollinators were numerous and diverse and also significantly varied between populations. In fact, pollinator assemblage was also different in other populations of this species in SE Spain (79 % of Diptera and 4 % of bees: Talavera et al. 1993). C. ladanifer has large flowers with an unrestrictive morphology that favors attraction to a high diversity of insects and also increases individual fitness in generalist plants (reviewed in Willmer 2011). This pollination system is shared with most of Cistaceae (Bosch 1992; Herrera 1992; Talavera et al. 1993, 2001) and may have worked as an adaptation to spatial–temporal variation in the relative abundances of most effective pollinators (Herrera 1996). Therefore, generalization is common in C. ladanifer as a relevant factor towards the adaptation to the high variability in pollination environment, favoring pollen transfer among individuals and, thus, a high percentage of fruit and seed production, which ultimately may favor the display of these large flowers in the Mediterranean ecosystem.
Sex differences and selection patterns
Larger flowers of C. ladanifer attracted higher visit rates and a different pollinator assemblage that, overall, favored pollen dispersal. Flowers of this species produce a high pollen amount and this pattern is also related to flower size (Herrera 1992; Teixido and Valladares 2013). This favors pollen dispersal in larger flowers not only by increasing pollinator visit rates and efficiency, but also by having more available pollen. Although in our study reduced flowers contained on average the same amount of pollen than control flowers since only size was experimentally manipulated, this process of pollen dispersal may not be uncommon in natural populations of this species. As a consequence, selection would tend to operate on a correlation between flower size and pollen amount.
Differential pollen dispersal in larger flowers in C. ladanifer provides some suggestion that pollinators play a role in selection on flower size through male fitness in the study populations. We are aware that pollen dispersal is only a component of male fitness and has been found to have little relationship to siring seeds in other systems (Conner 2006; Herrera et al. 2006). However, as we stated above, pollen dispersal is often measured as a representative component affecting male reproductive success and its measurement is valuable for elucidating the mechanisms of selection (Snow and Lewis 1993; Arista and Ortiz 2007). Although not all dispersed pollen will contribute male fitness, pollen collection and export should be proportional to pollen donation (Holland et al. 2004).
Following our data, visitation rate only explains male fitness estimates in El Escorial, even only for natural variation in flower size as measured in control flowers. Therefore, it is difficult to see how pollinators are driving selection in Tres Cantos, where there is no relationship between visitation and pollen dispersal. The absence of this relationship could imply that in some control flowers a few visits disperse higher pollen amount than more visits do in reduced flowers, which would be evidence for the importance of pollinator efficiency on pollen dispersal. In a generalist species as C. ladanifer it is likely that a high percentage of visitors are inefficient pollinators, as showed in the sister species, C. libanotis (Talavera et al. 2001). However, pollinator visit rates as well as dispersed pollen responded similarly to the treatment in both populations, so efficiency-dependent pollen dispersal appears not to be relevant. Either way, data suggest that pollinators are not the agents driving selection in this population and, likely, we failed in detecting pollinator-mediated phenotypic selection on flower size through pollen dispersal. Otherwise, in El Escorial, flowers were larger and had a higher amount of pollen, and pollinator visit rates were the same as in Tres Cantos. Therefore, there was a higher pollen dispersal limitation and, consequently, higher selection opportunity through male fitness. Most interestingly, this opportunity is also sizable taking into consideration only natural variation in flower size as recorded in control flowers, thus supporting the importance of this trait for increasing male reproductive success. Overall, our results entail that pollen limitation through male function may also be important and that the strength of selection on flower size through this sex may likewise vary among populations.
Several works have documented current phenotypic selection towards larger flowers through male fitness in different plant species (Stanton et al. 1986; Galen 1989; Maad and Alexandersson 2004; Arista and Ortiz 2007; Hodgins and Barrett 2008). Compared to the sister species C. salviifolius, the strength of selection through pollen dispersal in our study is somewhat low (see Arista and Ortiz 2007). We recorded that pollen dispersal was lower than 50 % (against 80 % in Arista and Ortiz’ work), which is far from satisfying male function. This suggests that pollen dispersal does not become saturated and plants may produce more pollen than that is potentially available, always favoring male function under benign pollination conditions. However, our data of pollen dispersal could be due to differences in the methodology carried out. Arista and Ortiz (2007) recorded pollen dispersal on flowers picked at sunset, in contrast with flowers picked at afternoon in our study. Other plausible explanations may also be due to differences in visit rates and/or efficiency of pollinators, but they did not record pollinator data, whereas we did not record their efficiency, so interpretations should be made with caution.
Flower size had less effect on female fitness in our study species. We only detected selection towards larger flowers through seed number in one population. Female function is usually dependent on ecological context, thus being pollen or resource-limited (Ashman and Morgan 2004). A general absence of a positive relationship between pollinator visit rates and female fitness suggests low levels of pollen limitation in C. ladanifer, a common pattern in other Cistus (Bosch 1992; Herrera 1992; Talavera et al. 1993, 2001; Arista and Ortiz 2007). Following this assumption, selection opportunity on flower size through female sex is potentially low, even for large differences in pollinator visit rates among individuals with contrasting flower size (Johnson 1996; Totland 2004). Absence of selection on flower size through female fitness components has been detected in other plant species, especially depending on ecological context (Totland 2001; Ashman and Morgan 2004) and also in other Cistaceae (C. salviifolius: Arista and Ortiz 2007; Halimium atriplicifolium: Teixido 2013).
However, we did detect a trend to produce more fruits and seeds in larger flowers in El Escorial. In fact, we also detected that differences in pollinator visit rates affected seed number in this population. This suggests that smaller flowers may be limited by pollen receipt, thus reducing the number of seeds per fruit. Most interestingly, phenotypic selection on flower size through seed number in this population was almost as strong as through pollen dispersal. This may be due to flowers in El Escorial being larger than in Tres Cantos. However, they received a similar number of visit rates and different composition of pollinators. Hence, both the number and the efficiency of pollinators were inadequate to fertilize all the ovules, thus increasing pollen limitation in smaller flowers and favoring directional selection on flower size. Previous studies have documented current phenotypic selection towards larger flowers through seed number associated with pollen limitation (Totland 2001; van Kleunen and Ritland 2004; Hodgins and Barrett 2008; Nattero et al. 2010b).
Jointly, resource limitation may also be important in this population and plants might allocate more resources to control, more visited flowers, than to reduced, less visited flowers. Under resource limitation conditions, a selective abortion in fruit and seed production in pollen-limited flowers may improve both the quantity and quality of offspring in pollen-saturated flowers on the same plant (Lloyd 1980; Haig and Westoby 1988). In fact, the pattern of resource investment between flowers and fruit and seed production is relevant in the reproductive function of C. ladanifer (Teixido and Valladares 2013). Nevertheless, this assumption should be taken with caution since we lack data of resource availability and allocation in the present study. Flower size is significantly correlated with ovule number in this species (Herrera 1992) and, in El Escorial, flowers were larger than in Tres Cantos, but they received the same pollinator visit rates and produced less fruits and seeds. Based on these results, pollen rather than resource limitation seems to be more important in this population.
Differences in pollinator assemblage, pollen limitation and their effects on seed number between populations could also respond to the effect of other biotic factors such as coflowering species (nurse plants: Ghazoul 2006; competitor plants: Caruso 2001), the effect of flower number, which may or may not increase pollinator visit rates (Thompson 2001; Harder and Johnson 2009; Brys and Jacquemyn 2010) and/or to selective pressures imposed by antagonists. For example, the incidence of florivores was significantly influenced by flower size in C. ladanifer (Teixido et al. 2011). Florivores may reduce fitness of both sexes by degrading the attractive properties of flowers and/or by direct consumption of available gametes (Krupnick et al. 1999; Cardel and Koptur 2010). In this context, florivores may exert negative selective pressures on the same floral traits positively selected by pollinators (McCall and Irwin 2006). Other plausible mechanisms could also be related to the effect of abiotic conditions giving rise to spatial variation in the patterns of selection through female fitness (Herrera 1995; Totland 2001, 2004). In this regard, floral costs and differential resource allocation between functions involves indirect effects on female function (Cresswell 1998). In C. ladanifer, flower size is significantly related to greater floral resource allocation (Herrera 1992; Teixido and Valladares 2014). This differential resource allocation to larger flowers has shown that flower size entails higher indirect costs in terms of fruit and seed production (Teixido and Valladares 2013). Overall, these patterns support the idea that selection on flower size is not only dependent on pollinators, but rather that this trait responds to adjustments between costs and benefits and that this balance is closely linked to sex.
When both sexes have different optima for those traits related to reproductive success, there may be a sexual conflict giving rise to traits in equilibrium (Chapman 2006). In C. ladanifer, flower size affected both sexes in El Escorial but only the male function in Tres Cantos. The result in the latter population implies differences in the evolutionary pressures of both sexes that could lead to sexual selection conflicts on this trait in this population. Taken together, our results show that differences in selective pressures between sexes are essential to understand the variation in flower size and the evolution of large flowers in Mediterranean environments. In order to improve our understanding about evolution of flower size in this large-flowered Mediterranean species, it would also be necessary to carry out further among-population variation and temporal studies to determine which sex differentially influences plasticity on this trait and more strongly affects phenotypic selection.
Conclusions
This work shows a positive relationship between flower size and pollinator visit rates and its effects on reproductive success in C. ladanifer. In addition, we detected sex- and context-dependent (i.e., spatial variation between populations) phenotypic selection on flower size. Overall, our results suggest that pollinators may play a role in selection on flower size in natural populations of this species, at least to a certain degree. The experimental manipulation of this trait and the use of pollen dispersal as an indirect estimate of male fitness showed adequate methods to successfully evaluate our objectives. The different patterns of selection on flower size verify the importance of estimating phenotypic selection through both sexes and different components of fitness together with differences in environmental conditions between populations. Taken together, these patterns support the “male function” hypothesis in our study species but also suggest that inherent conditions to each site have the potential to create among-population differences in flower size by local adaptations to climatic and pollinator environment (i.e. to resource and pollen limitation, respectively). As a consequence, optimal flower size through male fitness appears to be larger than through female one, in such a way that whether flower size were halfway between both optimal values, an actual sexual conflict would also act as a stabilizing mechanism of this trait. Therefore, flower size variation will ultimately be a sexual conflict modulated by the environment and mainly constrained by female fitness in this large-flowered Mediterranean species.
References
Aigner PA (2005) Variation in pollination performance gradients in a Dudleya species complex, can generalization promote floral divergence? Func Ecol 19:681–689
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Aust Ecol 26:32–46
Aragón CF, Escudero A, Valladares F (2008) Stress-induced dynamic adjustments of reproduction differentially affect fitness components of a semi-arid plant. J Ecol 96:222–229
Arista M, Ortiz PL (2007) Differential gender selection on floral size: an experimental approach using Cistus salviifolius. J Ecol 95:973–982
Ashman T-L, Majestic CJ (2006) Genetic constraints on floral evolution: a review and evaluation of patterns. Heredity 96:343–352
Ashman T-L, Morgan MT (2004) Explaining phenotypic selection on plant attractive characters: male function, gender balance or ecological context? Proc R Soc Lond B 271:553–559
Bateman AJ (1948) Intra-sexual variation in Drosophila. Heredity 2:349–368
Bartkowska MP, Johnston MA (2012) Pollinators cause stronger selection than herbivores on floral traits in Lobelia cardinalis (Lobeliaceae). New Phytol 193:1039–1048
Bell G (1985) On the function of flowers. Proc R Soc Lond B 224:223–265
Bosch J (1992) Floral biology and pollinators of three co-occurring Cistus species (Cistaceae). Bot J Linn Soc 109:39–55
Brys R, Jacquemyn H (2010) Floral display size and spatial distribution of potential mates affect pollen deposition and female reproductive success in distylous Pulmonaria officinalis (Boraginaceae). Plant Biol 12:597–603
Burd M, Callahan HS (2000) What does the male function hypothesis claim? J Evol Biol 13:735–742
Cardel YJ, Koptur S (2010) Effects of florivory on the pollination of flowers: an experimental study with a perennial plant. Int J Plant Sci 171:283–292
Caruso CM (2001) Differential selection on floral traits of Ipomopsis aggregata growing in contrasting environments. Oikos 94:295–302
Caruso CM, Peterson SB, Ridley CE (2003) Natural selection on floral traits of Lobelia (Lobeliaceae): spatial and temporal variation. Am J Bot 90:1333–1340
Chapman T (2006) Evolutionary conflicts of interest between males and females. Curr Biol 16:744–754
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E, Plymouth
Conner JK (2006) Ecological genetics of floral evolution. In: Harder LD, Barrett SCH (eds) Ecology and evolution of flowers. Oxford University Press, Oxford, pp 260–277
Conner JK, Rush S (1996) Effects of flower size and number on pollinator visitation to wild radish, Raphanus raphanistrum. Oecologia 105:509–516
Cresswell JE (1998) Stabilizing selection and the structural variability of flowers within species. Ann Bot 81:463–473
Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD (2004) Pollination syndromes and floral specialization. Annu Rev Ecol Syst 35:375–403
Fishman L, Willis JH (2008) Pollen limitation and natural selection on floral characters in the yellow monkeyflower, Mimulus guttatus. New Phytol 177:802–810
Galen C (1989) Measuring pollinator-mediated selection on morphometric floral traits: bumblebees and the alpine sky pilot, Polemonium viscosum. Evolution 43:882–890
Galen C (1996) Rates of floral evolution: adaptation to bumblebee pollination in an alpine wildflower, Polemonium viscosum. Evolution 50:120–125
Galen C (1999) Why do flowers vary? The functional ecology of variation in flower size and form within natural plant populations. Bioscience 49:631–640
Galen C (2005) It never rains but then it pours: the diverse effects of water on flower integrity and function. In: Reekie E, Bazzaz FA (eds) Reproductive allocation in plants. Elsevier Academic Press, San Diego, pp 77–95
Galen C, Sherry RA, Carroll AB (1999) Are flowers physiological sinks or faucets? Costs and correlates of water use by flowers of Polemonium viscosum. Oecologia 118:461–470
Ghazoul J (2006) Floral diversity and the facilitation of pollination. J Ecol 94:295–304
Guzmán B, Narbona E, Vargas P (2013) Investigating reproductive incompatibility barriers in a Mediterranean rockrose (Cistus ladanifer). Plant Bios. doi:10.1080/11263504.2013.801369
Haig D, Westoby M (1988) Inclusive fitness, seed resources, and maternal care. In: Lovett Doust J, Lovett Doust L (eds) Plant reproductive ecology: patterns and strategies. Oxford University Press, New York, pp 60–79
Halpern SL, Adler LS, Wink M (2010) Leaf herbivory and drought stress affect floral attractive and defensive traits in Nicotiana quadrivalvis. Oecologia 163:961–971
Harder LD, Johnson SD (2009) Darwin’s beautiful contrivances: evolutionary and functional evidence for floral adaptation. New Phytol 183:530–545
Herrera J (1992) Flower variation and breeding systems in the Cistaceae. Plant Syst Evol 179:245–255
Herrera CM (1995) Microclimate and individual variation in pollinators: flowering plants are more than their flowers. Ecology 76:1516–1524
Herrera CM (1996) Floral traits and plant adaptation to insect pollinators, a devil’s advocate approach. In: Lloyd DG, Barrett SCH (eds) Floral biology: studies on floral evolution in animal-pollinated plants. Chapman and Hall, New York, pp 65–87
Herrera CM, Castellanos MC, Medrano M (2006) Geographical context of floral evolution: towards an improved research programme in floral diversification. In: Harder LD, Barrett SCH (eds) Ecology and evolution of flowers. Oxford University Press, New York, pp 278–294
Hodgins KA, Barrett SCH (2008) Natural selection on floral traits through male and female function in wild populations of the heterostylous daffodil Narcissus triandrus. Evolution 62:1751–1763
Holland JN, Bronstein JL, DeAngelis DL (2004) Testing hypotheses for excess flower production and low fruit-to-flower ratios in a pollinating seed-consuming mutualism. Oikos 105:633–640
Johnson SD (1996) Pollination, adaptation and speciation models in the Cape flora of South Africa. Taxon 45:59–66
Johnson SD, Midgley JJ (1997) Fly pollination of Gorteria diffusa (Asteraceae), and a possible mimetic function for dark spots. Am J Bot 84:429–436
Jones AG (2008) On the opportunity for sexual selection, the Bateman gradient and maximum intensity of sexual selection. Evolution 63:1673–1684
Kingsolver JG, Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, Hill CE, Hoang A, Gilbert P, Beerli P (2001) The strength of phenotypic selection in natural populations. Am Nat 157:245–261
Krupnick GA, Weis AE, Campbell DR (1999) The consequences of floral herbivory for pollinator service to Isomeris arborea. Ecology 80:125–134
Lande R, Arnold SJ (1983) The measurement of selection on correlated characters. Evolution 37:1210–1226
Larcher W (2000) Temperature stress and survival ability of Mediterranean sclerophyllous plants. Plant Bios 134:279–295
Littell RC, Milliken GA, Stroup WW, Wolfinger RD (1996) SAS system for mixed models. SAS Institute Incorporation, New York
Lloyd DG (1980) Sexual strategies in plants. I. An hypothesis of serial adjustment of maternal investment during one reproductive success. New Phytol 86:69–79
Maad J, Alexandersson R (2004) Variable selection in Platanthera bifolia (Orchidaceae): phenotypic selection differed between sex functions in a drought year. J Evol Biol 17:642–650
McCall AC, Irwin RE (2006) Florivory: the intersection of pollination and herbivory. Ecol Lett 9:1351–1365
Muñoz-Garmendía F, Navarro C (1993) Cistaceae. In: Castroviejo S, Aedo C, Gómez-Campo M et al (eds) Flora Iberica. CSIC, Madrid, pp 318–436
Narbona E, Guzmán B, Arroyo J, Vargas P (2010) Why are fruits of Cistus ladanifer (Cistaceae) so variable? A multi-level study across the western Mediterranean region. Perspect Plant Ecol 12:305–315
Nattero J, Cocucci AA, Medel R (2010a) Pollinator-mediated selection in a specialized pollination system: matches and mismatches across populations. J Evol Biol 23:1957–1968
Nattero J, Sérsic AN, Cocucci AA (2010b) Patterns of contemporary phenotypic selection and flower integration in the hummingbird-pollinated Nicotiana glauca between populations with different flower-pollinator combinations. Oikos 119:852–863
Nattero J, Malerba R, Medel R, Cocucci A (2011) Factors affecting pollinator movement and plant fitness in a specialized pollination system. Plant Syst Evol 296:77–85
Ninyerola M, Pons X, Roure JM (2005) Atlas climático digital de la Península Ibérica. Metodología y aplicaciones en bioclimatología y geobotánica. Universidad Autónoma de Barcelona, Barcelona. ISBN 932860-8-7. http://opengis.uab.es/wms/iberia/. Accessed 20 July 2013
Parachnowitsch AL, Kessler A (2010) Pollinators exert natural selection on flower size and floral display in Penstemon digitalis. New Phytol 188:393–402
Quinn G, Keough M (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, ISBN 3-900051-07-0, http://www.R-project.org
Sahli HF, Conner JK (2011) Testing for conflicting and nonadditive selection: floral adaptation to multiple pollinators through male and female fitness. Evolution 65:1457–1473
Shykoff JA, Bucheli E, Kaltz O (1996) Flower lifespan and disease risk. Nature 379:779–780
Sletvold N, Ågren J (2010) Pollinator-mediated selection on floral display and spur length in the orchid Gymnadenia conopsea. Int J Plant Sci 171:999–1009
Sletvold N, Trunschke J, Wimmergren C, Ågren J (2012) Separating selection by diurnal and nocturnal pollinators on floral display and spur length in Gymnadenia conopsea. Ecology 93:1880–1891
Snow AA, Lewis PO (1993) Reproductive traits and male fertility in plants: empirical approaches. Ann Rev Ecol Syst 24:331–351
Stanton ML, Snow AA, Handel SN (1986) Floral evolution: attractiveness to pollinators increases male fitness. Science 232:1625–1627
Stinchcombe JR, Agrawal AF, Hohenlohe PA, Arnold SJ, Blows MW (2008) Estimating nonlinear selection gradients using quadratic regression coefficients: double or nothing? Evolution 62:2435–2440
Talavera S, Gibbs PE, Herrera J (1993) Reproductive biology of Cistus ladanifer (Cistaceae). Plant Syst Evol 186:123–134
Talavera S, Bastida F, Ortiz PL, Arista M (2001) Pollinator attendance and reproductive success in Cistus libanotis L. (Cistaceae). Int J Plant Sci 162:343–352
Teixido AL (2013) Indirect costs counteract the effects of pollinator-mediated phenotypic selection on corolla size in the Mediterranean shrub Halimium atriplicifolium. J Plant Ecol. doi:10.1093/jpe/rtt043
Teixido AL, Valladares F (2013) Large and abundant flowers increase indirect costs of corollas: a study of coflowering sympatric Mediterranean species of contrasting flower size. Oecologia 173:73–81
Teixido AL, Valladares F (2014) Disproportionate carbon and water maintenance costs of large corollas in hot Mediterranean ecosystems. Perspect Plant Ecol 16:83–92
Teixido AL, Méndez M, Valladares F (2011) Flower size and longevity influence florivory in the large-flowered shrub Cistus ladanifer. Acta Oecol 37:418–421
Thomas MM, Rudall PJ, Ellis AG, Savolainen V, Glover BJ (2009) Development of a complex floral trait: the pollinator attracting petal spots of the beetle daisy, Gorteria diffusa (Asteraceae). Am J Bot 96:2184–2196
Thompson JD (2001) How do visitation patterns vary among pollinators in relation to floral display and floral design in a generalist pollination system? Oecologia 126:386–394
Thompson JD (2005) Plant evolution in the Mediterranean. Oxford University Press, New York
Totland Ø (2001) Environment-dependent pollen limitation and selection on floral traits in an alpine species. Ecology 82:2233–2244
Totland Ø (2004) No evidence for a role of pollinator discrimination in causing selection on flower size through female function. Oikos 106:558–564
van Kleunen M, Ritland K (2004) Predicting evolution of floral traits associated with mating system in a natural plant population. J Evol Biol 17:1389–1399
Wade MJ (1979) Sexual selection and variance in reproductive success. Am Nat 114:742–747
Weiss J, Delgado-Benarroch L, Egea-Cortines M (2005) Genetic control of floral size and proportions. Int J Dev Biol 49:513–525
Willmer P (2011) Pollination and floral ecology. Princeton University Press, Princeton
Acknowledgments
We are grateful to J.F. Scheepens and an anonymous reviewer for providing constructive comments to improve the manuscript. A. Greylak reviewed the English. We also thank Drs. J.C. Moreno and V. Mazimpaka for laboratory assistance. We are also grateful to C. García-Agulló for fieldwork support. M.B. held a collaboration grant at Autónoma University of Madrid, Spain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barrio, M., Teixido, A.L. Sex-dependent selection on flower size in a large-flowered Mediterranean species: an experimental approach with Cistus ladanifer . Plant Syst Evol 301, 113–124 (2015). https://doi.org/10.1007/s00606-014-1058-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-014-1058-0