Abstract
Flower size is a key trait in the reproductive ecology of animal-pollinated plants. However, pollinator-mediated selection does not always modulate this trait and environmental conditions and/or antagonist interactions may favor smaller flowers. We evaluate the occurrence of a large-flowered family in a hot and dry Mediterranean environment, mediated by a cost-benefit balance and a male–female conflict. Large flowers have sizeable benefits in terms of pollination and reproductive success and pollinators mediate selection through male function, but female fitness is context-dependent. High floral production and maintenance costs and florivore incidence in large flowers limit female function, which counteracts pollinator-mediated selection. Large flowers are highly costly in the Mediterranean and flower size is mediated by a sexual conflict between the benefits of male function and the costs of the female one. However, a short floral longevity, occasional pollen limitation and selection through maleness keep the existence of large flowers in these environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animal-pollinated plants include a large diversity of flowers, varying in color spectrum, shape and design, scent, longevity, size and display. Historically, numerous botanists and evolutionary biologists have greatly been interested in the study of the processes driving such a diversification (Sprengel, 1793; Darwin, 1862, 1877; Stebbins, 1950, 1970). As a general rule, scientific of both disciplines agree on conferring to pollinators a key role in the evolution of floral variability occurring in natural populations. This assumption is supported by the fact that zoophilous plants rely on pollinators for reproduction and flowers are the structures designed to this purpose. Flowers play an essential role in attracting pollinators, thus providing them energetic and nutritive rewards, whereas pollinators transfer pollen among plants within populations. Overall, the taxonomic diversity of pollinators (birds, insects, mammals and reptiles), which includes a large morphological, functional and behavioral diversity, is matched to diversity of floral variation (Fenster et al., 2004; Aigner, 2005; Smith et al., 2008; Rosas-Guerrero et al., 2014; Gómez et al., 2015). Therefore, pollinators may potentially exert selection on floral traits in plant natural populations.
Flower size is a key trait in the reproductive ecology of animal-pollinated plants. This character is closely linked to pollinator attraction, as larger flowers are more easily detected (Bell, 1985; Ohashi & Yahara, 2001; Lázaro et al., 2013) and contain a greater amount of rewards, such as pollen and nectar (Cruden & Lyon, 1985; Herrera, 1992; Jones, 2001; Willmer, 2011). Flower size has consequently been related to increases in diversity (Andersson, 1988; Aigner, 2005; Cuautle & Thompson, 2010), frequency (Young & Stanton, 1990; Medel et al., 2007), number (Bell, 1985; Johnson et al., 1995; Liao et al., 2009), duration (Conner & Rush, 1996; Thompson, 2001; Nattero et al., 2011) and preference of floral visitors (Young & Stanton, 1990; Brody & Mitchell, 1997). Therefore, flower size greatly favors pollen transfer, thus increasing both male and female fitness (Bell, 1985; Conner & Rush, 1996; Aigner, 2005; Nattero et al., 2011; Barrio & Teixido, 2015). Consequently, many works have documented pollinator-mediated phenotypic selection towards larger flowers (e.g. Stanton et al., 1986; Galen, 1989, 1996; Totland, 2001; Maad & Alexandersson, 2004; Hodgins & Barrett, 2008; Sahli & Conner, 2011; Brothers & Atwell, 2014). Ultimately, processes of diversification in flower size should be followed by among-individual genetic variation so that evolution may occur. Indeed, natural plant populations show a high variability in flower size with a significant inheritability (Andersson & Widén, 1993; Galen, 1996; Ashman & Majestic, 2006). If pollinators exert selective pressures against small flowers and flower size is inherited, then flowers should show little variation in this trait (see Ushimaru et al., 2006). However, smaller flowers and flower size variation still remain within populations.
There are several possible explanations for the prevalence of smaller flowers and apparent variation in flower size. Overall, biotic factors other than pollinators (i.e. floral antagonists) together with abiotic factors such as resource availability and climate can potentially exert selective pressures on flower size and favor smaller flowers (Galen, 1999; Caruso et al., 2005; Strauss & Whittall, 2006; Campbell & Powers, 2015). The occurrence and strength of these factors as selective agents of flower size are mostly context-dependent in relation to environmental conditions and gender (i.e. pollen and/or resource limitation and their differential effects on male and female function; Ashman & Morgan, 2004). Theory predicts that smaller flowers will be potentially advantageous under benign pollination conditions, which reduce pollen limitation, and in hot and dry environments with low resource availability, especially in terms of water (Galen, 1999, 2005). Otherwise, larger flowers may be generally favored by increasing male fitness by means of higher pollen dispersal rates (Bell, 1985; Arista & Ortiz, 2007; Barrio & Teixido, 2015).
In this review, we aim to improve our understanding of possible causes that explain the subsistence of large flowers under hot and dry conditions. Full knowledge about the biotic and abiotic processes acting on the patterns of variation of flower size is essential to understand how plants achieve reproduction in these environments and, consequently, it is of extensive ecological and evolutionary importance. We begin our consideration with a brief introduction to the theoretical frameworks proposed to explain the causes and consequences of the variation in flower size and the expected predictions in hot and dry ecosystems. Second, we show a potential conflict between large flowers and hot and dry conditions by using rockroses (Cistaceae) as a model system, a large-flowered family inhabiting a Mediterranean environment. Finally, we provide data of the extent and variation of the balance between costs and benefits in this study system as well as its consequences about reproductive success to, subsequently, discuss the ecological and evolutionary implications for flower size.
Theoretical Background
Why Does Flower Size Vary?
In a seminal paper, Galen (1999) explained in detail that a unilateral point of view of pollinator-mediated flower size evolution is probably oversimplistic. Selection on flower size is rather a pluralistic process in which not only pollinators are involved, but also some plant enemies (e.g. herbivores, parasites, predators) together with another alternative aspects of plant abiotic environment. In this regard, Galen (1999) proposed both the “resource-cost” and the “enemy-escape” hypotheses to explain the variation of this trait and improve in the understanding about why less-attractive flowers may be evolutionarily favored, thus balancing their low attraction to pollinators by means of a reduction in resource allocation as well as a greater protection from flower enemies. Therefore, an important facet in the maintenance of flower size variation is that those benefits acquired by pollinator attraction and subsequent pollen transfer may be counteracted by an increase in costs of such an attraction.
Flowers require a sizeable amount of resources directly assigned for their production and maintenance (i.e., direct costs; see Chapin, 1989; Ashman & Schoen, 1997; Teixido & Valladares, 2014a). Carbon, water and other nutrients are necessary to increase the pollinator attraction and reward as well as to maintain flowers physiologically active while remaining open, with showy petals, pigments, aromatic compounds and nectar (Cruden & Lyon, 1985; Pyke, 1991; Galen et al., 1993, 1999; De la Barrera & Nobel, 2004a; Witt et al., 2013). Accordingly, larger flowers require greater allocation of biomass and water for their construction (Ågren, 1988; Galen, 1999; Méndez & Traveset, 2003; Herrera, 2009; Halpern et al., 2010; Teixido & Valladares, 2013) as well as higher maintenance costs due to the high respiration and transpiration rates (Vemmos & Goldwin, 1994; Galen et al., 1999; Lambrecht & Dawson, 2007; Teixido & Valladares, 2014a).
A fundamental tenet in the resource economy of plants is that costs derived of the direct allocation of resources are translated into indirect costs (Gulmon & Mooney, 1986; see also Chapin, 1989), that is, in negative effects on other functions, such as the reproductive output. Andersson (1999, 2000, 2001, 2005) quantified indirect costs of floral attractiveness in diverse species. Overall, under controlled conditions of pollination, he found an increase in fruit and seed production in experimentally perianth-removed flowers in relation to unmanipulated control flowers. However, indirect costs of flower size were no take into account, despite the evidence in this regard would provide a strong support to the prediction of Galen’s hypotheses (1999) based on resource allocation to flowers.
Larger-flowered plants have also to deal with additional costs imposed by antagonist animals, such as herbivores, parasites, seed predators and nectar robbers and thieves that obtain food and rewards from their floral visits without offering benefits to pollination (Shykoff et al., 1996; Krupnick et al., 1999; Irwin et al., 2001; Strauss & Whittall, 2006; Ruane et al., 2014). For example, floral herbivores (i.e., florivores) cause damage to open flowers and their structures (McCall & Irwin, 2006). Florivory incidence increases as traits of pollinator attractiveness do, thus increasing with flower size (Galen, 1999; Mosleh Arany et al., 2009; Oguro & Sakai, 2015). The resulting consume and degradation of flowers may reduce the reproductive success of the plant by (1) reducing the number of available flowers and/or permanence of smaller ones, (2) altering the attraction properties to pollinators or (3) directly consuming viable gametes (Schemske & Horvitz, 1988; Krupnick et al., 1999; McCall & Irwin, 2006; Cardel & Koptur, 2010; Althoff et al., 2013). From an evolutionary perspective, florivores may consequently exert negative selective pressures on flower size, making smaller flowers potentially advantageous against these floral enemies (Galen, 1999; Irwin et al., 2001; Irwin, 2006; Althoff et al., 2013).
What Does Theory Predict for Hot and Dry Environments?
Under hot and dry stressful conditions, animal-pollinated plants have to deal with two essential problems: first, water shortage may limit showiness of flowers and therefore pollinator attractiveness; second, high temperatures may induce higher floral physiological maintenance costs. Indeed, production and maintenance of corollas as showy structures to pollinators are particularly costly in terms of water (Galen et al., 1999; Lambrecht, 2013; Teixido & Valladares, 2014a). Many workers have provided experimental evidence for plants producing smaller flowers under drought conditions in a diverse range of species (Carroll et al., 2001; Elle & Hare, 2002; Caruso et al., 2005; Caruso, 2006; Halpern et al., 2010). Although those studies were not particularly focused on plants inhabiting hot and dry ecosystems and drought stress was artificially induced under controlled environmental conditions, results still show that xeric conditions may constrain selection for larger flowers by means a reduced soil water availability.
Additionally, flowers occurring in hot and dry environments may involve increased carbon costs and excessive evaporative demand. Galen and colleagues (Galen et al., 1999; Galen, 2000) reported that leaf photosynthetic rate at the time of flowering significantly declined with increasing corolla size in Polemonium viscosum under dry conditions, related to higher water use. Therefore, water demand by large corollas may influence leaf stomata closure, also constraining carbon gain (see also Lambrecht & Dawson, 2007). Likewise, floral overheating in hot climates can be damaging so transpirational cooling becomes crucial to minimize it (Patiño & Grace, 2002; Galen, 2005). However, water shortage in dry environments can lead to an inefficient thermoregulation. Heat and drought, acting together, can disrupt the normal performance of flowers, affecting both fruit and seed production (Konsens et al., 1991; Galen, 2000; Erickson & Markhart, 2002; Lambers et al., 2008; Fang et al., 2010).
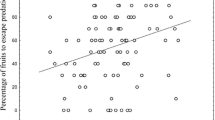
Following these assumptions, minimizing floral water loss by reducing corolla size should be potentially advantageous for plants living in these environments (Galen, 2000, 2005; Elle & Hare, 2002; Herrera, 2005). In fact, here we exemplified this pattern by surveying different plant communities occurring across a temperature and precipitation gradient in a Mediterranean ecosystem (Fig. 1). Species of communities inhabiting hotter and drier habitats significantly display smaller flowers than those ones flowering under wetter conditions (Fig. 1a). This pattern is interestingly taxonomic scale-independent as shows consistent repeatability both at family- and species-level, at least in Cistaceae, the family used as study system in this review (Fig. 1b, c). Hence, small flowers are not uncommon in hot and dry environments and seem to be the adaptative feature under these conditions.
However, some large-flowered plants do occur in hot and dry ecosystems. As a general rule, these species show adaptations to prevent overheating and excessive water loss. For example, nocturnal flowering and pollination are features of several caperbushes (Capparis sp.) of semi-arid areas (Rhizopoulou et al., 2006) and most large-flowered desert cacti (Valiente-Banuet et al., 1997; Fleming et al., 2001). Floral cooling mechanisms appeared to be critical for the reproductive success of large convolvulaceous flowers in hot tropical environments (Patiño & Grace, 2002). Likewise, floral longevity is usually ephemeral according to fast fitness accrual rates occurring in large flowers under hot and dry conditions (Primack, 1985; Ashman & Schoen, 1994; Galen, 2005; Teixido & Valladares, 2015). Still, a few species display diurnal-pollination large flowers under hot and dry conditions. Therefore, these species represent a good model system to understand the selective pressures acting on flower size and why large flowers occur in these environments.
Study System: Large-Flowered Mediterranean Plants
Living and Flowering in a Hot and Dry Environment
High temperatures and water shortage over the summer generally characterize the climate in the Mediterranean region (Blondel & Aronson, 1999; Quézel & Médail, 2003; Thompson, 2005; see Fig. 2). Annual rainfall is concentrated into a small number of events along a short seasonal peak and soil moisture becomes decreasingly available during the drought period. As the progressive drought sets in, stomatal control is probably the most effective means of regulating water loss due to transpiration (Joffre et al., 1999). Stomatal closure may provide an effective control on water stress by allowing plants to increase water use efficiency during summer drought. Stomatal closure in response to water stress will also cause reduced gas exchange and thus reduce photosynthesis. However, reduction in transpiration may result in overheating, a problem that can be exacerbated by high solar irradiance and temperature. If excessive, such overheating can cause photo-inhibitory damage (Joffre et al., 1999). In addition to these constraints, Mediterranean plants have also to deal with a nutrient deficiency and a high incidence of herbivore insects (Thompson, 2005). Therefore, several conditions can thus simultaneously limit plant growth, survival and reproduction in these environments.
Typical Mediterranean climatic diagram based on data recorded in Tres Cantos, Madrid, Spain, where several populations of Cistaceae were studied. N = 40 years (Ninyerola et al., 2005)
Under these circumstances, Mediterranean plants have developed an array of strategies to cope with such constraints. Specifically, phenologically and morphophysiologically conservative resource use strategies and water use efficiency appear to be adaptative and to play a key role in these environments (Joffre et al., 1999; Valladares et al., 2000, 2008; Larcher, 2000; Thompson, 2005; Zaragoza‐Castells et al., 2008). Thus, tolerance and avoidance of drought stress are two non-independent characteristic features of Mediterranean vegetation. For example, sclerophyllous evergreen habit of trees and shrubs seems to be a well-fitted trait to Mediterranean stress conditions (Duhme & Hinckley, 1992; Valladares et al., 2004). The combination of morphological and physiological traits associated with sclerophylly may facilitate tolerance of negative turgor pressure under water stress as well as a higher degree of stomatal regulation (Duhme & Hinckley, 1992). The nutrient acquisition and allocation strategy of sclerophyllous species may also permit growth in a nutrient-poor soil and their chemical defence may assure long leaf lifetime. Additionally, one of the most characteristic features in the Mediterranean is the production of secondary volatile compounds, such as essential oils (Ross & Sombrero, 1991; Seufert et al., 1995). These chemical compounds may likewise mediate in defence against plant enemies and improve tolerance of water constraints and high solar radiation (Joffre et al., 1999; Thompson, 2005).
During the reproductive stage, plants may avoid and tolerate water stress by speeding up their development, shortening flowering duration (Larcher, 2000; Thompson, 2005; Aragón et al., 2008) and, occasionally, delaying the initiation of flowering until the rainy season to maximize water use efficiency (Blionis et al., 2001; Verdú et al., 2002). Many other species show an annual life history, avoiding the summer drought as dormant seeds and only germinating and flowering during the rainy season. Large and highly persistent seed banks in opposition to individual long life spans are not uncommon in the driest Mediterranean areas (Caballero et al., 2005; Aragón et al., 2009; Olano et al., 2012). Additionally, low investment in nectar reward is another common drought-avoiding feature in many Mediterranean plant species (Herrera, 1985; Petanidou et al., 2000; Potts et al., 2001). Likewise, insect diversity in the Mediterranean is high, so floral attractiveness has also to deal with greater plant-animal antagonist interactions. In several Mediterranean species, pre-dispersal flower, fruit and seed predators have been reported to have significant impact on fitness, and thus potentially constrain selection on flower size related to pollinators (Herrera, 2000, 2002; Gómez, 2003).
If conservative water use strategies are critical for the plants to flower in the Mediterranean region and this required process implies a sizeable water allocation in terms of floral production and maintenance under hot and dry conditions, water investment-based arguments to explain the potential advantages of smaller flowers in this ecosystem would be expected (Galen, 1999). Indeed, three studies in the Mediterranean ecosystem showed that flowers of Narcissus triandrus (Barrett et al., 2004), Rosmarinus officinalis (Herrera, 2005) and Cistus salviifolius (Herrera, 2009) were smaller in hotter and drier populations. Our survey for this review goes further and shows that this pattern is not only apparent at specific-level, but also happens at both family- and community-level (Fig. 1). Therefore, variation in flower size follows an ecological gradient, increasing from warmer and drier to less stressful conditions. Likewise, antagonist-mediated selection has been reported to limit flower size in several Mediterranean species (Herrera, 1993, 2000, 2002; Gómez, 2003). From an evolutionary perspective, the presence of large and costly flowers contrasts with the expected mechanisms of “cost of resources” and “enemy-escape”, that would favour small flowers in these environments (Galen, 1999).
Cistaceae: a Large-Flowered Mediterranean Family
Cistaceae (so-called rockroses) is a family comprising eight genera (Cistus, Crocanthemum, Fumana, Halimium, Helianthemum, Hudsonia, Lechea and Tuberaria) and about 200 species of shrubs and herbs inhabiting temperate zones of Northern Hemisphere and South America (Arrington & Kubitzki, 2003). Its center of diversification is the Mediterranean region, especially in the Iberian Peninsula (up to 64 species), wherein occur widely distributed the five Mediterranean genera (i.e. Cistus, Fumana, Halimium, Helianthemum and Tuberaria; Muñoz-Garmendía & Navarro, 1993). The other three genera only appear in the American continent, from the middle east of the United States and southern Canada, to Central America, especially in Mexico, and temperate zones of South America (Arrington & Kubitzki, 2003). Around the Mediterranean region, species of this family usually occur in hot and open areas with rocky dry soils, calcareous or acidic, forming scrublands and herb understory of the sclerophyllous mixed formations dominated by Pinus and Quercus (Muñoz-Garmendía & Navarro, 1993; Arrington & Kubitzki, 2003).
Flowering occurs almost exclusively during the spring, at earliest in February, and can readily extend up to July (Muñoz-Garmendía & Navarro, 1993). There are some annual herbaceous species, but the vast majority are perennial and polycarpic shrubs. Flowers are hermaphrodites and typically chasmogamous, with five yellow, white or pink-purplish petals conforming a disc-shaped corolla. Anthesis occurs early in the morning and synchronously within populations (Herrera, 1992). Self-incompatibility is common across the family, but some herbs and smaller-flowered species can show self-pollination and cleistogamous flowers (Herrera, 1992; Talavera et al., 1993, 1997; Rodriguez-Perez, 2005; Aragón & Escudero, 2008; Guzmán et al., 2015).
Although flower size is somewhat variable, the perennial shrub genera Cistus and Halimium often show large flowers (Fig. 1c). Most interestingly, Cistus ladanifer is one of the largest-flowered species in the Mediterranean area, with diameters that can exceed 10 cm (Arrington & Kubitzki, 2003; Teixido et al., 2011). Stamens of these genera are usually numerous and their anthers contain large amounts of pollen grains, which is positively correlated with flower size (Bosch, 1992; Herrera, 1992; Arista & Ortiz, 2007). These species also contain high pollen-to-ovule ratios, consistent with outcrossing breeding systems (Bosch, 1992; Herrera, 1992). In fact, formal experimental studies have reported that these species are mostly self-incompatible and they depend on a vast spectrum of pollinators for reproduction (Brandt & Gottsberger, 1988; Bosch, 1992; Herrera, 1992; Talavera et al., 1993, 1997, 2001; Teixido & Valladares, 2014b; Guzmán et al., 2015). Overall, these Cistus and Halimium large-flowered species constitute a model study system to exhaustively evaluate the selective pressures operating on flower size and the conflicts faced by large flowers in a hot and dry environment such as the Mediterranean ecosystem.
Here we provide observational and empirical data by reviewing those studies focused on large-flowered rockroses comprising surveys of pollinators, reproductive success, phenotypic selection, direct costs of floral production and maintenance in terms of carbon and water, indirect costs in terms of fruit and seed production, and florivory (Table 1). Overall, our review includes seven species of large-flowered Cistaceae showing among- and within-population variation in flower size and occurring across 18 different populations with contrasting climatic conditions. Additionally, we show unpublished data collected from our own research in the relation between resource allocation for floral production with flower size among 37 species of Cistaceae. We analyse the effect of such variations in flower size and environmental conditions on the benefit-cost balance of displaying large flowers and, consequently, its ecological and evolutionary consequences at three different levels. First, at interspecific level, i.e., among species with contrasting flower size. Second, at intraspecific level or among populations within of a particular species. Third, among individuals showing variation in flower size within populations.
Understanding the Matter: Ecology and Evolutionary Aspects
Balance Between Costs and Benefits
Pollinators and Reproductive Success
The interactions between large-flowered Cistaceae and pollinating agents show the generalist character of these species. Large and showy flowers are rewarded for the visit of a high number and diversity of pollinators, including ants, bees, bumblebees, beetles, flies and wasps of different size and behaviour (Bosch, 1992; Talavera et al., 1997, 2001; Teixido & Valladares, 2014b). Interestingly, the amount of pollinating visitors significantly increase with interspecific variation in flower size (Fig. 3). Bosch (1992) reported higher indexes of attractiveness in the largest-flowered species, Cistus albidus, among three coflowering Cistus with contrasting flower size. Based on Talavera et al.’s (2001) study with Cistus libanotis and data from our own research with Cistus ladanifer (Teixido & Valladares, 2014b; Barrio & Teixido, 2015), we calculated a mean attractiveness index for these species following the Bosch’s (1992) methodology. A regression model for all the indexes of attractiveness on flower size for those five Cistus species reveals the significant benefits reached by larger-flowered species in terms of pollinator visit rates (Fig. 3). Specifically, a two-fold increase in flower size entails about an attractiveness index five-fold higher. Therefore, the largest-flowered species C. ladanifer shows a very beneficial attractiveness in relation to other smaller-flowered Cistus species.
Mean index of attractiveness for five Cistus species (C. albidus. C. ladanifer, C. libanotis, C. monspeliensis and C. salviifolius) with contrasting flower size. Indexes were calculated following Bosch (1992)
Overall, a direct consequence of the numerous and diverse plant-pollinator interaction in Cistus is a double benefit in sexual terms, as these species are hermaphrodites. On the one hand, this favours a high set of fruit and seed production, which readily exceeds 80 % (Bosch, 1992; Talavera et al., 2001; Arista & Ortiz, 2007; Guzmán et al., 2011; Teixido & Valladares, 2014b). On the other hand, flowers have a great amount of pollen grains and this noticeably facilitates their dispersal (Arista & Ortiz, 2007; Barrio & Teixido, 2015). In particular, this increased male and female reproductive output is sizeable in C. ladanifer, as flowers of this species produce a high pollen and ovule amount (Herrera, 1992; Talavera et al., 1993). This largely favours pollen dispersal and seed production, not only by increasing pollinator visit rates and diversity, but also by having more pollen and ovules available. Pollen production in this species can readily exceed 750,000 grains/flower whereas the number of ovules reaches up to 1500/flower (Talavera et al., 1993), which involves about a ten-fold increase in relation to other Cistus species (Herrera, 1992). Consequently, the values of male and female reproductive success in C. ladanifer are particularly high.
The abundance of pollinating insects visiting flowers and, subsequently, the high fruit and seed production, entails that there is an adequate pollen quantity deposited on stigmas. This is also noteworthy since may happen in only a few hours, as flowers generally last one day open (Teixido & Valladares, 2014c). In fact, when flower size was experimentally reduced in C. salviifolius and C. ladanifer, flowers still produce high percentages of fruits and seeds (Arista & Ortiz, 2007; Barrio & Teixido, 2015). Therefore, an additional advantage of flower size is that pollination visitation is promptly enough to fertilize all the ovules and thus easily reaching a high reproductive success.
However, pollen quantity limitation is not an uncommon pattern in large-flowered rockroses and here is where flower size variation within-populations becomes relevant. This variation plays a key role as larger-flowered individuals significantly increase pollinator visit rates (Teixido & Valladares, 2014b; Barrio & Teixido, 2015). In addition, flower size is positively related to ovule number (Herrera, 1992). Thus, smaller-flowered individuals not only have less available ovules, but also receive less pollen. Although pollinators are abundant and diverse, visitation decreases in relation to larger flowers and may not be sufficient to fertilize all the ovules. This involves that in pollen-limited populations pollinators may potentially exert selection towards larger flowers through female fitness (Arista & Ortiz, 2007; Teixido & Valladares, 2014b; see also Table 2). Therefore, fruit and seed production in larger-flowered individuals may be even more favoured by means a differential increase of offspring.
The positive relationship between flower size and pollinators also entails benefits to male function. Larger-flowered individuals have more anthers, disperse higher pollen amounts and could potentially sire a higher number of seeds. However, this hypothesis has not been accurately tested in our study system, mainly due to the complexity of measuring male function in natural populations. Molecular analysis with genetic markers, including DNA extraction and genotyping are needed to record direct estimates of male reproductive success (reviewed in Conner, 2006). Though indirect methods such as the amount of dispersed pollen do not necessarily imply paternity success, pollen dispersal is a representative component of male success and is a useful measure to disentangle the mechanisms, the strength and the direction of phenotypic selection through this sex (Snow & Lewis, 1993; Maad & Alexandersson, 2004; Kuriya et al., 2015). In this regard, formal studies with C. salviifolius (Arista & Ortiz, 2007) and C. ladanifer (Barrio & Teixido, 2015) have shown that pollinator-mediated selection towards larger flowers is frequent and intense through pollen dispersal (Table 2).
Direct Costs: Floral Production and Maintenance
However, the production of large and showy flowers requires a sizeable resource allocation and their maintenance a high energetic demand and water uptake to remain physiologically active. In Cistaceae, sexual structures (carpels and stamens) comprise important amounts of nitrogen and phosphorous, whereas perianth (calix and corolla) require a high investment in terms of carbon (dry mass) and water (fresh mass) (Herrera, 2009; Teixido & Valladares, 2013; Teixido, 2014). In our survey of 37 species of rockroses, we detected that floral resource allocation in terms of dry mass and nutrients exponentially increases with flower size (Fig. 4). This means that the investment in floral production follows an allometric pattern, i.e., larger flowers not only are more costly for being larger, but also allocate more resource by unit of area. Likewise, attractiveness to pollinators is correspondingly expensive, as corollas involve about one-third of floral dry mass with a high investment of carbon (Teixido & Valladares, 2013; Teixido, 2014) and about a half of allocation in terms of fresh mass (Herrera, 2009). Specifically, the largest-flowered species (C. ladanifer) requires up to a five-fold more resource allocation to petals than other large-flowered Cistus (Teixido & Valladares, 2013). Interestingly, investment to floral attractiveness may also show an intraspecific variation in relation to habitat type. Herrera (2009) found that upland populations of C. salviifolius displayed larger and heavier flowers with greater fresh mass allocation to corollas than lowland, drier populations. Therefore, water conservation-based local selective pressures may probably maintain considerable levels of intraspecific floral variation and reduce flower size in hotter and drier sites.
In terms of floral maintenance, water costs related to evapotranspiration are also high and allometrically increase in larger-flowered species, especially under hotter and drier conditions (Teixido & Valladares, 2014a). As shown in Fig. 5, mean floral transpiration rates in C. ladanifer are about six-fold higher than those of C. albidus, whereas difference in flower size between both species is about a two-fold magnitude. At intraspecific level, larger-flowered individuals of C. ladanifer significantly transpire more and, additionally, tend to spend about four-fold more water in sunny days than during cloudy days (Teixido & Valladares, 2014a). In fact, corollas of C. ladanifer reach transpiration rates similar to those of large-flowered cacti inhabiting arid climates (Nobel & De la Barrera, 2000; De la Barrera & Nobel, 2004b). Only the transpiration of these corollas, without considering other floral structures, entails the use of two litres of water during the flowering period. On a similar organ surface area basis, corollas also require more than a half of leave water use, which is noticeable because corollas remain open and functional only about 6 h (Teixido & Valladares, 2015).
In addition to water costs, floral energetic demands require maintenance costs in terms of carbon. Net exchange rates in petals are negative, which indicates low or non-existent photosynthetic production in relation to carbon loss mediated by respiration (Teixido & Valladares, 2014a). Respiratory demands recorded in corollas of C. ladanifer (−0.91 μmol CO2 m−2 s−1) reach up to 64 % of leaf respiration rates (−1.42 μmol CO2 m−2 s−1) per unit of area and time and quadruple those of species inhabiting cold habitats (Galen et al., 1993; Teixido & Valladares, 2014a). Higher respiration rates are expected to happen in hotter environments because plant respiration increases as a function of temperature (Atkin & Tjoelker, 2003). Still, floral net carbon exchange rate in C. ladanifer suggests high respiratory demands in large-flowered Mediterranean species. Besides petal respiration, flower size may also involve additional costs, such as nectar production and calix, carpel and stamen respiration.
Indirect Costs: Negative Effects on Reproductive Output
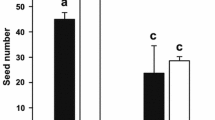
Direct resource allocation to floral production and maintenance impose significant limits to female reproductive success. Therefore, there also are indirect costs in terms of fruit and seed production, as detected under controlled conditions of pollination and experimentally removing floral attractiveness structures (Andersson, 1999, 2000, 2001, 2005). In Cistaceae, indirect costs were noticeable as the relative gain of fruits in petal-removed flowers ranged 11–33 % and that of seeds 8–16 % in relation to control unmanipulated flowers (Fig. 6). This range of variation was significantly related to interspecific and intraspecific differences in flower size. Furthermore, the intensity of relative gain of fruits showed significant differences with local climatic conditions. For example, C. ladanifer, the largest-flowered species, had the highest rates of relative gain of fruits and seeds, and this gain showed a two-fold increase in terms of fruits in the driest population (Fig. 6). For the smaller-flowered species with less interspecific variation in flower size, those occurring in drier sites showed a significant increase of reproductive output between petal-removed and control flowers in relation to wetter sites, at least in terms of fruits (i.e. C. albidus and H. atriplicifolium; Fig. 6; see also Table 1 for mean specific flower size and local climatic conditions).
Comparison of relative gain of fruits (grey bars) and seeds (white bars) for petal-removed and control flowers among different species of Cistaceae. Different letters between relative gain of fruits (grey bars) and between relative gain of seeds (white bars), respectively, indicate significant differences among species using an unequal HSD post hoc test (p < 0.05)
Indirect costs for fruit set also increased with flower size for all four studied species (Teixido & Valladares, 2013; Teixido, 2014). Most interestingly, the relationship between relative gain of fruits and flower size was also greater in the larger-flowered species in the sympatric coflowering pair C. ladanifer–C. laurifolius (Teixido & Valladares, 2013). Overall, these results suggest that the functional and physiological maintenance of corollas involves indirect costs in terms of fruit and seed production. This is especially relevant in larger-flowered species and/or under the driest and hottest, most stressful conditions. Therefore, petal-removed flowers may increase their reproductive output by saving water and other resources, such as carbon, to maintain petals physiologically active.
Ecological Costs in Terms of Florivory
Lastly, larger flowers entail additional costs to production and maintenance by increasing florivory incidence, which could involve more limitations to fruit and seed production. Besides, pollen consume and degradation of floral attractiveness derived from florivore damage may constrain male fitness. However, nothing is known about the effects of florivory on reproductive output or as selective mechanism on flower size in Cistaceae or any large-flowered Mediterranean species. To our best knowledge, Teixido et al. (2011) reported the only study about florivore incidence in relation to flower size variation focused on the large-flowered C. ladanifer. Several ant species picking stamens and beetles consuming petals and pollen were the main florivores (Fig. 7) and the incidence of florivory was significantly and positively influenced by flower size in three populations, with florivory probabilities of approximately 18–35 % on largest flowers. Although the impact of this incidence on fitness was not studied, the negative effects of florivores on reproductive output and their selective pressures on flower size reported by several studies (Galen, 1999; Irwin et al., 2001; Irwin, 2006; Althoff et al., 2013; Ruane et al., 2014) lead to suppose that smaller flowers may be potentially advantageous against these enemies.
Ecological Context and the Evolutionary Battle of the Sexes
Overall, we have pointed out that flower size in our study system is adjusted to a cost-benefit balance, but this process is closely linked to sex. Sexual allocation theory suggests that selection on flower size should be stronger through male fitness whereas the female one is resource-limited (“male function” hypothesis, see Wade, 1979; Burd & Callahan, 2000; Jones, 2008). Although this general statement is controversial and seems to be more dependent on ecological context (see Ashman & Morgan, 2004 for details), this is the pattern commonly reported in Cistaceae (Arista & Ortiz, 2007; Barrio & Teixido, 2015). Indeed, despite a predominance of a directional and positive phenotypic selection towards larger flowers through pollen dispersal, the costs derived from Mediterranean stressful conditions wherein rockroses occur may reduce female reproductive success and, ultimately, impose constrains to flower attractiveness.
In Cistaceae, it is not uncommon the absence of phenotypic selection on flower size through female fitness components (Arista & Ortiz, 2007; Teixido, 2014; Teixido & Valladares, 2014b; Barrio & Teixido, 2015). Furthermore, resource limitation resulting from floral physiological maintenance and subsequent indirect costs in terms of fruit and seed production (i.e. female fitness) involve processes of stabilizing selection on flower size (Teixido, 2014; Table 2). This implies that must exist a combined action of pollen limitation with resource limitation and subsequent floral costs. In this regard, small increases in flower size within a given population would increase fruit and seed set by reducing pollen limitation until flower size becomes too large and, then, elevated floral costs counteract the benefits of high rates of pollen deposition, thus reducing fruit and seed production.
When both sexes have different optima for those traits related to reproductive success, there may be a sexual conflict giving rise to traits in equilibrium (Chapman, 2006). Therefore, pollinator attractiveness itself in natural populations of Cistaceae does not always involve selection towards larger flowers and evolution on this trait may be limited by opposite evolutionary pressures of both sexes that could lead to sexual selection conflicts on flower size. Specifically, these opposite selective pressures could be also acting on female fitness components, related to the strength of pollen and resource limitation and, ultimately, influenced by an ecological context. We detected that flower size showed temporal variation in two populations of C. ladanifer in response to phenotypic selection on fruit set or seed number in the year before, increasing flower size when there was positive directional selection and decreasing when was stabilizing (Teixido & Valladares, 2014b). Hence, total selection exerted on flower size in this large-flowered species may depend strongly on whether plants are more pollen or resource limited in their female reproduction, respectively. This pattern suggests the important role that female function, the more resource-limited gender, plays in the plasticity and temporal variation of flower size and in the potential of buffering costs and modulating this trait in Cistaceae.
Concluding Remarks
Understanding how large flowers occur in hot and dry environments such as the Mediterranean, an anomalous trait in this ecosystem, is of extensive ecological and evolutionary importance. Flowers are the most diverse structures produced by angiosperms and knowledge about plant-animal and plant-environment interactions are essential to explain their patterns of diversification and the mechanisms, limitations and processes carried out to achieve reproduction. Additionally, increases in temperature and decreases in precipitation induced by climate change in the Mediterranean could disrupt the plant-pollinator interactions and damage flowers, thus limiting their reproductive success and, consequently, imposing selective constraints to flower size. There are abundant evidence about the growth and survival limitations to which Mediterranean plants are subjected. However, little is known about flowering costs and the effects of flower size, an additional cost potentially awkward for plants inhabiting this ecosystem. Considering this knowledge gap, we addressed this question by reviewing the scarce experimental and observational data on this topic using a large-flowered family to reach a broader perception about the benefits, costs and functional limitations of large flowers in this stressful environment.
Here, we confirm that flower size is a relevant trait in animal-pollinated hermaphroditic plants as large flowers are exposed to strong selective pressures imposed by a cost-benefit balance and a sexual conflict in a stressful environment. Overall, high reproductive benefits achieved by large-flowered Cistaceae can be counteracted by the environmental stress inherent to the Mediterranean ecosystem, which limits the advantages of flower size (especially through the female fitness). Therefore, our review supports the idea that the relative strength and direction of selection on flower size is not only pollinator-dependent. However, pollinator-mediated selection for increasing flower size through both components of fitness is feasible, keeping large flowers in this system. Specifically, we have identified several conclusive assumptions as well as several open questions that deserves future research:
-
(1)
Large flowers obtain benefits in terms of pollinator visit rates and diversity with direct effects on fruit and seed production, which is usually high, so pollen limitation on female fitness is not uncommon in this study system. However, pollen availability may show different levels between populations and years according to pollinator environment and selection through female function components is feasible to occur. Otherwise, the differential pollinator visitation on variation in flower size always translates into selection on larger flowers through male fitness. Despite this evidence, we propose that molecular analyses with genetic markers would be needed to record direct estimates of male reproductive success.
-
(2)
Floral production and maintenance costs are especially high in large flowers, especially in terms of water physiological use by transpiration under the hottest and driest conditions. Overall, these costs inherent to flower attractiveness and functional activity involve indirect costs in terms of fruit and seed production, which significantly increases in both larger-flowered individuals within-populations and larger-flowered species. Therefore, floral carbon and water costs limit female reproductive output and, consequently, exert abiotic negative selective pressures on flower size. It would be especially informative to study the relative importance of indirect costs as selective agents in relation to pollinators, especially between coflowering species with contrasting flower size.
-
(3)
Short floral longevity reported in the largest-flowered species C. ladanifer is in accordance with the elevated floral costs of this species. This pattern suggests that large-flowered plants blooming under hot and dry environments have to be short-lived, mediated by a fast fitness accrual rate, which involves an early and efficient pollination and a decrease in floral maintenance costs. Overall, this ephemeral floral longevity together with the high fruit and seed production, ecological context-dependent female pollen limitation and selection through maleness may keep the existence of large-flowered species in a Mediterranean environment.
-
(4)
Lastly, larger flowers also entail costs in terms of florivory as are more likely attacked by antagonist insects, such as pollen- and petal-eater ants and beetles. Although we did not know the effects of this interaction on reproductive output and evolutionary consequences on flower size, florivores could readily exert relevant selective pressures on this trait, counteracting the positive effects of pollinators. It would be interesting to analyse this pattern as well as its relative importance in a multivariate fitness function estimating selection generated by pollinators and florivores on flower size through male and female function.
Literature Cited
Ågren, J. 1988. Sexual differences in biomass and nutrient allocation in the dioecious Rubus chamaemorus. Ecology 69: 962–973.
Aigner, P. A. 2005. Variation in pollination performance gradients in a Dudleya species complex, can generalization promote floral divergence? Functional Ecology 19: 681–689.
Althoff, D. M., W. Xiao, S. Sumoski & K. A. Segraves. 2013. Florivore impacts on plant reproductive success and pollinator mortality in an obligate pollination mutualism. Oecologia 173: 1345–1354.
Andersson, S. 1988. Size-dependent pollination efficiency in Anchusa officinalis (Boraginaceae): causes and consequences. Oecologia 76: 125–130.
——— 1999. The cost of floral attractants in Achillea ptarmica (Asteraceae), evidence from a ray removal experiment. Plant Biology 1: 569–572.
——— 2000. The costs of flowers of Nigella degenii inferred flower and perianth removal experiments. International Journal of Plant Sciences 16: 903–908.
——— 2001. Fitness consequences of floral variation in Senecio jacobaea (Asteraceae): evidence from a segregating hybrid populations and a resource manipulation experiment. Biological Journal of the Linnean Society 74: 17–24.
——— 2005. Floral costs in Nigella sativa (Ranuncualceae): compensatory responses to perianth removal. American Journal of Botany 92: 279–283.
——— & B. Widén. 1993. Pollinator-mediated selection on floral traits in a synthetic population of Senecio intergrifolius (Asteraceae). Oikos 66: 72–79.
Aragón, C. F. & A. Escudero. 2008. Mating system of Helianthemum squamatum (Cistaceae), a gypsophile specialist of semi-arid Mediterranean environments. Botanica Helvetica 118: 129–137.
———, ——— & F. Valladares. 2008. Stress-induced dynamic adjustments of reproduction differentially affect fitness components of a semi-arid plant. Journal of Ecology 96: 222–229.
———, M. Méndez & A. Escudero. 2009. Survival costs of reproduction in a short-lived perennial plant: live hard, die young. American Journal of Botany 96: 904–911.
Arista, M. & P. L. Ortiz. 2007. Differential gender selection on floral size: an experimental approach using Cistus salviifolius. Journal of Ecology 95: 973–982.
Arrington, J. M. & K. Kubitzki. 2003. Cistaceae. Pp 62–70. In: K. Kubitzki, C. Bayer, & P. F. Stevens (eds). The families and genera of vascular plants vol. V. Springer, Berlin.
Ashman, T.-L. & C. J. Majestic. 2006. Genetic constraints on floral evolution: a review and evaluation of patterns. Heredity 96: 343–352.
——— & M. T. Morgan. 2004. Explaining phenotypic selection on plant attractive characters: male function, gender balance or ecological context? Proceedings of the Royal Society of London B Series 271: 553–559.
——— & D. J. Schoen. 1994. How long should flowers live? Nature 371: 788–791.
——— & ———. 1997. The cost of floral longevity in Clarkia tembloriensis: an experimental investigation. Evolutionary Ecology 11: 289–300.
Atkin, O. K. & M. G. Tjoelker. 2003. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in Plant Science 8: 343–351.
Barrett, S. C. H., L. D. Harder & W. W. Cole. 2004. Correlated evolution of floral morphology and mating-type frequencies in a sexually polymorphic plant. Evolution 58: 964–975.
Barrio, M. & A. L. Teixido. 2015. Sex-dependent selection on flower size in a large-flowered Mediterranean species: an experimental approach with Cistus ladanifer. Plant Systematics and Evolution 301: 113–124.
Bell, G. 1985. On the function of flowers. Proceedings of the Royal Society of London B Series 224: 223–265.
Blionis, G. J., J. M. Halley & D. Vokou. 2001. Flowering phenology of Campanula on Mt Olympos, Greece. Ecography 24: 696–706.
Blondel, J. & J. Aronson. 1999. Biology and wildlife of the Mediterranean region. Oxford University Press, Oxford.
Bosch, J. 1992. Floral biology and pollinators of three co-occurring Cistus species (Cistaceae). Botanical Journal of the Linnean Society 109: 39–55.
Brandt, U. & G. Gottsberger. 1988. Flower phenology, pollinating insects and breeding systems in Cistus, Halimium and Tuberaria species in Portugal. Lagascalia 15: 625–634.
Brody, A. K. & R. J. Mitchell. 1997. Effects of experimental manipulation of inflorescence size on pollination and pre-dispersal seed production in the hummingbird-pollinated plant Ipomopsis aggregata. Oecologia 110: 86–93.
Brothers, A. N. & J. W. Atwell. 2014. The role of pollinator-mediated selection in the divergence of floral traits between two closely related plant species. International Journal of Plant Sciences 175: 287–295.
Burd, M. & H. S. Callahan. 2000. What does the male function hypothesis claim? Journal of Evolutionary Biology 13: 735–742.
Caballero, I., J. M. Olano, A. L. Luzuriaga & A. Escudero. 2005. Spatial coherence between seasonal seed banks in a semi-arid gypsum community: density changes but structure does not. Seed Science Research 15: 153–160.
Campbell, D. R. & J. M. Powers. 2015. Natural selection on floral morphology can be influenced by climate. Proceedings of the Royal Society of London B Series 282: 20150178.
Cuautle, M. & J. N. Thompson. 2010. Diversity of floral visitors to sympatric Lithophragma species differing in floral morphology. Oecologia 2010: 71–80.
Cardel, Y. J. & S. Koptur. 2010. Effects of florivory on the pollination of flowers: an experimental study with a perennial plant. International Journal of Plant Sciences 171: 283–292.
Carroll, A. B., S. G. Pallardy & C. Galen. 2001. Drought stress, plant water status, and floral trait expression in fireweed, Epilobium angustifolium (Onagraceae). American Journal of Botany 88: 438–446.
Caruso, C. M. 2006. Plasticity of inflorescence traits in Lobellia siphilitica (Lobeliaceae) in response to soil water availability. American Journal of Botany 93: 531–538.
———, D. L. D. Remington & K. E. Ostergren. 2005. Variation in resource limitation of plant reproduction influences natural selection on floral traits of Asclepias syriaca. Oecologia 146: 68–76.
Chapin, F. S., III. 1989. The cost of tundra plant structures: evaluation of concepts and currencies. American Naturalist 133: 1–19.
Chapman, T. 2006. Evolutionary conflicts of interest between males and females. Current Biology 16: 744–754.
Conner, J. K. 2006. Ecological genetics of floral evolution. Pp 260–277. In: L. D. Harder & S. C. H. Barrett (eds). Ecology and evolution of flowers. Oxford University Press, Oxford, UK.
——— & S. Rush. 1996. Effects of flower size and number on pollinator visitation to wild radish, Raphanus raphanistrum. Oecologia 105: 509–516.
Cruden, R. W. & D. L. Lyon. 1985. Patterns of biomass allocation to male and female functions in plants with different mating system. Oecologia 66: 299–306.
Darwin, C. 1862. On the various contrivances by which British and foreign orchids are fertilized by insects. John Murray, London, UK.
——— 1877. The different forms of flowers on plants of the same species. John Murray, London, UK.
De la Barrera, E. & P. S. Nobel. 2004a. Nectar: properties, floral aspects, and speculations on origin. Trends in Plant Science 9: 65–69.
——— & ———. 2004b. Carbon and water relations for developing fruits of Opuntia ficus-indica (L.) Miller, including effects of drought and gibberellic acid. Journal of Experimental Botany 55: 719–729.
Duhme, F. & T. M. Hinckley. 1992. Daily and seasonal ariation in water relations of macchia shrubs and trees in France (Montpellier) and Turkey (Antalya). Vegetatio 100: 185–198.
Elle, E. & J. D. Hare. 2002. Environmentally induced variation in floral traits affects the mating system in Datura wrightii. Functional Ecology 16: 79–88.
Erickson, A. N. & A. H. Markhart. 2002. Flower development stage and organ sensivity of bell pepper (Capsicum annuum L.) to elevated temperature. Plant Cell and Environment 25: 123–130.
Fang, X., N. C. Turner, G. Yan, F. Li & K. H. M. Siddique. 2010. Flower numbers, pod production, pollen viability, and pistil function are reduced and flower and pod abortion increased in chickpea (Cicer arietinum L.) under terminal drought. Journal of Experimental Botany 61: 335–345.
Fenster, C. B., W. S. Armbruster, P. Wilson, M. R. Dudash & J. D. Thomson. 2004. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution and Systematics 35: 375–403.
Fleming, T. H., C. T. Sahley, J. Nathaniel-Holland, J. D. Nason & J. L. Hamrick. 2001. Sonoran desert columnar cacti and the evolution of generalized pollination systems. Ecological Monographs 71: 511–530.
Galen, C. 1989. Measuring pollinator-mediated selection on morphometric floral traits, bumblebees and the alpine sky pilot, Polemonium viscosum. Evolution 43: 882–890.
——— 1996. Rates of floral evolution: adaptation to bumblebee pollination in an alpine wildflower, Polemonium viscosum. Evolution 50: 120–125.
——— 1999. Why do flowers vary? The functional ecology of variation in flower size and form within natural plant populations. Bioscience 49: 631–640.
——— 2000. High and dry, drought stress, sex-allocation trade-offs, and selection on flower size in the alpine wildflower Polemonium viscosum (Polemoniaceae). American Naturalist 156: 72–83.
——— 2005. It never rains but then it pours: the diverse effects of water on flower integrity and function. Pp 77–95. In: E. Reekie & F. A. Bazzaz (eds). Reproductive allocation in plants. Elsevier Academic Press, San Diego, USA.
———, T. E. Dawson & M. L. Stanton. 1993. Carpels as leaves: meeting the carbon cost of reproduction in an alpine buttercup. Oecologia 95: 187–193.
———, R. A. Sherry & A. B. Carroll. 1999. Are flowers physiological sinks or faucets? Costs and correlates of water use by flowers of Polemonium viscosum. Oecologia 118: 461–470.
Gómez, J. M. 2003. Herbivory reduces the strength of pollinator-mediated selection in the Mediterranean herb Erysinum mediohispanicum: consequences for plant specialization. American Naturalist 162: 242–256.
———, F. Perfectti & J. Lorite. 2015. The role of pollinators in floral diversification in a clade of generalist flowers. Evolution 69: 863–878.
Gulmon, S. L. & H. A. Mooney. 1986. Costs of defence and their effects on plant productivity. Pp 681–698. In: T. J. Givnish (ed). On the Economy of Plant Form and Function. Cambridge University Press, London, UK.
Guzmán, B., E. Narbona & P. Vargas. 2011. Similar reproductive success of the two petal colour polymorphisms of Cistus ladanifer (Cistaceae). Plant Biosystems 145: 931–937.
———, ——— & ———. 2015. Investigating reproductive incompatibility barriers in a Mediterranean rockrose (Cistus ladanifer). Plant Biosystems 149: 1–6.
Halpern, S. L., L. S. Adler & M. Wink. 2010. Leaf herbivory and drought stress affect floral attractive and defensive traits in Nicotiana quadrivalvis. Oecologia 163: 961–971.
Herrera, C. M. 1993. Selection on floral morphology and environmental determinants of fecundity in a hawk moth-pollinated violet. Ecological Monographs 63: 251–275.
——— 2000. Measuring the effects of pollinators and herbivores: evidence for non-additivity in a perennial herb. Ecology 81: 2170–2176.
——— 2002. Interaction of pollinators and herbivores on plant fitness suggests a pathway for correlated evolution of mutualism- and antagonism-related traits. Proceedings of the Natural Academy of Sciences USA 99: 16823–16828.
Herrera, J. 1985. Nectar secretion patterns in southern Spanish Mediterranean shrublands. Annals of the Missouri Botanical Garden 74: 69–78.
——— 1992. Flower variation and breeding systems in the Cistaceae. Plant Systematics and Evolution 179: 245–255.
——— 2005. Flower size variation in Rosmarinus officinalis: individuals, populations and habitats. Annals of Botany 95: 431–437.
——— 2009. Visibility vs. biomass in flowers: exploring corolla allocation in Mediterranean entomophilous plants. Annals of Botany 103: 1119–1127.
Hodgins, K. A. & S. C. H. Barrett. 2008. Natural selection on floral traits through male and female function in wild populations of the heterostylous daffodil Narcissus triandrus. Evolution 62: 1751–1763.
Irwin, R. E. 2006. The consequences of direct versus indirect species interactions to selection on traits: pollination and nectar robbing in Ipomopsis aggregata. American Naturalist 167: 315–328.
———, A. K. Brody & N. M. Waser. 2001. The impact of floral larceny on individuals, populations and communities. Oecologia 129: 161–168.
Joffre, R., S. Rambal & C. Damesin. 1999. Functional attributes in Mediterranean-type ecosystems. Pp 347–380. In: F. I. Pugnaire & F. Valladares (eds). Handbook of functional plant ecology. Marcel Dekker, Inc., New York, USA.
Johnson, S. G., L. F. Delph & C. L. Elderkin. 1995. The effect of petal-size manipulation on pollen removal, seed set, and insect-visitor behavior in Campanula americana. Oecologia 102: 174–179.
Jones, A. G. 2008. On the opportunity for sexual selection, the Bateman gradient and maximum intensity of sexual selection. Evolution 63: 1673–1684.
Jones, K. N. 2001. Pollinator-mediated assortative mating: causes and consequences. Pp 259–273. In: L. Chittka & J. D. Thomson (eds). Cognitive ecology of pollination: animal behaviour and floral evolution. Cambridge University Press, Cambridge, UK.
Lambers, H., F. S., III Chapin & T. L. Pons. 2008. Plant physiological ecology, ed. 2nd. Springer, New York.
Konsens, I., M. Ofir & J. Kigel. 1991. The effect of temperature on the production and abscission of flowers and pods in snap bean (Phaseolus vulgaris L.). Annals of Botany 67: 391–399.
Krupnick, G. A., A. E. Weis & D. R. Campbell. 1999. The consequences of floral herbivory for pollinator service to Isomeris arborea. Ecology 80: 125–134.
Kuriya, S., M. Hattori, Y. Nagano & T. Itino. 2015. Altitudinal flower size variation correlates with local pollinator size in a bumblebee-pollinated herb, Prunella vulgaris L. (Lamiaceae). Journal of Evolutionary Biology 28: 1761–1769.
Lambrecht, S. C. 2013. Flower water costs and size variation in the highly selfing Leptosiphon bicolor (Polemoniaceae). International Journal of Plant Sciences 174: 74–84.
——— & T. E. Dawson. 2007. Correlated variation of floral and leaf traits along a moisture availability gradient. Oecologia 151: 574–583.
Larcher, W. 2000. Temperature stress and survival ability of Mediterranean schlerophyllous plants. Plant Biosystems 134: 279–295.
Lázaro, A., A. Jakobsson & Ø. Totland. 2013. How do pollinator visitation rate and seed set relate to species’ floral traits and community context? Oecologia 173: 881–893.
Liao, W.-J., Y. Hu, B.-R. Zhu, X.-Q. Zhao, Y.-F. Zeng & D.-Y. Zhang. 2009. Female reproductive success decreases with display size in monkshood, Aconitum kusnezoffii (Ranunculaceae). Annals of Botany 104: 1405–1412.
Maad, J. & R. Alexandersson. 2004. Variable selection in Platanthera bifolia (Orchidaceae): phenotypic selection differed between sex functions in a drought year. Journal of Evolutionary Biology 17: 642–650.
McCall, A. C. & R. E. Irwin. 2006. Florivory: the intersection of pollination and herbivory. Ecology Letters 9: 1351–1365.
Medel, R., A. Valiente, C. Botto-Mahan, G. Carvallo, F. Pérez, N. Pohl & L. Navarro. 2007. The influence of insects and hummingbirds on the geographical variation of the flower phenotype in Mimulus luteus. Ecography 30: 812–818.
Méndez, M. & A. Traveset. 2003. Sexual allocation in single-flowered hermaphroditic individuals in relation to plant and flower size. Oecologia 137: 69–75.
Mosleh Arany, A., T. J. de Jong & E. van der Meijden. 2009. Herbivory and local genetic differentiation in natural populations of Arabidopsis thaliana (Brassicaceae). Plant Ecology 201: 651–659.
Muñoz-Garmendía, F. & C. Navarro. 1993. Cistaceae. In: S. Castroviejo, C. Aedo, & M. Gómez-Campo (eds). Flora Iberica III: 318 − 436. CSIC, Madrid.
Nattero, J., R. Malerba, R. Medel & A. Cocucci. 2011. Factors affecting pollinator movement and plant fitness in a specialized pollination system. Plant Systematics and Evolution 296: 77–85.
Ninyerola, M., X. Pons & J.M. Roure. 2005. Atlas climático digital de la Península Ibérica [online]. UAB, Barcelona. http://opengis.uab.es/wms/iberia/espanol/es_cartografia.htm [accessed September 2011].
Nobel, P. S. & E. De la Barrera. 2000. Carbon and water balances for young fruits of platyopuntias. Physiologia Plantarum 109: 160–166.
Ohashi, K. & T. Yahara. 2001. Behavioral responses of pollinators to variation in floral display size and their influences on the evolution of floral traits. Pp 274–296. In: L. Chittka & J. D. Thomson (eds). Cognitive ecology of pollination, animal behavior and floral evolution. Cambridge University Press, Cambridge, UK.
Oguro, M. & S. Sakai. 2015. Relation between flower head traits and florivory in Asteraceae: A phylogenetically controlled approach. American Journal of Botany 102: 407–416.
Olano, J. M., I. Caballero & A. Escudero. 2012. Soil seed bank recovery occurs more rapidly than expected in semi-arid Mediterranean gypsum vegetation. Annals of Botany 109: 299–307.
Patiño, S. & J. Grace. 2002. The cooling of convolvulaceous flowers in a tropical environment. Plant Cell and Environment 25: 41–51.
Petanidou, T., V. Goethals & E. Smets. 2000. Nectary structure of Labiatae in relation to their nectar secretion and characteristics in a Mediterranean shrub community—Does flowering time matter? Plant Systematics and Evolution 225: 103–118.
Potts, S. G., A. Dafni & G. Ne’eman. 2001. Pollination of a core flowering shrub species in Mediterranean phrygana: variation in pollinator diversity, abundance and effectiveness in response to fire. Oikos 92: 71–80.
Primack, R. B. 1985. Longevity of individual flowers. Annual Review of Ecology and Systematics 16: 15–37.
Pyke, G. H. 1991. What does it cost a plant to produce floral nectar? Nature 350: 58–59.
Quézel, P. & F. Médail. 2003. Ecologie et biogéographie des forêts du bassin méditerranéen. Elsevier, Paris.
Rhizopoulou, S., E. Ioannidi, N. Alexandredes & A. Argiropoulus. 2006. A study on functional and structural traits of the nocturnal flowers of Capparis spinosa L. Journal of Arid Environments 66: 635–647.
Rodriguez-Perez, J. 2005. Breeding system, flower visitors and seedling survival of two endangered species of Helianthemum (Cistaceae). Annals of Botany 95: 1229–1236.
Rosas‐Guerrero, V., R. Aguilar, S. Martén‐Rodríguez, L. Ashworth, M. Lopezaraiza‐Mikel, J. M. Bastida & M. Quesada. 2014. A quantitative review of pollination syndromes: do floral traits predict effective pollinators? Ecology Letters 17: 388–400.
Ross, J. D. & C. Sombrero. 1991. Pp 83–94. Environmental control of essential oil production in Mediterranean plants. Ecological chemistry and biochemistry of plant terpenoids. Clarendon, Oxford.
Ruane, L. G., A. T. Rotzin & P. H. Congleton. 2014. Floral display size, conspecific density and florivory affect fruit set in natural populations of Phlox hirsuta, an endangered species. Annals of Botany 113: 887–893.
Sahli, H. F. & J. K. Conner. 2011. Testing for conflicting and nonadditive selection: floral adaptation to multiple pollinators through male and female fitness. Evolution 65: 1457–1473.
Schemske, D. W. & C. Horvitz. 1988. Plant-animal interactions and fruit production in a neotropical herb: a path analysis. Ecology 69: 1128–1137.
Seufert, G., D. Kotzias, C. Sparta & B. Versino. 1995. Volatile organics in Mediterranean shrubs and their potential role in a changing environment. In Global change and Mediterranean-type ecosystems (pp. 343 − 370). Springer New York.
Shykoff, J. A., E. Bucheli & O. Kaltz. 1996. Flower lifespan and disease risk. Nature 379: 779–780.
Smith, S. D., C. Ané & D. A. Baum. 2008. The role of pollinator shifts in the floral diversification of Iochroma (Solanaceae). Evolution 62: 793–806.
Snow, A. A. & P. O. Lewis. 1993. Reproductive traits and male fertility in plants: empirical approaches. Annual Review of Ecology and Systematics 24: 331–351.
Sprengel, C. K. 1793. Das entdeckte geheimnis der natur im bau und in der befruchtung der blumen. Friedrich Vieweg, Berlin, Germany.
Stanton, M. L., A. A. Snow & S. N. Handel. 1986. Floral evolution: attractiveness to pollinators increases male fitness. Science 232: 1625–1627.
Stebbins, G. L. 1950. Variation and evolution in plants. Columbia University Press, New York, USA.
——— 1970. Adaptive radiation of reproductive characteristics in angiosperms: I. Pollination mechanisms. Annual Review of Ecology and Systematics 1: 307–326.
Strauss, S. Y. & J. B. Whittall. 2006. Non-pollinator agents of selection on floral traits. Pp 120–138. In: L. D. Harder & S. C. H. Barrett (eds). Ecology and evolution of flowers. Oxford University Press, Oxford, UK.
Talavera, S., F. Bastida, P. L. Ortiz & M. Arista. 2001. Pollinator attendance and reproductive success in Cistus libanotis L. (Cistaceae). International Journal of Plant Sciences 162: 343–352.
———, P. E. Gibbs & M. Arista. 1997. Reproductive biology of Halimium atriplicifolium (Lam.) Spach and H. halimifolium (L.) Willk. (Cistaceae). Lagascalia 19: 571–578.
———, ——— & J. Herrera. 1993. Reproductive biology of Cistus ladanifer (Cistaceae). Plant Systematics and Evolution 186: 123–134.
Teixido, A. L. 2014. Indirect costs counteract the effects of pollinator-mediated phenotypic selection on corolla size in the Mediterranean shrub Halimium atriplicifolium. Journal of Plant Ecologyogy 7: 364–372.
——— & F. Valladares. 2013. Large and abundant flowers increase indirect costs of corollas: a study of coflowering sympatric Mediterranean species of contrasting flower size. Oecologia 173: 73–81.
——— & ———. 2014a. Disproportionate carbon and water maintenance costs of large corollas in hot Mediterranean ecosystems. Perspectives in Plant Ecologyogy, Evolution and Systematics 16: 83–92.
——— & ———. 2014b. Pollinator-mediated phenotypic selection does not always modulate flower size and number in the large-flowered Mediterranean shrub Cistus ladanifer (Cistaceae). Botanical Journal of the Linnean Society 176: 540–555.
——— & ———. 2014c. Large flowers tend to be short-lived in Mediterranean ecosystems: insights from three Cistus species. Plant Biosystems 148: 1211–1220.
——— & ———. 2015. Temperature-limited floral longevity in the large-flowered Mediterranean shrubCistus ladanifer (Cistaceae). International Journal of Plant Sciences 176: 131–140.
———, M. Méndez & F. Valladares. 2011. Flower size and longevity influence florivory in the large-flowered shrub Cistus ladanifer. Acta Oecologica 37: 418–421.
Thompson, J. D. 2001. How do visitation patterns vary among pollinators in relation to floral display and floral design in a generalist pollination system? Oecologia 126: 386–394.
——— 2005. Plant evolution in the Mediterranean. Oxford University Press, New York.
Totland, Ø. 2001. Environment-dependent pollen limitation and selection on floral traits in an alpine species. Ecology 82: 2233–2244.
Ushimaru, A., S. Kikuchi, R. Yonekura, A. Maruyama, N. Yanagisawa, M. Kagami, M. Nakagawa, S. Mahoro, Y. Kohmatsu, A. Hatada, S. Kitamura & K. Nakata. 2006. The influence of floral symmetry and pollination systems on flower size variation. Nordic Journal of Botany 24: 593–598.
Valiente-Banuet, A., A. Rojas-Martínez, A. Casas, M. C. Arizmendi & P. Dávila. 1997. Pollination biology of two winter-blooming giant columnar cacti in the Tehuacán Valley, México. Journal of Arid Environments 37: 1–11.
Valladares, F., E. Martínez-Ferri, L. Balaguer, E. Pérez-Corona & E. Manrique. 2000. Low leaf-level response to light and nutrients in Mediterranean evergreen oaks: a conservative resource-use strategy? New Phytologist 148: 79–91.
———, A. Vilagrosa, J. Peñuelas, R. Ogaya, J.J. Camarero, L. Corcuera, S. Sisó & E. Gil-Pelegrín. 2004. Estrés hídrico, ecofisiología y escalas de la sequía. Pp. 163 − 190. In: F. Valladares (ed.), Ecología del bosque mediterráneo en un mundo cambiante. Ministerio de Medio Ambiente, EGRAF, S.A, Madrid, Spain.
———, J. Zaragoza-Castells, D. Sánchez-Gómez, S. Matesanz, B. Alonso, A. Portsmouth, A. Delgado & O. K. Atkin. 2008. Is shade beneficial for Mediterranean shrubs experiencing periods of extreme drought and late-winter frosts? Annals of Botany 102: 923–933.
Vemmos, S. N. & G. K. Goldwin. 1994. The photosynthetic activity of Cox’s orange pippin apple flowers in relation to fruit setting. Annals of Botany 73: 385–391.
Verdú, M., J. Barrón-Sevilla, A. Valiente-Banuet, N. Flores-Hernández & P. García-Fayos. 2002. Mexical plant phenology: is it similar to mediterranean communities? Botanical Journal of the Linnean Society 138: 297–303.
Wade, M. J. 1979. Sexual selection and variance in reproductive success. American Naturalist 114: 742–747.
Willmer, P. 2011. Pollination and floral ecology. Princeton University Press, Princeton, NJ, USA.
Witt, T., A. Jürgens & G. Gottsberger. 2013. Nectar sugar composition of European Caryophylloideae (Caryophyllaceae) in relation to flower length, pollination biology and phylogeny. Journal of Evolutionary Biology 26: 2244–2259.
Young, H. J. & M. L. Stanton. 1990. Influences of floral variation on pollen removal and seed production in wild radish. Ecology 71: 536–547.
Zaragoza‐Castells, J., D. Sánchez‐Gómez, I. P. Hartley, S. Matesanz, F. Valladares, J. Lloyd & O. K. Atkin. 2008. Climate‐dependent variations in leaf respiration in a dry‐land, low productivity Mediterranean forest: the importance of acclimation in both high‐light and shaded habitats. Functional Ecology 22: 172–184.
Acknowledgments
We thank A. Escudero, J.M. Iriondo, J. Arroyo, P. García-Fayos, A. Sánchez, A. Traveset, S. Karrenberg, J. Ågren, N. Sletvold, A.L. Parachnowitsch, C.M. Caruso, J. Herrera, J. Ollerton, J.F. Scheepens, J. Těšitel and M.A. Rodríguez-Gironés for the comments provided during the first versions of the manuscript. We are also grateful to Y. Valiñani and E. Galisteo for lab assistance and to J.P. González-Varo, J. Güemes, E. Carrió, E. Triano and R. Torices for collecting flower buds for analyses of floral production costs. J. Herrera kindly provided some data of flower size for several Mediterranean species.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Teixido, A.L., Barrio, M. & Valladares, F. Size Matters: Understanding the Conflict Faced by Large Flowers in Mediterranean Environments. Bot. Rev. 82, 204–228 (2016). https://doi.org/10.1007/s12229-016-9168-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12229-016-9168-8