Abstract
The flora on the afro-alpine sky islands is renowned for extreme fragmentation, representing a unique natural experiment in biogeography. Here we address the roles of isolation and gene flow, in particular across the narrow Rift Valley (the RV barrier) that cuts through the Ethiopian Highlands (EH), and across the vast low-lying landscape that separates EH from the East African mountains (the EH–EA barrier). We inferred the history of two species with different dispersal mechanisms, but with similar geographic ranges and habitats based on Amplified fragment length polymorphisms (AFLPs). Contrary to our predictions, we found that the populations from opposite sides of the RV barrier were less similar than those from opposite sides of the EH–EA barrier, and that only the supposedly short distance-dispersed species (Trifolium cryptopodium) showed a strong signal of secondary gene flow across the RV barrier. In the wind-dispersed Carduus schimperi, we rather found an evidence for the gene flow between differentiated populations inhabiting different EA mountains. Both species harbored little genetic diversity but considerable genetic rarity in several individual mountains, suggesting long-term isolation and bottlenecks during climatically unfavorable periods. Our genetic data corroborate a division of C. schimperi into three subspecies, but with new delimitation of their ranges, and of T. cryptopodium into two intraspecific taxa. Our findings support the idea that stochasticity may play a major role in shaping extremely fragmented ecosystems such as the afro-alpine. After initial colonization of different mountains, periods of isolation may alternate with unpredictable episodes of intermountain gene flow.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The afro-alpine sky island flora, which occurs above ~3,500 m on the tall African mountains, is renowned not only for its peculiarity and inaccessibility, but also for its extreme fragmentation. It thus represents a unique, but still amazingly little explored natural biogeographical experiment. The afro-alpine vegetation is structurally and to a large degree also floristically distinct from other African vegetation types, such as the montane forests that occupy lower altitudes on the same mountains, and in particular that of the intervening drier, lower-lying terrain that is inhabited by distant evolutionary lineages and efficiently isolate many of the mountain peaks from each other (Hedberg 1957, 1969). The afro-alpine system is therefore excellently suited to address general biogeographical problems, including colonization sources and processes, spatial and temporal variability in isolation and diversification, and rates and correlates of gene flow among fragments during different climatic periods.
The afro-alpine floristic region in the strict sense (Hedberg 1986) is confined to Ethiopia and tropical East Africa (mainly Kenya, Uganda, and Tanzania; Fig. 1). The Ethiopian and the East African parts are separated by the vast Kenyan Highlands, which extend over 250 km at an altitude of ~1,000 m (Brühl 1997) and are believed to have served as an efficient barrier against gradual migration of afro-alpine plants even during the Pleistocene cold periods, when afro-alpine vegetation extended to lower altitudes and occupied larger areas than today (Hedberg 1969, 1986). The topography and connectivity within the Ethiopian and East African systems are also conspicuously different. In East Africa, many mountains or mountain groups occur as isolated high peaks up to 5,895 m (Mt Kilimanjaro) and 5,199 m (Mt Kenya), protruding from lower-lying areas at altitudes of 1,000–2,000 m. Most of the mountain groups originated by volcanic activity connected to the formation of the Great Rift Valley system (Fig. 1). In contrast, Ethiopia provides much more extensive areas of afro-alpine vegetation. The Ethiopian Highlands (EH) consist of vast, continuous areas of which almost 80 % are situated above 3,000 m and with scattered mountain ranges such as the Bale Mts (4,377 m, >100 km2 of afro-alpine vegetation) and the rugged and spectacular Simen Mts (4,620 m; Yalden 1983). The EH are however divided into a northwestern and a southeastern plateau by the Rift Valley, which from 750 m of altitude in the northeast progressively raises southwest-wards to 1,700 m and then drops to 1,100 m in the south (Chorowicz 2005; Fig. 1). Even during the Pleistocene cold periods, when afro-alpine vegetation may have extended as much as 1,000 m downwards (Coetzee 1964), the Rift Valley in Ethiopia is believed to have acted as an important biogeographic barrier (e.g., Arctander et al. 1999; Gottelli et al. 2004).
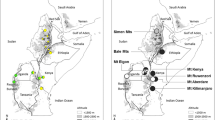
Sampling sites, AFLP-based estimates of genetic diversity (D) and frequency-down-weighted marker value (DW) as a measure of genetic rarity in Carduus schimperi (a) and Trifolium cryptopodium (b). The size of the circles is proportional to within-mountain gene diversity and the shading reflects genetic rarity. The different circle sizes and circle shadings (from white–minimum to black–maximum) represent one quartile each of the distribution of D and DW, respectively. The stippled lines represent the Great Rift Valley system
Although quite a few afro-alpine plant species are endemic to a single African mountain or cluster of neighboring mountains, many of them are more widespread and several are found on both sides of the Rift Valley in Ethiopia as well as in East Africa (Hedberg 1957, 1969). Intermountain migration is believed to be mainly dependent on long-distance dispersal via strong winds or birds, resulting in apparent randomness in the distribution pattern of many afro-alpine taxa and in the species composition of ‘vicarious’ afro-alpine plant communities (Hedberg 1970, 1986). The patterns of initial colonization of mountains and subsequent isolation and potential secondary gene flow among mountains are likely influenced by a number of factors such as the geographic distance among mountains, the ecological requirements of each particular species and thus the suitability of the intervening habitat separating particular mountains throughout different climatic periods, and the dispersal ability of each particular species. So far only a few plant species of the afro-montane forest and the intermediate ericaceous belt have been studied for their phylogeographic history (Lobelia giberroa Hemsl., Kebede et al. 2007; Hagenia abyssinica (Bruce) J.F. Gmel, Ayele et al. 2009; Prunus africana (Hook.f.) Kalkman, Kadu et al. 2011, 2013; Erica arborea L., Désamoré et al. 2011; Erica spp., Gizaw et al. 2013. The widespread arctic-alpine Arabis alpina L. is so far the only species studied from the uppermost afro-alpine vegetation belt (Koch et al. 2006; Assefa et al. 2007; Ehrich et al. 2007). This species originated in Eurasia and appear to have spread twice into the afro-alpine region via mountains in northern Ethiopia.
Here, we selected two widespread afro-alpine species to address patterns of isolation and gene flow among mountains, in particular across the narrow Rift Valley (the RV barrier) that cuts through the EH, and across the vast lowland barrier between the EH and the East African tall mountains (the EH–EA barrier). The two species, Carduus schimperi Sch.Bip. (Asteraceae) and Trifolium cryptopodium Steud.ex A.Rich. (Fabaceae), were selected because they are phylogenetically distant and occupy similar geographic ranges as well as habitats, but whereas one (C. schimperi) is wind-dispersed; the other (T. cryptopodium) has no obvious morphological adaptations to long-distance dispersal. In contrast to the arctic-alpine and afro-alpine A. alpina (Assefa et al. 2007; Ehrich et al. 2007), both species selected for this study result from in situ afro-alpine species radiations; i.e., each of them have likely originated in a single mountain and subsequently spread to the others.
The nine native afro-montane/afro-alpine species of the otherwise Eurasian thistle genus Carduus L. (Asteraceae) have been referred to a distinct endemic subgenus, Afrocarduus Kazmi (Kazmi 1963; Jeffrey 1968). Two of them (C. nyassanus (S.Moore) R.E.Fries and C. keniensis R.E.Fries) were retrieved as a monophyletic group but most closely related to the genus Cirsium Mill. in a recent molecular phylogenetic study (Barres et al. 2013). Our target species, C. schimperi, is a spiny, perennial herb with a thick tap root, easily distinguished from the other afro-alpine members of the subgenus by its lack of stems (Jeffrey and Beentje 2000; Tadesse 2004). It has clusters of sessile, large (up to 4.5-cm long and 2.5-cm wide) capitula in the center of basal leaf rosettes, which typically are appressed to the ground and can reach 80 cm in diameter. The capitula have numerous florets that are white to pale lilac and sweet scented, and the cypsela has a long (up to 44 mm) pappus (Jeffrey and Beentje 2000; Tadesse 2004), suggesting insect pollination and efficient wind dispersal. The species was first described from the Simen Mts in Ethiopia and occurs from (1,800-)2,000 m to 4,600 m in grasslands, moorlands, and rocky areas in East Africa [Tanzania, Kenya, Uganda, and Democratic Republic of the Congo (DRC)] and northeastern Africa (Ethiopia and Sudan; Hedberg 1957; Jeffrey and Beentje 2000; Tadesse 2004). It has been reported as tetraploid with 2n = 32 in Mt Kenya (as C. chamaecephalus (Vatke) Oliv. & Hiern.; Hedberg and Hedberg 1977).
Three subspecies are currently recognized in C. schimperi (Jeffrey and Beentje 2000). They were formerly recognized at the species level by Fries (1923) and Hedberg (1957), but the latter found that the material available of these very spiny and thus not very popular collection targets was too limited for a conclusive taxonomic treatment. Subspecies platyphyllus (R.E.Fr.) C. Jeffrey, reported as endemic to a single mountain (Mt Kenya; 3,000–4,600 m), appears to be the most distinct of the three, distinguished by its obovate to broadly ovate leaf outline in addition to its short corolla (15–17 mm). The other two subspecies are recorded as more widespread, and both have leaves that are oblong, elliptic, or oblanceolate-oblong/elliptic in outline, but reported to differ in corolla length (26–40 mm in ssp. schimperi and 19–24 mm in ssp. nanus (R.E.Fr.) C. Jeffrey according to Jeffrey and Beentje (2000); note however, that Tadesse (2004) reported that the corolla length in Ethiopian material of ssp. schimperi is 18–35 mm). In the north, from where it originally was described, subspecies schimperi has been recorded from many areas in the EH (from 2,000 to above 4,000 m) and also from Sudan (Tadesse 2004), and in East Africa, it has apparently only been reported from a few quite low-lying sites (three in Kenya and two in Tanzania), from 1,800 m to 2,650 m (Jeffrey and Beentje 2000). Subspecies nanus has been reported from 2,550–4,050 m in Mt Elgon (both on the Ugandan and Kenyan sides), Mt Aberdare in Kenya, and a few Western Rift mountains on the DRC side (Hedberg 1957; Jeffrey and Beentje 2000). No obvious ecological differences seem to have been reported among the three subspecies.
The large, mainly temperate genus Trifolium L. (Fabaceae) is most diverse in the Mediterranean, where it is suggested to have originated (Ellison et al. 2006), but it is also remarkably rich in species in Ethiopia (32 spp.; Thulin 1989) and tropical East Africa (22 spp.; Gillett et al. 1971). The sub-Saharan African species seem to have originated from three independent dispersal events from the Mediterranean, but otherwise their interrelationships were poorly resolved in a molecular phylogeny of the genus (Ellison et al. 2006). Our study species, T. cryptopodium, is a typically mat-forming perennial herb with a woody rootstock, creeping stems that often root at the nodes, and globose inflorescences up to 18-mm wide with many purple to mauve flowers and dehiscent pod that usually has single seed up to 1.6-mm long (Gillett et al. 1971; Thulin 1989). It is, thus, probably insect pollinated and seems to lack particular adaptations to long-distance dispersal. Also this species was first described from the Simen Mts in northern Ethiopia, and it has later been reported from grassland, often on rocky and moist ground in montane forests and alpine regions across the EH from 2,000 to 4,300 m and in some East African mountains from 1,800 to 4,100 m (Mt Elgon, both on the Ugandan and Kenyan side, and a few mountains in Kenya and Tanzania (Hedberg 1957; Gillett et al. 1971; Thulin 1989). It has been reported as hexaploid with 2n = 48 from the Bale Mts and Mt Elgon (Hedberg and Hedberg 1977). A diploid count (2n = 16) has also been reported for T. cryptopodium, as for most other afro-alpine as well as other species in the genus (Ellison et al. 2006).
Trifolium cryptopodium shows a considerable morphological variation, especially in relative lengths of the calyx and corolla and in prominence of the calyx nerves, but this variation was regarded as too continuous to allow recognition of intraspecific taxa in the Flora of Tropical East Africa (Gillett et al. 1971). The plants from Mt Kilimanjaro were however originally described as a distinct species, and Gillett (1952) recognized all specimens from East Africa as var. kilimandscharicum (Taub.) Gillett and also as distinct from the Ethiopian var. cryptopodium, of which he only referred to material from the Simen Mts. He distinguished var. kilimandscharicum by having more (10–11) calyx nerves and a shorter standard relative to the calyx (<twice as long) than var. cryptopodium (<10 nerves, standard twice as long as calyx).
For this study, we sampled both species across most of their ranges to assess their genetic structuring and infer their phylogeographic history from Amplified Fragment Length Polymorphisms (AFLPs, Vos et al. 1995). Because of the extensive area of unsuitable habitat between the EH and the East African mountains (the EH–EA barrier), we expected to find a stronger phylogeographic break corresponding to this area than to the more narrow Rift Valley in Ethiopia, i.e., in each species, the populations from opposite sides of the RV barrier were expected to be genetically more similar than the populations from opposite sides of the EH–EA barrier. We also predicted that there is more detectable gene flow across the RV barrier in the wind-dispersed C. schimperi than in the supposedly short distance-dispersed T. cryptopodium. Finally, we addressed whether the proposed infraspecific taxonomy of the species is reflected in their patterns of genetic diversity.
Materials and methods
Sampling and AFLP fingerprinting
We collected 24 populations of C. schimperi and 19 populations of T. cryptopodium in the field from seven mountains/mountain systems in Ethiopia, Kenya, and Tanzania between 2007 and 2009 (Appendix 1; Fig. 1). Whenever possible, four distantly separated populations of each species were sampled per mountain system. From each population, fresh young leaf samples were collected from five individual plants occurring at the corners and in the center of a 100 m × 100 m plot, and dried using silica gel. Three of the five plants were collected and deposited as voucher specimens in the following herbaria: one in the Natural History Museum, University of Oslo (O), Norway; one in the National Herbarium, Addis Ababa University (ETH), Ethiopia; and the third voucher was deposited according to country of collection, i.e., in the East African Herbarium (EA), Kenya, or at the Sokoine University of Agriculture (SUA), Tanzania.
Total genomic DNA was extracted from silica-dried leaf tissue using an automated GeneMole® robot and the Mole Strip™ Plant DNA Kit following the manufacturer’s instructions (QIAGEN, Nordic, Oslo, Norway). The quality of the extracted DNA was checked on 1 % TBE–agarose gels. The AFLP protocol followed Gaudeul et al. (2000), except that the PCR reaction volumes were reduced by 50 % and pre-selective PCR products were diluted ten times. For each species, 12 primer combinations were tested on eight individuals from different mountains. Two or three primer combinations, which provided many and well separated polymorphic bands, were selected for final analyses of the total material: EcoRI ACA (6-FAM)-MseI CAC, EcoRI ACT (6-FAM)-MseI CAG, and EcoRI ACG (VIC)-MseI CTC for C. schimperi, and EcoRI AGA (6-FAM)-MseI CTC, and EcoRI ACA (VIC)-MseI CAC for T. cryptopodium. For each sample, 2.0 μL 6-FAM, and 2.0 μL VIC labeled selective PCR products were mixed with 11.7-μL formamide and 0.3-μL GeneScan™ 500 ROX™ internal-lane size standard and run on an ABI 3100 sequencer (Applied Biosystems).
The raw data were analyzed using the ABI prism GeneScan analysis software version 3.7 (Applied Biosystems) and imported for scoring into GeneMapper version 4.0 (Applied Biosystems). AFLP fragments in the size range 50–500 base pairs (bp) were automatically scored as present (1) or absent (0). Ambiguous peaks of low intensity were excluded. Reproducibility was calculated based on ten duplicated samples of C. schimperi and on 20 duplicated samples of T. cryptopodium as the average proportion of correctly replicated bands (Bonin et al. 2004). Error rate calculations and data cleaning were done separately for each primer combination. The duplicates were removed and matrices for all primers were combined for each species to obtain the two final data matrices.
AFLP data analyses
Pairwise similarity among AFLP multilocus genotypes was calculated using Dice’s coefficient in NTSYSpc version 2.11a (Rohlf 2000) and visualized using Principal Coordinate Analyses (PCoAs). We used the R-script AFLPdat (Ehrich 2006) to estimate the proportion of polymorphic markers (P) and Nei’s gene diversity (D; estimated as the average proportion of pairwise differences among genotypes, Kosman 2003). Genetic rarity was estimated as frequency-down-weighted marker values (DW or rarity) according to Schönswetter and Tribsch (2005) using AFLPdat (Ehrich 2006). The level of gene diversity and genetic rarity was calculated for each species in total, for each population, for each mountain, and for identified genetic (STRUCTURE) groups. To estimate among-mountain differentiation, Φst values taking into account the molecular distance between haplotypes (number of pairwise differences) were computed in ARLEQUIN version 3 (Excoffier and Schneider 2005) with significance estimated by 1,000 permutations. Neighbor-joining (NJ) analyses were performed based on Nei and Li’s (1979) genetic distance using TreeCon version 1.3b (Van de Peer and De Wachter 1994). The trees were midpoint rooted, and support for branches was estimated from 1,000 bootstrap replicates.
Genetic structure analyses were performed in STRUCTURE v 2.3.3 (Pritchard et al. 2000) to identify genetically homogeneous groups within each species based on Bayesian clustering algorithms. We tested the admixture model with correlated allele frequencies as well as the no admixture model with uncorrelated allele frequencies. The results of the preliminary analyses of each data set were almost identical, and we therefore selected the admixture model with correlated allele frequencies for the final analyses. The recessive allele model was used to take into account the dominant nature of AFLP data (Falush et al. 2007). The analyses were carried out at the Bioportal, University of Oslo (http://www.bioportal.uio.no) for K = 1–10 and 10 replicate runs for each K, using a burn-in period of 2 × 105 and 106 iterations. We used the R-script STRUCTURE-SUM (Ehrich 2007) to summarize the results and determine the most likely value of K based on the estimated posterior log probability of the data, L(K), and the rate of change in the probability between successive runs, ∆K, as a function of K, calculated according to Evanno et al. (2005). For each selected value of K, the average estimate of individual admixture values among the replicated runs was calculated using the program CLUMPP (Jakobsson and Rosenberg 2007) and the result was visualized using the program DISTRUCT (Rosenberg 2004). Analyses of Molecular Variance (AMOVAs) were performed to investigate degree of differentiation between populations and groups of populations using ARLEQUIN version 3 (Excoffier and Schneider 2005).
Results
Carduus
The final data set comprised a total of 107 plants from 24 populations and 541 fragments, of which 426 (78.74 %) were polymorphic. Reproducibility of the AFLP markers was 98.2 %.
In the STRUCTURE analysis, the average value of the log-likelihood increased rapidly up to K = 3, and then leveled off until K = 8. However, the results among the ten replicated runs for each K showed large variation except for K = 3. The rate of change in the probability between successive Ks, ∆K, clearly identified K = 3 as the most likely number of genetic groups in the total data set (Figs. 2a, 3a). Two of the genetic groups showed restricted geographical distributions; one was confined to the Simen Mts in northwestern Ethiopia (the Simen group) and one to Mt Kenya in East Africa (the Kenya group; Figs. 1a, 3a). The third group had a wider distribution, occurring in the Bale Mts in southeastern Ethiopia as well as in Mt Aberdare and Mt Elgon in East Africa (the Bale/EA group).
Detection of the number of groups (K) in STRUCTURE analyses of the AFLP datasets for Carduus schimperi (a) and Trifolium cryptopodium (b). The graphs show the mean value of log probability of the data, L(K), as function of K ranging from 1 to 10 as estimated by the program STRUCTURE, and the rate of change in the probability between successive runs, ∆K, as a function of K, calculated according to Evanno et al. (2005)
Mid-point rooted neighbor-joining trees based on Nei and Li’s (1979) distance (left, with bootstrap values above 50 % estimated from 1,000 replicates), graphical representation of STRUCTURE result (right, each individual is represented by a horizontal bar and different populations are separated by black lines) and Principal Coordinates Analyses (PCoAs, middle) based on Dice’s coefficient of similarity among the AFLP phenotypes observed in 107 plants (24 populations) of Carduus schimperi (a) and 66 plants (17 populations) of Trifolium cryptopodium (b). Shape of symbols shows geographic origin of the accessions (see Appendix 1) and colors represent the genetic groups inferred by STRUCTURE, except that the orange color in (b) identifies the ten plants from the Simen Mts that showed a high degree of admixture between the Choke/Simen and the Bale/EA groups. Arrows and encircled individuals indicate the most admixed plants from Mt Kenya in (a) and one admixed plant from the Bale Mts in (b)
The first two PCoA axes explained 30.8 % of the total variation and clearly separated the three genetic groups identified in the STRUCTURE analysis, except that five of the plants from Mt Kenya (representing five of the ten populations analyzed from that mountain) appeared as intermediate between the Kenya group and the Bale/EA group (Fig. 3a). The first PCoA axis (19.6 %) separated the Simen group from the Kenya group with the Bale/EA group in an intermediate position; the latter group was separated along the second axis (11.2 %). The third axis (5.8 %) separated the plants from the Bale Mts from the remainder of the Bale/EA group.
In the NJ tree, the Simen group formed a cluster with 100 % bootstrap support, and the Kenya and Bale/EA groups formed another cluster with 98 % support, but with limited support for internal resolution. In the Kenya—Bale/EA cluster, the five intermediate (as indicated in the PCoA) plants from Mt Kenya appeared somewhat divergent (Fig. 3a). When these five Mt Kenya plants were excluded in another NJ analysis, the Kenya group formed a separate cluster with 95 % support, whereas no support was obtained for the Bale/EA group (result not shown).
In agreement with the PCoA and the NJ analyses, the visualization of individual admixture in the STRUCTURE analysis showed that the five intermediate Mt Kenya plants represented considerable admixture between the Kenya and Bale/EA genetic groups (Fig. 3a). Some degree of admixture was also indicated for other plants in the STRUCTURE analysis (Fig. 3a). Notably, slight admixture across the Rift Valley in Ethiopia was indicated; two plants from the Bale Mts appeared to be admixed with the Simen genetic group.
A non-hierarchical AMOVA of the total material assigned 56.53 % of the overall genetic variation to variation within populations, but this proportion increased to more than 90 % in separate analyses for the Kenya and for the Simen genetic groups (Table 2). In hierarchical AMOVAs, 40.04 % of the total variation was assigned to variation among the five sampled mountains (P < 0.0001), and 35.4 % of the total variation was assigned to variation among the three genetic groups (P < 0.0001). We performed additional AMOVAs to further explore the unexpected relationship of the Bale populations. Whereas the among-group variation for the Ethiopian Bale vs. Simen Mts was as high as 47.1 % (P = 0.0362), the among-group variation for the Bale Mts vs. the East African mountains Elgon/Aberdare was 21.1 % (P = 0.0606); however, these values were not significant or only scarcely so, probably because of low sample size of the Bale populations (Table 2). The genetic differentiation estimates among the five mountains were however highly significant (P < 0.0001). According to pairwise comparisons of the Φst values, the Simen populations were the most differentiated ones (Table 3).
The total gene diversity pooled over all genotyped individuals was D = 0.097. The average within-population gene diversity was 0.054 (SD = 0.014) and the average proportion of polymorphic loci was P = 11.1 % (range 5.36–16.64; Appendix 1). The widespread Bale/EA group was most diverse, with average within-population gene diversity D = 0.061 (P = 12.6 %), whereas less diversity was observed in the Kenya (D = 0.056, P = 11.4 %) and Simen (D = 0.043, P = 8.7 %) genetic groups (Appendix 1). When pooled by individual mountains, gene diversity and rarity were clearly higher in the mountains Bale, Elgon, and Aberdare (the Bale/EA group; D = 0.089–0.104, DW = 5.11–5.62) than in Mt Kenya (D = 0.080, DW = 3.59) and, in particular, the Simen Mts (D = 0.057, DW = 2.79; Fig. 1a; Table 1)
.
Trifolium
A total of 66 individual plants from 19 populations were successfully analyzed. The final data set contained 297 fragments, of which 237 (79.8 %) were polymorphic. Reproducibility of the AFLP markers was 98.3 %. Since two of the populations (KN0232and KN0323) only consisted of a single successfully analyzed plant, they were merged with a neighboring population (KN0322) in some of the following analyses (Appendix 1).
In the STRUCTURE analysis, L(K) increased rapidly to K = 2 and then gradually until K = 5 (Fig. 2b). The results of the ten replicated runs for K > 2 showed however large differences. The rate of change in the probability between successive Ks, ∆K, also identified K = 2 as the most likely number of genetic groups (Figs. 2b, 3b). One group was restricted to the two sampled mountains in northwestern Ethiopia (the Choke/Simen group), and the other had a wider distribution ranging from the Bale Mts in southeastern Ethiopia to the three East African mountains Aberdare, Elgon, and Kilimanjaro (the Bale/EA group; Figs. 1b, 3b).
Both the PCoA and NJ analyses (Fig. 3b) indicated a structuring of the AFLP variation that was largely similar to that obtained in the STRUCTURE analysis. Two major clusters corresponding to the two genetic groups were retrieved in the NJ tree, although with limited bootstrap support (67–78 %). The majority of the plants from the Simen Mts (hereafter named the ‘pure Simen populations’) appeared at one extreme of PCoA axis 1 (17.9 %), and all but one plant from the Bale/EA genetic group appeared at the other extreme of this axis. The remaining ten of the Simen plants, originating from four populations, and those from Mt Choke, along with a single plant from the Bale Mts, appeared in intermediate positions along axis 1 and were further separated along axis 2 (7.5 %).
In agreement with the intermediate positions of these latter plants along PCoA axis 1, the visualization of the individual admixture values from the STRUCTURE analysis showed that they represented considerable admixture between the Choke/Simen and the Bale/EA genetic groups (Fig. 3b). The ten plants from the Simen Mts showed admixture between the Choke/Simen and the Bale/EA groups with ~0.6 and ~0.4 average proportion of individual membership to each group, respectively. Similarly, the plants from Mt Choke showed admixture with ~0.8 average proportion of individual membership to the Choke/Simen group and ~0.2 to the Bale/EA group.
A non-hierarchical AMOVA of the total dataset assigned 68.1 % of the overall genetic variation to variation within populations (Table 2)
. In hierarchical AMOVAs, 36.4 % of the total variation was assigned to variation among the six sampled mountains (P < 0.0001), and 31.9 % of the total variation was assigned to variation between the two genetic groups (P < 0.0001). In agreement with the other data analyses, additional AMOVAs indicated stronger differentiation between the Bale Mts and the other Ethiopian mountains (Simen and Choke) than between the Bale Mts and the East African mountains, but the sample size for the Bale Mts was low and these comparisons were not significant (Table 2). The genetic differentiation estimates among the mountains also showed that the populations from the Simen Mts and Mt Choke were strongly differentiated from all other populations (Table 3)
.
The total gene diversity pooled over all genotyped individuals of T. cryptopodium was D = 0.113. The mean within-population gene diversity was 0.079 (SD = 0.025) and the mean proportion of polymorphic loci was P = 13.9 % (range 4.04–22.22 %; Appendix 1). Genetic diversity and rarity were much higher in the widespread Bale/EA group (D = 0.099, DW = 6.94) than in the geographically restricted Choke/Simen group (D = 0.070, DW = 2.67; Appendix 1). Genetic diversity pooled by mountains was also highest in all individual Bale/EA mountains (up to D = 0.127 in the Bale Mts) except in Mt Kilimanjaro, which showed low diversity (D = 0.071; Fig. 1b; Table 1). Genetic rarity was also highest and quite similar among the mountains in the widespread group, ranging from DW = 5.93 in Mt Elgon to 8.07 in Mt Kilimanjaro. In the Choke/Simen group, both diversity and rarity were low in both mountains (D = 0.078–0.080, DW = 2.40–3.63; Fig. 1b; Table 1).
Discussion
Crossing afro-alpine barriers: a major role of stochasticity?
Contrary to our predictions, it appeared that in each of the studied afro-alpine species the populations from opposite sides of the narrow Rift Valley (the RV barrier) in Ethiopia were less similar than those from opposite sides of the extensive low-lying barrier separating the EH from the East African mountains (the EH–EA barrier). In all analyses of our genetic data, the plants from the Bale Mts in southeastern Ethiopia grouped with the plants from mountains in East Africa rather than with those from the northwestern Ethiopian mountains. This was unexpected because the EH–EA gap was thought to represent by far the strongest barrier against plant migration because of its great geographic extension as well as on the ecological unsuitability of the intervening habitat, even during the Pleistocene cold periods (Hedberg 1969). Gradual migration of afro-alpine plants across the EH–EA barrier therefore seems difficult; bridging this gap seems most likely to have happened via direct long-distance dispersal. We cannot exclude with certainty, however, that the particular species studied here may gradually have crossed this barrier during cold periods, since they have been observed to occur down to 2,000 m in Ethiopia and down to 1,800 m in East Africa under the current climate.
Notably, the disjunction between the EH and the East African mountains is reflected in the floristic division of the afro-alpine region into two corresponding subregions (Hedberg 1986), which might have been hypothesized to be paralleled by major genetic subdivision within individual widespread species as well. The Rift Valley barrier cutting through the EH, on the other hand, represents a much shorter geographic distance (<75 km) between two areas, each of which potentially provided more or less continuous, extensive suitable habitat during cold climatic periods (cf. map in Gottelli et al. 2004), and somewhat larger distance and less continuous habitat under the present climate. Thus, direct dispersal could be assumed to have occurred more frequently across the RV barrier. In addition, the valley bottom may have allowed for some gradual migration during very cold periods (Gottelli et al. 2004).
Both our study species belong to species groups that have radiated in situ in the afro-alpine region. However, the phylogenetic and biogeographic relationships within each group are still unresolved (Ellison et al. 2006; Barres et al. 2013). The geographic origin of the two species examined here is therefore unknown. Nevertheless, both the afro-alpine Trifolium and Carduus groups appear to be derived from ancestors that entered Africa from the north (Ellison et al. 2006; Barres et al. 2013), likely via mountainous parts of the Arabian Peninsula, thus rendering northwestern Ethiopia as the most likely starting point of both of these afro-alpine species radiations (see also Koch et al. 2006 for A. Alpina and Popp et al. 2008 for Lychnis L., hypothesizing this migration route). Notably, in both species the current populations in northwestern Ethiopia form their own distinct genetic group, suggesting long-term isolation (Fig. 3a, b). The current southeastern Ethiopian populations, on the other hand, form another genetic group together with all or most of the East African populations, which may suggest recent colonization of East Africa from southeastern Ethiopia. However, we consider this explanation unlikely given that we observed high genetic rarity in several East African mountains, pointing to long-term persistence also in this area. Thus, the fact that the current northwestern Ethiopian populations are genetically distinct, whereas the southeastern Ethiopian populations belong to the same genetic group as the East African populations rather suggest that secondary gene flow in both directions has occurred between the latter areas one or several times, since they originally were colonized. Another possible scenario is that the two species originated in the south and colonized first southeastern Ethiopia and then northwestern Ethiopia, but also in this case their current genetic structuring suggests secondary gene flow across the EH–EA barrier.
We found that the main geographic structuring of the genetic variation was similar in the two species and thus does not seem to reflect their different adaptations to dispersal. Although our sampling did not include the lowermost occurrences of the two species, which both are recorded down to 2,000 m in Ethiopia and down to 1,800 m in East Africa (Hedberg 1957; Gillett et al. 1971; Jeffrey and Beentje 2000; Tadesse 2004; Thulin 1989), this should not affect the following conclusions. In both species, secondary bidirectional gene flow across the extensive EH–EA barrier seems to have been sufficiently strong to prevent differentiation into different genetic groups and, therefore not detectable in our STRUCTURE analyses, whereas gene flow across the narrow RV barrier appears to have been more limited and allowed for genetic differentiation. Our AFLP analyses nevertheless provided evidence for some dispersal across the RV barrier in Ethiopia, unexpectedly most strongly in the species that appears to lack particular adaptations to long-distance dispersal (T. cryptopodium; Fig. 3b). Dispersal appears to have been mainly unidirectional from the Bale Mts into the Choke and Simen Mts. In the wind-dispersed C. schimperi, on the other hand, we found only subtle signs of dispersal across the RV barrier, but rather evidence for gene flow between distinctly differentiated populations inhabiting different East African mountains. In this species, the Mt Kenya populations were identified as a distinct genetic group in accordance with their distinct morphological characteristics, suggesting that the species colonized this mountain a long time ago, followed by long-term isolation. However, individual plants in several populations in Mt Kenya showed clear signals of admixture with the other genetic group occurring in other East African mountains, suggesting mainly unidirectional gene flow into Mt Kenya (Fig. 3a). This pattern suggests that gene flow has occurred recently relative to the time of the original colonization and diversification of the Mt Kenya populations. In C. schimperi, it is also noteworthy that we found clear genetic differentiation between the Mt Kenya plants and the plants from the two other EA mountains sampled, Mt Aberdare and Mt Elgon, in spite of the fact that Mt Aberdare and Mt Elgon are separated by a much greater geographic distance than Mt Aberdare and Mt Kenya (Fig. 1a).
Our findings, thus, seem to indicate that secondary intermountain gene flow between different pairs of afro-alpine mountains or mountain groups has happened repeatedly in different time periods, but the pattern appears to be unpredictable from factors such as geographic distance between mountains, suitability of the intervening habitat, and dispersal adaptations. Footprints of old episodes of intermountain gene flow may have been eradicated by genetic swamping, especially in presumably outcrossing species such as the ones studied here, and resulted in a closer genetic relationship between pairs of contemporary mountain populations than would have been expected from the time elapsed since the initial colonization of the mountains. Thus, several findings in this study support the hypothesis that stochasticity may play a major role in shaping extremely fragmented ecosystems such as the afro-alpine. After initial colonization of different mountains, periods of isolation may alternate with periods of unpredictable intermountain gene flow. Our results on intraspecific genetic relationships are, thus, in agreement with the floristic and vegetational relationships observed by Hedberg (1970, 1986), who suggested that intermountain migration was mainly dependent on long-distance dispersal via strong winds or birds and, thus, could result in randomness in the distribution pattern of afro-alpine taxa and in the species composition of ‘vicarious’ afro-alpine plant communities.
Phylogeographic structure and infraspecific taxonomy
Both species showed low genetic diversity but still considerable genetic rarity in several individual mountains, suggesting long-term isolation involving bottlenecks during climatically unfavorable periods. Considerable proportions of the AFLP diversity were observed within populations, suggesting an outcrossing mating system (Appendix 1; cf. Nybom 2004). We found distinct geographic structuring of the genetic variation in both species, which appear to correspond to the proposed divisions of C. schimperi into three subspecies (but with new delimitation of their geographic ranges) and of T. cryptopodium into two intraspecific taxa.
The three subspecies of C. schimperi have mainly been delimited based on corolla length and leaf shape (Jeffrey and Beentje 2000; Tadesse 2004). We had only limited flowering material available of the species, but the corolla lengths (calculated based on the mean of five corollas measured per specimen) in our flowering specimens were roughly in agreement with previous literature reports (Jeffrey and Beentje 2000, Tadesse 2004), except that corolla lengths never appear to have been reported explicitly from the Bale Mts. In our material, the shortest corollas were found in Mt Kenya (mean 11.5 mm, range 8.0–13.0 mm, n = 3) and the longest ones in the Simen Mts (35 mm, n = 1), whereas intermediate ones were found in Mt Aberdare (mean 17.0 mm, range 14.0–20.5 mm, n = 2), Mt Elgon (20.5 mm, n = 1), and the Bale Mts (mean 25 mm, range 20.0–29.0 mm, n = 3). We also found the leaf shape variation to be consistent with previous reports. Thus, our morphological observations are in agreement with our genetic data, corroborating that three subspecies can be recognized in C. schimperi, of which the Mt Kenya endemic ssp. platyphyllus is the most distinct one based on morphology. However, in contrast to earlier reports, our results suggest that the Ethiopian Bale Mts populations must be referred to the otherwise East African ssp. nanus and not to ssp. schimperi, a final conclusion must await further studies.
In T. cryptopodium, we identified two genetic groups, one confined to northwestern Ethiopia (Simen and Choke Mts) and the other occurring in southeastern Ethiopia (Bale Mts) and in the East African mountains, but with considerable intermixing across the RV barrier. Also in this species, the genetic division we observed appears to correspond to the previously reported taxonomic variation, recognized at either the species or varietal level (Gillett 1952), but not accepted in the Flora of Tropical East Africa (Gillett et al. 1971). As Gillett (1952) only examined Ethiopian material from the Simen Mts, a morphological study of more extensive material is needed to assess whether the delimitation of our genetic groups corresponds to the reported distinction between var. cryptopodium, originally described from the Simen Mts, and var. kilimandscharicum, described from Kilimanjaro and later reported from other mountains in East Africa (Gillett 1952).
References
Arctander P, Johansen C, Coutellec-Vreto MA (1999) Phylogeography of three closely related African bovids (tribe Alcelaphini). Mol Biol Evol 16:1724–1739
Assefa A, Ehrich D, Taberlet P, Nemomissa S, Brochmann C (2007) Pleistocene colonization of afro-alpine ‘sky islands’ by the arctic-alpine Arabis alpina. Heredity 99:133–142
Ayele TB, Gailing O, Umer M, Finkeldey R (2009) Chloroplast DNA haplotype diversity and postglacial recolonization of Hagenia abyssinica (Bruce) J.F. Gmel in Ethiopia. Plant Syst Evol 280:175–185
Barres L, Sanmartin I, Anderson C, Susanna A, Buerki S, Galbany-Casals M, Vilatersana R (2013) Reconstructiong the evolution and biogeographic history of Tribe Cardueae (Compositae). Am J Bot 100:867–882
Bonin A, Bellemain E, Eidesen PB, Pompanon F, Brochmann C, Taberlet P (2004) How to track and assess genotyping errors in population genetics studies. Mol Ecol 13:3261–3273
Brühl C (1997) Flightless insects: a test case for historical relationships of African mountains. J Biogeogr 24:233–250
Chorowicz J (2005) The East African rift system. J Afr Earth Sci 43:379–410
Coetzee JA (1964) Evidence for a considerable depression of the vegetation belts during the upper Pleistocene on the East African Mountains. Nature 204:564–566
Désamoré A, Laenen B, Devos N, Popp M, González-Mancebo JM, Carine MA, Vanderpoorten A (2011) Out of Africa: north-westwards Pleistocene expansions of the heather Erica arborea. J Biogeogr 38:164–176
Ehrich D (2006) AFLPdat: a collection of R functions for convenient handling of AFLP data. Mol Ecol Notes 6:603–604
Ehrich D (2007) Structure-sum v. 2007: A series of R functions for summarizing the outputs of the program Structure ver. 2.2. Unpublished, available from the author upon request
Ehrich D, Gaudeul M, Assefa A, Koch MA, Mummenhoff K, Nemomissa S, Brochmann C (2007) Genetic consequences of Pleistocene range shifts: contrast between the Arctic, the Alps and the East African mountains. Mol Ecol 16:2542–2559
Ellison NW, Liston A, Steiner JJ, Williams WM, Taylor NL (2006) Molecular phylogenetics of the clover genus (Trifolium–Leguminosae). Mol Phylogen Evol 39:688–705
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Excoffier LLG, Schneider S (2005) ARLEQUIN version 3.0: an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Falush D, Stephens M, Pritchard J (2007) Inference of population structure using multilocus genotype data: dominant markers and null allele. Mol Ecol Notes 7:574–578
Fries TCE (1923) Beiträge zur Kenntnis der Flora des Kenia, Mt. Aberdare und Mt. Elgon. Notizblatt des Königl Botanischen Gartens und Museums zu Berlin 8:389–423
Gaudeul M, Taberlet P, Till-Bottraud I (2000) Genetic diversity in an endangered alpine plant, Eryngium alpinum L. (Apiaceae), inferred from amplified fragment length polymorphism markers. Mol Ecol 9:1625–1637
Gillett JB (1952) The genus Trifolium in southern Arabia and in Africa south of the Sahara. Kew Bull 7:367–404
Gillett JB, Polhill RM, Verdcourt B (1971) Leguminosae IIII. In: Milne-Redhead EE, Polhill RM eds. Flora of Tropical East Africa. London: crown agents for overseas governments and Administrations, pp 1016–1036
Gottelli D, Marino J, Sillero-Zubiri C, Funk SM (2004) The effect of the last glacial age on speciation and population genetic structure of the endangered Ethiopian wolf (Canis simensis). Mol Ecol 13:2275–2286
Hedberg O (1957) Afroalpine vascular plants. a taxonomic revision. Symb Bot Ups 15:1–411
Hedberg O (1969) Evolution and speciation in a tropical high mountain flora. Bot J Linn Soc 1:135–148
Hedberg O (1970) Evolution of the afroalpine flora. Biotropica 2:16–23
Hedberg O (1986) Origin of the afroalpine flora. In: Vuilleumier F, Monastero M (eds) High altitude tropical biogeography. Oxford University Press, New York, pp 443–468
Hedberg I, Hedberg O (1977) Chromosome numbers of afroalpine and afromontane angiosperms. Bot Not 130:1–24
Jakobsson M, Rosenberg NA (2007) CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 23:1801–1806
Jeffrey C (1968) Notes on Compositae: III. The cynareae in east tropical Africa. Kew Bull 22:107–140
Jeffrey C, Beentje HJ (2000) Compositae I. In: Beentje HJ (ed) Flora of tropical East Africa. Balkema, Rotterdam, pp 46–54
Kadu CA, Schueler S, Konrad H, Muluvi GM, Eyog-Matig O, Muchugi A, Williams VL, Ramamonjisoa L, Kapinga C, Foahom B, Katsvanga C, Hafashimana D, Obama C, Geburek T (2011) Phylogeography of the Afromontane Prunus africana reveals a former migration corridor between East and West African highlands. Mol Ecol 20:165–178
Kadu CA, Konrad H, Schueler S, Muluvi GM, Eyog-Matig O, Muchugi A, Williams VL, Ramamonjisoa L, Kapinga C, Foahom B, Katsvanga C, Hafashimana D, Obama C, Geburek T (2013) Divergent pattern of nuclear genetic diversity across the range of the Afromontane Prunus africana mirrors variable climate of African highlands. Ann Bot 111:47–60
Kazmi SMA (1963) Revision der Gattung Carduus (Compositae), Teil I. Sonderdruck aus den Mitteilungen der Botanischen Staatssamlung München 5:139–198
Kebede M, Ehrich D, Taberlet P, Nemomissa S, Brochmann C (2007) Phylogeography and conservation genetics of a giant lobelia (Lobelia giberroa) in Ethiopian and Tropical East African mountains. Mol Ecol 16:1233–1243
Koch MA, Kiefer C, Ehrich D, Vogel J, Brochmann C, Mummenhoff K (2006) Three times out of Asia Minor: the phylogeography of Arabis alpina L. (Brassicaceae). Mol Ecol 15:825–839
Kosman E (2003) Nei’s gene diversity and the index of average differences are identical measures of diversity within populations. Plant Pathol 52:533–535
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA 76:5269–5273
Nybom H (2004) Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol Ecol 13:1143–1155
Popp M, Gizaw A, Nemomissa S, Suda J, Brochmann C (2008) Colonization and diversification in the African ‘sky islands’ by Eurasian Lychnis L. (Caryophyllaceae). J Biogeogr 35:1016–1029
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Rohlf F (2000) NTSYSpc: Numerical Taxonomy and Multivariate Analysis System. Version 2.11a. Setauket (NY): Exeter Software
Rosenberg NA (2004) DISTRUCT: a program for the graphical disply of population structure. Mol Ecol Notes 4:137–138
Schönswetter P, Tribsch A (2005) Vicariance and dispersal in the alpine perennial Bupleurum stellatum L. (Apiaceae). Taxon 54:725–732
Tadesse M (2004) Asteraceae (Compositae). In: Hedberg I, Friss I, Edwards S (eds) Flora of Ethiopia and Eritrea. Addis Ababa University, The National Herbarium, Addis Ababa
Thulin M (1989) Subfamily Papilionoideae (Faboideae). In: Edwards S, Hedberg I (eds) Flora of Ethiopia and Eriteria. The National Herbarium, Addis Ababa University/Department of Systematic Botany, Uppsala University, Uppsala, Addis Ababa
Van de Peer Y, De Wachter R (1994) TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci 10:569–570
Vos P, Hogers R, Bleekwe M, Reijians M, Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP a new techinique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Gizaw A, Kebede M, Nemomissa S, Ehrich D, Bekele B, Mirré V, Popp M, Brochmann C (2013) Phylogeography of the heathers Erica arborea and E. trimerain the afro-alpine ‘sky islands’ inferred from AFLP and plastid DNA sequences. FLORA 208:453–463
Yalden DW (1983) The extent of high ground in Ethiopia compared to the rest of Africa. SINET 6:35–38
Acknowledgments
This study is part of the project ‘AFROALP-II—Afro-alpine ‘sky islands’: genetic versus taxonomic biodiversity, climate change, and conservation’ funded by The Norwegian Programme for Development, Research and Higher Education (NUFU; project no 2007/1058) to S. Nemomissa and C. Brochmann. We thank the other members of the AFROALP-II team for discussions and help during the fieldwork. Sincere thanks are due to Mats Thulin and Manuel Pimentel who determined/verified the identity of our Trifolium and Carduus collections, respectively, and to Desalegn Chala for producing the base map.
Author information
Authors and Affiliations
Corresponding author
Additional information
T. Wondimu and A. Gizaw should be considered shared first authors.
S. Nemomissa and C. Brochmann should be considered shared senior authors.
Appendix 1
Appendix 1
See Table 4.
Rights and permissions
About this article
Cite this article
Wondimu, T., Gizaw, A., Tusiime, F.M. et al. Crossing barriers in an extremely fragmented system: two case studies in the afro-alpine sky island flora. Plant Syst Evol 300, 415–430 (2014). https://doi.org/10.1007/s00606-013-0892-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-013-0892-9