Abstract
In this study, the genetic diversity of 15 Turkish natural Capparis populations was screened using the randomly amplified polymorphic DNA analysis (RAPD) technique. Ten RAPD primers produced 98 loci, 73 of which were polymorphic. The binary RAPD data were computed using the POPGENE (version 1.31), a genetic data analysis software program. According to genetic diversity analysis at locus level, the total genetic diversity (H T) and genetic diversity within population (H s) were detected as 0.16 and 0.12, respectively. The genetic differentiation (G ST) and gene flow (N m) between populations were observed as 0.22 and 1.79, respectively. The mean number of allele per locus (n a), the mean number of effective allele (n ea), and the mean value of genetic diversity (H e) were determined as 2, 1.20, and 0.16, respectively. According to Pearson’s correlation analysis, the mean number of allele had a strong negative correlation with wind and a strong positive correlation with rain. According to multiple regression analysis, eco-geographical factors had a significant effect on the mean number of allele, the mean number of effective allele, and the mean value of genetic diversity. The principal component analysis revealed 87.42 % of total genetic variation. The principal coordinate analysis displayed the separation of population according to genetic distances based on dissimilarities matrix values on a scattered plot graph. Five different varieties, Capparis spinosa L. var. spinosa, var aegyptia and var. canescens, and Capparis ovata Desf. var. palaestina, and var. herbacea were identified in this study. Intermediate forms of plants were observed among the specimens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Capparis belongs to section Capparis L. in the genus Capparis L. from Capparaceae and includes more than 250 species (Jacobs 1965; Mabberley 1987: Cited in Fici 1993) grown in the tropical and subtropical areas of the Old and New World (Fici 2011). In the Mediterranean region, five species (Capparis spinosa L., Capparis sicula Veill., Capparis aegyptia (Lam.) Boiss., Capparis orientalis Veill., and Capparis ovata Desf.) have been recorded (Inocencio et al. 2006). In Turkey, two species (C. spinosa and C. ovata) of Capparis L. grow and each species is represented by three varieties. These varieties are: C. spinosa var. spinosa, C. spinosa var. inermis Turra., C. spinosa var. aegyptia (Lam) Boiss, and; C. ovata var. palaestina Zoh., C ovata var. herbacea (wild) Zoh., and C. ovata var. canescens (Coss.) Heywood (Zohary 1960; Davis 1965–1985).
The flowers of the Capparaceae family display a great evolutionary plasticity for adaptation to different pollinating agents. In the large genera Cleome and Capparis, bees, hummingbirds, hawk moths, and bats are involved in pollination. On the other hand, protandry and self-compatibility have been reported in the case of Capparis pittieri (Bawa et al. 1985: cited in Kers 2003).
Coode (1965) reported the intermediate forms having leaves rounded and elliptic on the one plant between var. spinosa and C. ovata Desf. var. canescens (Coss.) Heywood. Barbera and Di Lorenzo (1984) mentioned that hybridisation was easy between C. spinosa and C. ovata, therefore, there were many intermediate types in culture. Zohary (1960) noted that he had seen intermediates between var. aegyptia and C. ovata var. canescens. Higton and Akeroyd (1991) stated that there were tentative intermediates between C. spinosa L. and C. ovata Desf. and parallel variations were frequent.
There is very scarce molecular study about section Capparis L. in the literature. Inocencio et al. (2005) investigated the relationship between Capparis spp. (wild populations, presumed hybrids and local cultivars) using Amplified Fragment Length Polymorphism (AFLP) fingerprinting technique, while Abdel-Mawgood et al. (2006) applied the RAPD technique for the conservation of an isolated population of Capparis decidua. Vyas et al. (2009) analysed the genetic diversity of Capparis decidua (Forssk.) Edgew. according to biochemical and RAPD markers. Abdel-Mawgood et al. (2010) studied the genetic structure and diversity within, and among, six populations of Capparis decidua (Forssk.) Edgew. from Saudi Arabia using RAPD markers. The genetic diversity of the caper plant (Capparis L. ssp.) recorded employing ISSR markers sourced from North Morocco was reported by Saifi et al. (2011). A report of taxonomic considerations among, and within, some Egyptian taxa of Capparis and related genera (Capparaceae) as revealed by RAPD fingerprinting was published by Moubasher et al. (2011). More recently, Nosrati et al. (2012) detected an effect of population size on Capparis spinosa (Capparaceae) using RAPD markers.
There are many studies about Capparis but all are about phytochemical properties, or the different types of vegetative propagation of the plant in Turkey. Therefore, this report will be the first in the literature to address the molecular data of Turkish Capparis. Analyses were carried out to estimate the following genetic parameters: genetic diversity (H e), genetic diversity within (H S) and overall populations (H T), and genetic differentiation between populations (G ST). In addition, the effects of climate (temperature T, humidity HU, wind W, and rainfall R) and geography (altitude AL, latitude LT, and longitude LN) on the genetic diversity of 15 Turkish Capparis populations.
Materials and methods
Plant material
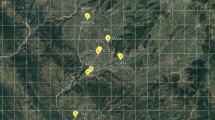
Fifteen Turkish natural populations of Capparis were analysed genetically in terms of randomly amplified polymorphic DNA (RAPD) diversity in this study. Genetic analyses were carried out using caper seeds. The seeds were collected in the August and September of 2010 from the Adıyaman, Antalya, Aydın, Balıkesir, Batman, Burdur, Çorum, Denizli, Diyarbakır, İzmir, Mardin and Şanlıurfa provinces, which are located at different altitudes ranging from 46 to 1,034 m (Fig. 1), by Dr. Fetullah Tekin, Dr. Özlem Özbek and Aslı Kara. The plants were identified by Prof. Dr. Şinasi Yıldırımlı.
Table 1 shows the variety names and the altitudes of locations from where the seeds were collected. Also shown are the name of the collection locations and the population codes that were used in this study. Throughout the text, we identified the different populations based on these codes. Population size was determined according to the number of plant in each population. If the numbers of plants are in the range of 1–25, it was considered to be a small population size; 26–50 indicates a medium population size, and >50 signifies a large population size. We randomly selected 10 accessions per population, in total 150 for randomly amplified polymorphic DNA (RAPD) analysis. Accessions code numbers were named by the population codes with numbers from 1 to 10; such as AN1, AN2 … AN10 and so on.
Morphological characterisation
For morphological characterisation we followed the taxonomical treatments of previous authors (Coode 1965; Zohary 1960; Higton and Akeroyd 1991). The capparis plants were identified according to their morphological characters (Table 2). For morphological characterization 17 morphological characters [Plant habit (PH), stipule shape (SS), stipule base (SB), stipule orientation (SO), stipule colour (SC), stipule hardness (SH), stem colour (STC), leaf shape (LS), base of leaf (BS), leaf apex (LA), fruit shape (FS), pulp colour (PC), seed colour (SEC), midrib (MI), mucronate (MU), stem hair (STH), leaf hair (LH), seed shape (SS)] were used. When the seeds were collected, there were very few numbers of flowers, which could not be possible to conserve for long term, therefore, the flower’s features were not included for morphological characterisation in this study.
DNA extraction
For DNA extraction, caper seeds, which go through periods of dormancy and exhibit troubles in germination, were used. Therefore, firstly, we applied pre-treatments to the seeds to break their dormancies. The seeds were embedded in tap water at room temperature overnight and the seeds, which sank in water, were collected and dried. Next, the seeds were treated with HCl (37 %) for 20 min to degenerate the testa (seed coats) of the seeds. After that, the seeds were washed with tap water for 1 min and left to dry at room temperature. Dried seeds were rubbed with sand paper to gently break the surface layer of testa and expose the endosperm. The seeds were then soaked in 2,000 ppm gibberellic acid for 24 h. Finally, the seeds were transferred to petri dish plates, in which three copies of 3 mm wetted chromatography paper were placed and left to germinate under room conditions for 48 h. DNA was extracted from the embryo (2n) and endosperm (3n) of the seed according to the protocol of the Nucleospin Plant II Mini Kit.
DNA amplification
For DNA amplification, RAPD assays were carried out in a 20 μL reaction mixture containing template 2 μL 10× Taq buffer (complete), 0.5 μL dNTP mixture (25 mM each), 0.35 μL of Taq DNA polymerase (500 u/μL, Bioron), 0.5 μL primer (100 pmol, Operon Technologies Inc.), 5 μL DNA (10–20 ng) and distilled water up to 20 μL. The sequences of the primers applied are provided in Table 2. Amplification was performed in a Thermo Scientific thermocycler PCR system. DNA amplification was obtained as follows: 94 °C for 5 min, 45 cycles of 94 °C for 1 min, 32 °C for 1 min, and 72 °C for 2 min, followed by one cycle of 72 °C for 10 min. The amplified DNA products were resolved on 1 % agarose gel (Sigma), visualised by ethidium bromide (10 mg/mL) staining, and photographed under UV light (DNR bio-imaging system). The RAPD bands were scored from photographs and gels into a binary data matrix as 1 (present) or 0 (absent).
Estimation of reproducibility rate of RAPD bands
We selected an accession randomly from each population, in total 15, and formed a repeat group in this study. The same PCR conditions and primers used in the study were then applied to the repeat group to determine the reproducibility rates of RAPD bands. Finally, the RAPD bands of the repeat group and the first run of the same samples were compared. We counted 223 and 173 RAPD bands in the first run and in the repeat group, respectively. Among these bands, 157 were repeated and the reproducibility of RAPD bands was 70.40 %. A representative figure of repeated RAPD-PCR results was obtained and their first run is detailed in Fig. 2.
Statistical analysis
RAPD bands are biallelic and dominant markers. The data considered to be diploid and dominant were analysed using POPGENE version 1.31 (Yeh et al. 1997). Genetic diversity (H e) was estimated according to Nei (1973). The mean number of alleles per locus (n a) and the mean number of effective alleles per locus (n ea) were also calculated. The percentage of the existing alleles in the 15 populations was estimated based on the overall sample size analysed for each locus, and the mean number of the sample size was considered.
Genetic differentiation between populations is often estimated with Nei’s Coefficient G ST (Nei 1972) for dominantly inherited DNA markers. This coefficient may tell us how genetic variation is partitioned within, and between, populations. The gene flow (N m) was calculated from G ST to determine its effect on gene diversity in this study. A dendrogram, displaying phylogenetic relationships between populations, was constructed according to genetic distances, based on RAPD data (Fig. 3).
A dendrogram representing phylogenetic relationships between 15 Capparis populations, grown in Turkey according to genetic distances (Nei 1972) between populations
Pearson’s correlation (r P) was computed to relate the following genetic indices with geographical (AL, LT, and LN) and climatic (R, T, HU, and W) variables: n a, n ea, and H e. Multiple regression analysis (MR) was used to display the effects of environmental factors on the variance of genetic indices. MR employed the genetic variables as dependent and the environmental variables as independent. Pearson correlation and multiple regression analysis were carried out using IBM SPSS (version 11) for Windows.
In this study, PCA was performed using the following genetic indices: n a, n ea, and H e as well as geographical (AL, LT, and LN) and climatic (R, T, HU, and W) data as variables according to the Pearson’s (one-tailed) correlation matrix. For principal coordinates analysis (PCoA), dissimilarity matrix values, which were calculated using a binary data matrix with Jaccard’s coefficient, were used to ordinate accessions from 15 Turkish Capparis populations on a scattered plot. The genetic distances values were used for hierarchical cluster analysis using UPGMA (Unweighted Pair-Group Average) agglomeration method using POPGENE (version 1.31). PCA and PCoA were carried out using XLSTAT (version 2013 Addinsoft).
Results
Morphological characterisation of 15 Capparis populations
Fifteen Capparis populations analysed in this study were characterised according to general morphological characters (Supplemantary Material Table S1). Description of populations are as follows:
Antalya (AN)
Obovate to round shaped leaves were glabrous, obtuse leaf base, retuse apex shape without mucro formation and raised midrib (Fig. 4). Strong, long, and golden-yellow coloured stipules curved and oriented retrorse (Fig. 5). Purple coloured stem was hairless and procumbent habit. Elliptic shaped fruit had yellow coloured pulp formation. Brown coloured seeds had oblong, obovoid-pyriform or renal form. This population is considered as Capparis spinosa L. var. spinosa.
Aydın/Didim (AD)
Rounded to subcordate shaped leaves were glabrous, cordate leaf base, rounded apex shape without mucro formation, and raised midrib (Fig. 6a). Moderate to weak and orange coloured stipules were rare, curved, and oriented retrorse (Fig. 7a). Purple to green coloured stem was hairless, and erect to pendulous habit. Obovate shaped fruit had yellow coloured pulp formation. Dark brown coloured seeds had oblong, obovoid-pyriform or renal form. This population is considered as Capparis spinosa L. var. spinosa.
Mardin/Beyazsu (BS)
Rounded shaped leaves were glabrous, obtuse leaf base, rounded apex shape without mucro formation, and raised midrib (Fig. 6b). Strong and orange coloured stipules were oriented straight (Fig. 7b). Green coloured stem is hairless and erect habit. Obovate shaped fruit had red coloured pulp formation. Dark brown coloured seeds had oblong, obovoid-pyriform or renal form. This population is considered as Capparis spinosa var. aegyptia (Lam) Boiss.
İzmir (IZ)
Ovate to elliptic shape leaves were hairy, obtuse leaf base, obtuse to tapering apex shape without mucro formation and raised midrib (Fig. 6c). Strong to weak, short to long and orange to yellow coloured stipules were curved to erect and oriented antrorse to retrorse (Fig. 7c). Green coloured stem covered with hairs, and erect habit. Obovate shaped fruit had red coloured pulp formation. Brown coloured seeds had oblong, obovoid-pyriform or renal form. This population is considered as Capparis spinosa L. var. spinosa.
Denizli (DE)
Rounded to subcordate shaped leaves were glabrous, cordate leaf base, rounded apex shape, with- or without mucro formation and raised midrib (Fig. 6d). Moderately hard and golden-yellow coloured stipules curved and oriented antrorse (Fig. 7d). Green coloured stem covered with hairs, and procumbent habit. Obovate shaped fruit had red coloured pulp formation. Brown coloured seeds had oblong, obovoid-pyriform or renal form. This population is considered as Capparis spinosa L. var. spinosa.
Adıyaman/Eski Kahta (EK)
Ovate to rounded shape leaves were hairy, obtuse to tapering leaf base, obtuse apex without mucro formation, and raised midrib (Fig. 8a). Strong and golden-yellow coloured stipules curved and oriented retrorse (Fig. 9a). Green coloured stem is hairy and erect habit. Obovate shaped fruit had red coloured pulp formation. Dark brown coloured seeds had oblong, obovoid-pyriform, orbicular or renal form. Capparis spinosa L. var. spinosa.
Çorum/Osmancık (OS)
Ovate to rounded shaped leaves were hairy, obtuse to cordate shape leaf base, tapering apex shape with mucro formation and raised midrib (Fig. 8b). Strong, long, and orange coloured stipules curved, oriented straight and slightly upward (Fig. 9d). Green coloured stem covered with hairs and erect to procumbent habit. Obovate shape fruit had red coloured pulp formation. Dark brown coloured seeds had oblong, obovoid-pyriform, orbicular, or renal form. Capparis spinosa var. herbaceae (Willd.) Zoh.
Şanlıurfa/Harran (HA)
Obovate to elliptic shaped leaves were glabrous to hairy, obtuse leaf base, obtuse to tapering apex shape with mucro formation and raised midrib (Fig. 8c). Moderately hard and golden-yellow coloured stipules curved and oriented antrorse (Fig. 9b). Green coloured stem was hairless to hairy and procumbent to pendulous habit (Fig. 10). Obovate shape fruit had red coloured pulp formation. Dark brown coloured seeds had oblong, obovoid-pyriform or renal form. This population is considered as Capparis spinosa var. aegyptia (Lam) Boiss.
Balıkesir (BE)
Obovate to elliptic shaped leaves were glabrous, obtuse leaf base, tapering apex shape with- or without mucro formation and raised midrib (Fig. 11a). Weak and orange coloured stipules curved and oriented antrorse (Fig. 9e). Green coloured stem covered with hairs, and erect habit. Obovate shaped fruit had red coloured pulp formation. Brown coloured seeds had oblong, obovoid-pyriform or renal form. This population is considered as Capparis spinosa L. var. canescens (Coss.).
Şanlıurfa/Bozova
Elliptic shaped leaves were hairy, obtuse leaf base, tapering apex shape with weak spiny mucro formation, and raised midrib (Fig. 11b). Weak and golden-yellow coloured stipules were curved and oriented antrorse (Fig. 9c). Green coloured stem is hairless and pendulous habit. Obovate shaped fruit and red coloured pulp formation. Dark brown coloured seeds had oblong, obovoid-pyriform or renal form. This population is considered as Capparis spinosa var. aegyptia (Lam) Boiss.
Burdur (BU)
Ovate, rounded, or subcordate shape leaves were glabrous, obtuse to cordate leaf base, rounded apex shape with mucro formation, and raised midrib (Fig. 12a). Moderate to weak and golden-yellow coloured stipules were curved, oriented straight and slightly upward (Fig. 13a). Green coloured stem covered with hairs, and procumbent habit. Obovate shaped fruit and red coloured pulp formation. Dark brown coloured seeds had oblong, obovoid-pyriform or renal form. This population is considered as Capparis spinosa var. herbacea (Willd.) Zoh.
Diyarbakır/Tepecik (DT)
Elliptic to obovate shape leaves were hairy, obtuse leaf base, tapering apex shape with strong spiny mucro formation, and raised midrib (Fig. 12b). Strong, short, and orange-yellow coloured stipules curved and oriented retrorse (Fig. 13b). Green coloured stem was hairless, and erect habit. Obovate shaped fruit had red coloured pulp formation. Dark brown coloured seeds had had oblong, obovoid-pyriform, orbicular, or renal form. This population is considered as Capparis spinosa var. palaestina Zoh.
Batman/Hasankeyf (HK)
Obovate shaped leaves were hairy, obtuse leaf base, tapering apex shape with weak mucro formation, and raised midrib (Fig. 14a). Moderately hard and yellow coloured stipules were oriented straight (Fig. 13c). Whitish-grey coloured stem covered with hairs, and procumbent to pendulous habit (Fig. 15). Obovate shaped fruit had red coloured pulp formation. Dark brown coloured seeds had oblong, obovoid-pyriform, or renal form. This population is considered as Capparis spinosa var. palaestina Zoh.
Savur (SA)
Elliptic shaped leaves were hairy, obtuse leaf base, tapering apex shape with spiny mucro formation, and raised midrib (Fig. 14b). Strong, short, and orange coloured stipules curved and oriented retrorse (Fig. 13e). Green coloured stem covered with hairs, and procumbent habit. Obovate shaped fruit had red coloured pulp formation. Brown coloured seeds had oblong, obovoid-pyriform or renal form. This population is considered as Capparis spinosa var. palaestina Zoh.
Diyarbakır Üçtepe (DU)
Elliptic to ovate shaped leaves were hairy, obtuse leaf base, tapering apex with- or without mucro formation, and raised midrib (Fig. 14c). Strong and orange-yellow coloured stipules curved and oriented straight (Fig. 13d). Whitish-grey coloured stem was hairless and erect to pendulous habit (Fig. 16). Obovate shaped fruit had red coloured pulp formation. Dark brown coloured seeds had oblong, obovoid-pyriform or renal form. This population is considered as Capparis spinosa var. palaestina Zoh.
Polymorphism at RAPD loci
In this study, genetic diversity was analysed in 15 Turkish Capparis L. populations by RAPD method using 10 OPA primers, which produced 98 polymorphic loci (100 %). If the frequency of the most frequent allele of a gene is equal or less than 0.95, or 0.99, it is considered as polymorphic in population genetics. We considered the most frequent allele of a gene as polymorphic if its frequency was less than 0.95. Therefore, in this study, 73 loci out of 98 were polymorphic (74.49 %) and 25 loci (25.51 %) were theoretically polymorphic, but the most frequent allele of these loci had frequency equal to, or higher than, 0.95, thus, they were considered as monomorphic. A representative of RAPD band patterns that were produced using the primer OPA1, which was observed in population BS, is provided in Fig. 17.
The primer OPA18 produced the highest number of locus (12), while the primer OPA1 produced the lowest number of locus (5) (Supplementary Material Table S2). The highest number of polymorphic locus was determined to be 12 (100 %) in the case of the OPA18 primer, while the lowest polymorphic locus was determined to be four (80 %) with the OPA01 primer. On the other hand, when we looked exclusively at the percentage of the polymorphic locus rates of primers, the highest percentage of polymorphism was shown by OPA18 to be 100 %, while the lowest percentage of polymorphism was shown by OPA04 to be 45.45 %. The molecular weights of RAPD bands ranged between 100 and 2,000 bp. When we considered the total number of locus, and number of polymorphic locus produced by primers, OPA03, OPA05, OPA06, OPA13, and OPA18 displayed more polymorphism than other primers.
At population level, the highest number of polymorphic locus, 66 (67.35 %), was observed in population AN, while the lowest number of polymorphic locus, 18 (18.37 %), was observed in population IZ (Data not shown).
Analysis of genetic diversity
The mean values of genetic diversity within population (H S) and total (H T ) were 0.124 and 0.158, respectively (data not shown). The highest H S value was obtained at OPA18/3 locus as 0.281, while the lowest H S value was obtained at OPA5/1 locus as 0.055. The highest H T value was observed at OPA6/12 locus as 0.351, while the lowest H T value was observed at OPA4/7 locus as 0.068. The mean values of genetic differentiation (G ST), and gene flow (N m) between populations were calculated as 0.218, and 1.792, respectively. The highest G ST value was determined at OPA3/9 locus as 0.654, while the lowest G ST value was determined at OPA13/5 locus as 0.053. The highest N m value was found at OPA9/3 locus as 6.895, while the lowest N m value was found at OPA3/9 locus as 0.264. The highest genetic distance value (D = 0.091) was observed between the populations BO and DT, while the lowest genetic distances value (D = 0.007) was observed between the populations AD and BS (data not shown).
The highest number of allele (n a), the highest number of effective allele (n ea), and the highest value of expected gene diversity (H e) were observed as 1.674, 1.283, and 0.180, respectively, in population AN (Table 2). The lowest number of allele, the lowest number of effective allele, and the lowest value of expected gene diversity were observed as 1.184, 1.072, and 0.048, respectively, in population IZ.
Pearson’s correlation analysis
According to Pearson’s correlation (2-tailed) analysis, genetic diversity and the number of effective allele did not correlate directly with eco-geographical factors (Supplementary Material Table S3). However, wind (using 2009 and 2010 data) had strong negative correlation with allele number, displaying correlation values of r P = −0.549 (p = 0.034) and r P = −0.557 (p = 0.031) at p < 0.05 significant level, respectively. A strong positive correlation was also detected between rainfall (2009) and allele number with a correlation value of r P = 0.542 (p = 0.037) at p < 0.05 significant level.
Multiple regression analysis
The results of MR analysis showed that the combination of altitude, latitude, longitude, in addition to the 2009 and 2010 data recording temperature, rainfall, humidity, and wind, accounted for a considerable proportion of the variation observed in RAPD loci (Supplementary Material Table S4). When these seven variables combined, they had effects ranging from moderate to overly strong levels on the variance of the n a (92.5 %), n ea (35 %) and H e (90.7 %).
Principal component analysis (PCA)
Principal component analysis (PCA) accounted for 87.42 % of the total genetic variation based on five components (Supplementary Material Table S5). The first component, which accounted for 28.53 % of the total variance, was formed with n a, n ea, H e, W09, W10 and R09 (data not shown). The second component, representing 23.83 % of the total variance, was formed with H09, H10, R09, R10, AL, and LN. The third component, which was 15.93 % of the total variance, was composed of T09, T10, and LA. The fourth component, which comprised 10.93 % of the total variance, was composed of H09, H10, R09, and R10. The fifth component, which accounted for 8.20 % of the total variance, was formed by W09 and W10.
Principal coordinate analysis (PCoA)
According to PCoA results, the codes of the accessions from each population were plotted on the two components corresponding to different regions from where the 15 Turkish Capparis populations were collected (Fig. 18). In the scattered plot graph, accessions from populations BU, DT, DU, HK, and SA were grouped in circle 1.
The accessions from populations AD, BS, EK, and OS were grouped with some accessions from populations AN, BE, BO, DE, HA, and IZ in circle 2, while some accessions from populations AN, BE, BO, DE, HA and IZ were grouped in circle 3. The PCoA graph was consistent with the results of the dendrogram, according to which populations clustered into two main groups. In the first main group the populations BU, DT, DU, HK, and SA were clustered together, while in the second main group the populations AD, AN, BE, BO, BS, DE, EK, HA, IZ and OS were clustered together. In the second main group the populations AD, AN, BS, DE, EK, HA, IZ and OS were grouped in a separated subgroup and the population AN was the distant population to these populations in the dendrogram.
Discussion
Heywood (1964) reported two species C. spinosa L. and C. ovata Desf. in his publication of Flora Europa. Zohary (1960) recognised six species and 15 varieties in Mediterranean region and near east, while Jacobs (1965) recognised C. spinosa as a single species, which covers all of Zohary’s taxa in a revision of mainly East Asian material (Higton and Akeroyd 1991). Previous authors (Zohary 1960; Coode 1965; Higton and Akeroyd 1991) reported that two subspecies, C. spinosa L. and C. ovata Desf. of Capparis presented in Turkey and each subspecies represented by three varieties. We also observed two subspecies C. spinosa L. and C. ovata Desf. for species C. spinosa L. in Turkey. Five varieties were identified, three (var. spinosa, var. aegyptia, and var. canesecens) for subspecies C. spinosa L. and two (var. palaestina and var. herbacea) for C. ovata Desf. Some previous authors recognised var. canescens as a variety of spp. C. ovata Desf., but it was morphologically similar to spp. C. spinosa L. and according to molecular data it was clustered with varieties of spp. C. spinosa L. Therefore, we counted it as the variety of spp. C. spinosa L.
In line with previous studies (Zohary 1960; Coode 1965; Higton and Akeroyd 1991; Inocencio et al. 2006), we observed great morphological (leaf and seed morphology) variation among the populations even within populations. This is the first study of Capparis in Turkey in terms of using molecular techniques. We do not have much information about both morphology and molecular structure of Capparis in Turkey to compare the results obtained in this study. Mainly morphological characters of leaves with presence or absence of thorny stipules and fruits are used to differentiate Capparis plants. However, we observed thorny stipules in all specimens concerned in this study. The differences about the stipules were their colours, orientation, and somewhat hardness. The taxonomical treatments for both species and their varieties seemed to be problematic because morphological variability was confusing very much to identify varieties according to diagnostic features reported by previous authors (Zohary 1960; Davis 1965; Jacobs 1965; Al-Gohary1982; Higton and Akeroyd 1991; Heywood 1993; Fici and Gianguzzi 1997; El-Karemy 2001: cited in Moubasher et al. 2011).
Although, the seeds are thought to be the least plastic parts of capparis (Harper 1970), we observed great variability on its morphological characters (dimensions and shape, weight of 1,000 seeds) (data not shown). Another finding was that the seed weight of 1,000 seeds had higher values in C. spinosa L. populations than in C. ovata Desf. populations as it was reported by Tilli et al. (2011). According to seed morphology the varieties of subspecies C. spinosa L. and the subspecies C. ovata Desf. could be differentiated roughly (data not shown). We concluded that the seed was bearing important taxonomical treatment characters, which could be used to differentiate Capparis populations prior to other detailed analysis (Fig. 19).
In the literature there is very scarce information regarding the genetic data analysis of genus Capparis L. When the number of primers (10-mer oligonucleotide), number of plant samples (150 accessions), and the genetic data analysis undertaken with 15 Turkish Capparis L. populations in this study were taken account, this is the very first comprehensive molecular study on Capparis L. in the literature. When we compared the proportion of these polymorphic loci (74.49 %), our results were quite higher than in previous studies (Abdel-Mawgood et al. 2006, 2010; Vyas et al. 2009; Inocencio et al. 2005). However, Saifi et al. (2011) analysed genetic relationships between 18 populations (90 accessions) using five ISSR primers and revealed 75.51 % polymorphic fragments, which is consistent with our results.
RAPD data were computed using POPGENE version 1. 31 (Yeh et al. 1997) for genetic analysis, which displayed significant information about the genetic structure of Capparis populations from Turkey. The mean values of genetic diversity within population (H S) and total (H T) might be considered relatively low. This might be related to several factors. Firstly, we have little knowledge about the breeding system of Capparis L. (Saifi et al. 2011). According to Zhang and Tan (2009), C. spinosa L. has a breeding system of andromonoecious, in which plants have both perfect and male flowers. In male flowers (Fig. 20), the ovary is aborted and never produces fruit. The perfect flowers have an elongated pistil and viable pollen (Fig. 21). When male flowers are compared with perfect flowers (Figs. 20, 21), they have larger anthers and pollen grains. Consequently, male flowers invest the resources to increase a larger male function instead of developing the female reproductive structure. The faster pollen tube growth and/or more success in fertilising ovules displayed by larger pollen grains result in a higher success rate with regard to siring (Skogsmyr and Lankinen 2002). In habitats with extremely hot summers, anthesis is nocturnal, and fast pollen tube growth ensures completeness of fertilisation before extreme temperatures are reached.
Also according to the findings of Zhang and Tan (2009), C. spinosa has a mixed mating system and it is capable of spontaneous self-pollination, which was almost 10 % in C. spinosa, while C. spinosa L. grows in an extremely hot and dry habitat, pollination may not be guaranteed due to the short time of anthesis. This might be a result of a decrease in both seed production and male reproductive opportunities. Therefore, spontaneous self-pollination might serve as a mechanism of reproductive assurance (Fausto et al. 2001: cited in Zhang and Tan 2009). The observation of a low level of genetic diversity values is most probably related to the breeding system of Capparis L. in this study. Although we did not measure self-pollination rate, the low level of genetic variation might be reflecting the effects of self-pollination.
Kers (2003) also reported that pollination agents in Capparis L. seed dispersal included ants, hummingbirds and so on in Capparidaceae. The scarcity of pollinators might also affect the degree of genetic diversity. In addition, seeds were deposited and consumed by ants as winter survival food. Capparis L. seeds have strong dormancy. When both of these stress factors are combined, the number of seeds having chances to germinate may decrease and affect indirectly the level of genetic diversity.
The amount of genetic diversity seems to be related to population size (Nosrati et al. 2012), although we did not correlate them statistically in this study. Some small (BS and BT) and medium (IZ) populations had low level of genetic diversity, while large populations (AN and BO) had high level of genetic diversity. The low level of genetic diversity detected in fragmented and small-sized populations of Capparis could result in genetic drift, high inbreeding depression and low evolutionary potential (Reed and Frankham 2003: cited in Nosrati et al. 2012). Failure in pollination is often observed in small and fragmented plant populations, which are less attractive for pollinators (Jennersten 1988; Wilcock and Neiland 2002; Andrieu et al. 2009). Consequently, this might result in a decrease in fruit/seed set (Keller and Waller 2002). In addition, the intensive harvest of caper fruits by inhabitants around the area for commercial purposes has resulted in an effective decrease of fruit/seed dispersal in nature, and, consequently, a reduction in recombination and sexual reproduction (Nosrati et al. 2012). On the other hand, in some large and medium populations, we also determined a low level of genetic diversity. Differentiation in genetic diversity among populations might also be related to the eco-geographical conditions of locations, and where the populations are collected, a contention supported by the MR and Pearson’s correlation analyses conducted in this study.
In general, when the level of genetic diversity observed in 15 Turkish Capparis populations is considered, it was expected to be higher than the level we obtained because, embryo (2n) and endosperm (3n) DNA were used for genetic analysis. This is most probably related to the possibility that homozygous genotypes might be higher than heterozygous genotypes due to the self-compatibility in the plant material analysed in this study. In addition, aneuploid, and polyploid changes reported by Kers (2003) should be taken into account.
RAPD analysis indicated that the genetic distances among the 15 Turkish Capparis populations studied were very low, as reported by Saifi et al. (2011). The mean values of genetic differentiation (G ST) indicated that 78.2 % of variation was within populations and 21.8 % of variation was between populations. This level of genetic differentiation is a large amount especially for outcrossing and perennial plant species (Lienert 2004). This might be related with also the high percentage of self-compatibility in Turkish Capparis populations.
According to Pearson’s correlation (2-tailed) analyses, allele number had a strong negative correlation with wind (using 2009 and 2010 data), and a positive correlation with rainfall (2009). Rainfall might have had an effect on the regeneration of the over ground parts of plants, what was an expected result. Capparis spinosa L. is an andromonoecious plant species, which has both perfect and male flowers. Wind might increase the percentage of self-pollination rates in reproduction and help to avoid the recombination of alleles. Capparis spinosa L. is reported to be an insect pollinated plant species. Therefore, during the short anthesis time, a strong wind might decrease the number of pollinators and also the numbers of pollen grains their body parts bear thus decreasing the rate of cross-pollination.
Although eco-geographical factors, with the exception of rainfall and wind, had no correlation directly with genetic indices, according to multiple regression analysis when they combined, they had a substantial effect on genetic indices. Capparis plants are usually grown under stringent conditions such as extremely cold or high temperatures, dry, and in poor soils. In addition, the habitat’s elevations are quite changeable. Consequently, eco-geographical factors have a significant effect on the genetic diversity of Capparis.
PCA results are also complementary to the MR results. According to the five principal components extracted, the first component roughly includes dependent variables (n a, n ea, H) and W09, W10 and R09 explained 28.527 % of total variation, and rest of the principal components were composed of independent variables of eco-geographical factors and explained 58.895 % of total variation. The PCA also displayed the substantial effect of eco-geographical factors on genetic diversity of natural Capparis populations from Turkey.
The results of PCoA displayed that populations were grouped into three different regions. In the first region, the accessions from the populations (DT, DU, HK, and SA) of C. ovata Desf. var. palaestina and the population BU representing C. ovata Desf. var. herbacea were located distinctly away from the accessions representing the populations of C. spinosa L. varieties. This result was overlapped with the result of dendrogram based on molecular data, and assessment according to morphological characters. This shows that C. ovata Desf. var. palaestina is well differentiated according to morphological characters and molecular assessment from the other varieties of Capparis (Coode 1965). In this region, the accessions from the population SA, circled by dotted line, were located between C. ovata Desf. var. palaestina and C. spinosa L. varieties as it was in the dendrogram. The population of BS was recognised as the variety of C. spinosa L. var. aegyptia. The places where both of the populations BS and SA collected are much closed to each other. It is possible that there might be some introgression by gene flow between these two varieties. Therefore, the population SA was placed between the var. palaestina and var. aegyptia.
According to Inocencio et al. (2005) C. spinosa L. occasionally detected in the wild, mostly its known populations were cultivated and displayed intermediate morphological characters between C. orientalis and C. sicula. As a result of all these, they suggested that this taxon might be of hybrid origin and C. spinosa was a cultigen derived out of C. orientalis with some introgression from C. sicula rather than being a true species. In this regard, the accessions from the populations of spp. C. spinosa L. varieties were distributed to two different regions on the PCoA plot in this study. The majority of the accessions from the populations (BS, BO, EK, and HA) representing the variety of C. spinosa L. var. aegyptia were grouped together with some accessions from the populations (AD, AN, DE, IZ and, BE, and OS) representing the C. spinosa L. var. spinosa, C. spinosa L. var. canescens, and C. spinosa L. var. herbacea, respectively. Almost, half percent of the accessions from population AN, BE, HA, and IZ, and a few accessions from the populations BE and BO were grouped in the third region. The accessions from AN, BE, HA and IZ in the third region might belong to C. spinosa L. var. spinosa, while the accessions from AN, BE, HA and IZ in the second region might be hybrid forms between C. spinosa L. and C. ovata Desf., or two different varieties which could not be distinguishable according to morphological characters were coexisted in the same area. Because, two different leaf types on the same plant were observed in populations AD, DE, and OS (rounded and cordate), BE (obovate and elliptic), HA (obovate and rounded) and BU (elliptic and ovate) in this study. This might be the indication of intermediate forms as reported by previous studies (Coode 1965; Zohary 1960; Higton and Akeroyd 1991). In the third region, the accessions from the population BO might be also the hybrid plants between var. spinosa and var. aegyptia. In fact, during the seed germination processes, we had some difficulties about the germination of the seeds and plant growth. Perhaps this was not attributed to only seed dormancy. Most probably, we had the seeds collected from hybrid plants. But in literature, we do not have any information about reproductivity of hybrid Capparis plants, which is an interest of another study.
Coode (1965) reported that var. inermis Turra presented in Antalya. The leaf morphology of the population AN was similar to C. spinosa L. var. inermis Turra., which had no thorny stipules, but the population AN had. Therefore, the population AN was recognised as C. spinosa L. var. spinosa. Higton and Akeroyd (1991) referred the region, near where the populations AD and DE collected as the location of C. spinosa var. inermis Turra. on a map of Turkey. Although, the leaf morphological descriptions of the populations AD and DE were overlapped taxonomically with C. spinosa var. inermis Turra., nevertheless, they have strong thorny stipules, which are not found in var. inermis Turra. Therefore, these populations were also recognised as C. spinosa L. var. spinosa.
Both of the populations BU and OS were recognised as C. spinosa L. var. herbacea in this study. But, the population BU grouped with the populations recognised as var. palaestina, while the population OS grouped with var. spinosa, var. aegyptia, and var. canescens. We could not find any report about which variety or varieties were found in the area, where these populations collected, in the literature. But, the leaf and stipule structures of these populations were overlapped with the taxonomical treatment of previous studies. However, we observed intermediate forms in both of the populations. Most probably, when we were sampling, we sampled the hybrid genotypes, which had predominantly the genetic upload of C. ovata Desf. in population BU, and C. spinosa Desf. in population OS.
In this study, the RAPD method displayed 70.40 % of reproducibility. This is a considerably high level of reproducibility, in addition to its advantages of being fast, easy, cheap, and requiring less labour. The RAPD analysis displayed good performance in explaining genetic diversity within, and between, populations. The RAPD analysis showed that the method was successful to differentiate the varieties of C. spinosa L. and C. ovata Desf. from each other. When the RAPD technique is used with sufficient morphological characters such as reproductive parts, its success to differentiate the Capparis varieties will be increased, and can be used to characterise the genetic structure of the natural populations of Capparis.
Consequently, the PCoA result indicated that spp. C. spinosa L. has greater intraspecific variation than spp. C. ovata Desf. The leaf morphology and stipule characters are not sufficient to distinguish the different varieties in the species C. spinosa L. Moreover, we have little knowledge about the morphological variation, ecological requirements, reproduction type, and intra and interspecific genetic diversity of natural Capparis populations in Turkey. It is confirmed that for the species C. spinosa L. five varieties, var. spinosa, var. aegyptia, var. canescens, var. herbacea, and var. palaestina, sampled and analysed in this study. As a conclusion more complicated research projects should be planned to figure out appropriately the taxonomy of Capparis section in Turkey. Therefore, the results of this study are preliminary for those other further more specific studies.
References
Abdel-Mawgood AL, Assaeed AM, Al-Abdallatif TI (2006) Application of RAPD technique for the conservation of an isolated population of Capparis decidua Alexandria. J Agric Res 51:171–177
Abdel-Mawgood AL, Assaeed AM, Jakse J (2010) Genetic Structure and Diversity Within and Among Six Populations of Capparis decidua (Forssk.) Edgew. in Saudi Arabia. Plant Syst Evol 9(36):79–93
Al-Gohary IH (1982) Morphological studies on the Capparidaceae in Egypt. M.Sc. Thesis, Faculty of Science, Ain Shams University, Cairo
Andrieu E, Dornier A, Rouifed S, Schatz B, Cheptou PO (2009) The town Crepis and the country Crepis: how does fragmentation affect a plant-pollinator interaction? Acta Oecologica 35:1–7. doi:10.1016/j.actao.2008.07.002
Barbera G, Di Lorenzo RD (1984) The caper culture in Italy. Acta Horticulturae 144:167–171
Bawa KS, Bulloch SH, Perry DR, Coville RE, Grayum MH (1985) Reproduction biology of tropical lowland rain forest tree. II. Pollination system. Am J Bot 72:346–356
Coode MJE (1965) Capparis L. In: Davis PH (ed.) Flora of Turkey and the East Aegean Islands, vol 1. Edinburgh University Press, Edinburgh, pp 496–498
Davis PH (1965–1985) Flora of Turkey and the East Aegean Island, vol 1–9. Edinburg University Press, Edinburg
El-Karemy ZAR (2001) Capparaceae in the flora of Egypt. Taeckholmia 21:257–267
Fausto JA Jr, Eckhart VM, Geber MA (2001) Reproductive assurance and the evolutionary ecology of self-pollination in Clarkia xantiana(Onagraceae). Am J Bot 88:1794–1800
Fici S (1993) Taxonomic and Chorological notes on the genera Boscia Lam. Cadaba Foressk and Capparis L. (Capparaceae) in Somalia. Webbia 47(1):149–162
Fici S (2011) Capparis tchaourembensis, a new species of Capparaceae from Mayotte, Comororian Archipelago. Kew Bull 66:299–301
Fici S, Gianguzzi L (1997) Diversity and conservation of wild and cultivated Capparis in Sicily. Bocconea, 7:437–443. ISSN 1120-4060
Harper JL (1970) The shapes and sizes of seeds. Annu Rev Ecol Syst 1:327–356
Heywood VH (1964) Capparis L. In: Tutin TG, Heywood VH, Burges NA, Valentine D H, Walters SM, Webb DA (eds) Flora Europaea vol 1. Cambridge University Press, Cambridge, p 259
Heywood VH (1993) Flowering plants of the world. Oxford University Press, New York
Higton RN, Akeroyd JR (1991) Variation in Capparis spinosa L. in Europe. Flora Europaea 106(2):104–112
Inocencio C, Cowan RS, Alcaraz F, Rivera D, Fay ML (2005) AFLP fingerprinting in Capparis subgenus Capparis related to the commercial sources of capers. Genet Resour Crop Evol 52(2):137–144
Inocencio C, Rivera D, Obón MC, Alcaraz F, Barreña A (2006) A systematic revision of Capparis section Capparis (Capparaceae). Ann Mo Bot Gard 93(1):122–149
Jacobs M (1965) The genus Capparis (Capparaceae) from the Indus to the Pacific. Blumea 12(3):385–541
Jennersten O (1988) Pollination in Dianthus deltoids (Caryophyllaceae): effects of habitat fragmentation on visitation and seed set. Conserv Biol 2:359–366
Keller LF, Waller DM (2002) Inbreeding effects in wild populations. Trends Ecol Evol 17:230–241
Kers LE (2003) Capparaceae. In: Kubitzki K (Series Editor): The families and genera of vascular plants, vol 5: Kubitzki K, Bayer C (eds) Springer, Berlin, pp 36–56. ISBN 3-540-42873-9
Lienert J (2004) Habitat fragmentation effects on fitness of plant populations—a review. J Nat Conserv 12:53–72
Mabberley DJ (1987) The Plant-Book. A Portable Dictionary of the Higher Plants. Cambridge University Press, Cambridge
Moubasher H, Abd El-Ghani MM, Kamel W, Mansi M, El-Bous M (2011) Taxonomic considerations among and within some Egyptian taxa of Capparis and related genera (Capparaceae) as revealed by RAPD fingerprinting. Collectanea Botanica 30:29–35. doi:10.3989/collectbot.2011.v30.003
Nei M (1972) Nei’s original measures of genetic identity and genetic distance. Am Nat 106:283–292
Nei M (1973) Analysis of gene diversity in subdivided populations (population structure/genetic variability/heterozygosity/gene differentiation). Proc Natl Acad Sci USA 70(12):3321–3323
Nosrati H, Feizi MAH, Mazinani M, Haghighi AR (2012) Effect of population size on genetic variation levels in Capparis spinosa (Capparaceae) detected by RAPDs. Eur Asian J BioSci 6:70–75
Reed DH, Frankham R (2003) Correlation between fitness and genetic diversity. Conserv Biol 17:230–237
Saifi N, Ibijbijen J, Echchgadda D (2011) Genetic diversity of caper plant (Capparis ssp.) from North Morocco. J Food Agric Environ 9(3, 4):299–304
Skogsmyr I, Lankinen A (2002) Sexual selection: an evolutionary force in plants? Biol Rev Camb Philos Soc 77:537–562. doi:10.1017/S1464793102005973
Tilli N, Saadaoui E, Sakouhi F, Elfalleh W, El-Gazzah M, Triki S, Khaldi A (2011) Morphology and chemical composition of Tunisian caper seeds: variability and population profiling. Afr J Biotechnol 10(10):2112–2118
Vyas GK, Sharma R, Kumar V, Sharma TB, Khandelwal V (2009) Diversity analysis of Capparis decidua (Forssk.) Edgew. using biochemical and molecular parameters. Genet Resour Crop Evol 56:905–911
Wilcock C, Neiland MRM (2002) Pollination failure in plants: why it happens and when it matters. Trends Plant Sci 7:270–277
Yeh FC, Yang RC, Boyle T, Ye ZH, Mao JX (1997) POPGENE (version 1.32): The 523 user-friendly shareware for population genetic analysis. Molecular Biology and 524 Biotechnology Centre, University of Alberta Canada
Zhang T, Tan DY (2009) An examination of the function of male flowers in an andromonoecious shrub Capparis spinosa. J Integr Plant Biol 51:316–324
Zohary M (1960) The species of Capparis in the Mediterranean and the near eastern countries. Bull Res Counc Israel 80:49–65
Acknowledgments
This is the master thesis project of Aslı Kara and supported by a grant, which was provided by the Scientific Research Projects Department (BAP) of Hitit University with project number of FEF01.10.004. We are grateful for the collaboration of the General Directorate of Agricultural Research and Policy unit of the Republic of Turkey, Ministry of Food, Agriculture, and Livestock for providing technical assistance for field studies and special thanks to Dr. Fetullah Tekin for his contribution in the collection of Capparis seeds with us. We are also thankful to Prof. Dr. Şinasi Yıldırımlı from Hacettepe University for contribution to identification of plants according to Hacettepe University Herbarium (HUB) material. We are also giving special thanks to Dr. Silvio Fici from University of Palermo in Italy for his great contribution to improvement of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Özbek, Ö., Kara, A. Genetic variation in natural populations of Capparis from Turkey, as revealed by RAPD analysis. Plant Syst Evol 299, 1911–1933 (2013). https://doi.org/10.1007/s00606-013-0848-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-013-0848-0