Abstract
Multivariate analysis of leaf shape, anatomy, and Fourier-transform infrared (FTIR) data of 27 Camellia species with secretory structures (sects. Archecamellia, Stereocarpus, Furfuracea, Chrysantha), together with three species from related genera, Gordonia and Tutcheria (Theacea), was conducted to clarify some taxonomic problems. Our results show that crystals occurring in adaxial epidermal cells are firstly observed in Chrysantha species, and the secretory structures described are in fact cork warts. Furthermore, we introduce a form coefficient (F c) to assess the shape of epidermal cells, since they are usually irregular and difficult to describe. Pearson correlation analysis indicates that F c is useful to assess epidermal cell shape. Principal component analysis (PCA) of leaf shape indicates that two species from section Archecamellia and two species from section Stereocarpus are significantly different from those in section Furfuracea. Cluster analysis of FTIR data visualizes the degree of affinity among the 30 species examined here, which is consistent with the cluster analysis (CA) of anatomical data, as illustrated in the dendrogram. Therefore, our study indicates that integrated leaf characters based on leaf shape, anatomy, and FTIR data are useful in the taxonomic treatment of Camellia species with secretory structures. Taxonomic controversies among the Camellia species with secretory structures could thus be successfully addressed using only a few intact or small portions of leaves. Moreover, our results tend to support that Chrysantha species should not be merged into section Archecamellia, and that section Heterogenea should not be recognized in taxonomic treatments of Camellia species with secretory structures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
This is one of a series of studies on the genus Camellia, which hopes to improve our understanding of the taxonomic treatments of this genus (Lu et al. 2008a, 2008b; Lin et al. 2008). Secretory structures are common in the genus Camellia and are present in all species of sections Archecamellia Sealy, Stereocarpus (Pierre) Sealy, Furfuracea H.T. Chang, and Chrysantha H.T. Chang (Chang 1981, 1998). Notably, the above four sections represent groups of significant taxonomic controversy and uncertain affinities (Table 1). Based on morphological characters, Sealy (1958) first proposed the sections Archecamellia and Stereocarpus. He recognized seven species in section Archecamellia and one species in section Stereocarpus. H.T. Chang (1981) retained Sealy’s (1958) sections Archecamellia and Stereocarpus in his taxonomic treatments. However, he transferred four species from section Archecamellia into a new section Chrysantha. Furthermore, he set up another new section Furfuracea characterized by a large leaf, rough capsule, and solitary, large flower. In 1996, sect. Archecamellia and sect. Stereocarpus were reviewed by Chang, in which the monotypic sect. Stereocapus was maintained. However, in 1998, the taxonomic treatments of these two sections were returned to those of 1981. Ming (1999), based on Sealy’s (1958) and Chang’s (1981, 1998) work, produced a new taxonomic treatment. In his classification, Ming (1999) resurrected section Heterogenea from Sealy’s classification, comprising section Furfuracea and several Archecamellia and Stereocarpus species. He also disagreed with section Chrysantha established in Chang’s classification, and section Chrysantha was merged with section Pleurocarpus into section Archecamellia. Moreover, section Stereocarpus was significantly revised, in that C. dormoyana was retained therein, and section Luteoflora and C. pubipetala (from section Chrysantha) were added. These taxonomic controversies of Camellia species with secretory structures indicate that only traditional morphological characters are insufficient for species classification in Camellia (Sealy 1958; Chang 1981, 1998; Ming 1999, 2000). Therefore, it is necessary to seek other evidence for reexamination of the classification of Camellia species with secretory structures.
The leaf is a vegetative organ whose morphology, anatomy, and biochemistry have proven to be of great use for taxonomic treatments in higher plants. Although leaf morphology, especially leaf macromorphology, had been regarded as a common diagnostic character in Camellia taxonomy, the difficulty is still apparent in current Camellia classifications, because of changing leaf morphology of plants and the difficulty and inconsistency of their character description (Sealy 1958; Chang 1981, 1998; Ming 1999, 2000). Thus, it is necessary to introduce a quantitative method to describe leaf morphological characters to reduce the taxonomic confusion due to subjective descriptions (Meade and Parnell 2003). Multivariate analysis of leaf shape may have great potential to identify and classify Camellia species with secretory structures.
Additionally, leaves provide many anatomical characters which can be regarded as a classical source of data used in plant taxonomy (Stace 1966; Baranova 1972, 1987; Lubke and Phipps 1973; Upchurch 1984; Kong 2001; Yang and Lin 2005; Lu et al. 2008a, 2008b). Based on leaf anatomic characters, we successfully revised sections Oleifera, Tuberculata, and Thea of genus Camellia (Lin et al. 2008; Lu et al. 2008a, 2008b). However, little contribution has been made to the description of leaf anatomic characters in Camellia species with secretory structures (Ao et al. 2007).
Fourier-transform infrared spectroscopy (FTIR) is a rapid, simple, high-resolution analytical method which can provide biochemical profiles containing overlapping signals from a majority of the compounds that are present when whole cells are analyzed (Naumann et al. 1991; Mouille et al. 2003). Since plant leaves contain a wide range of different molecular components, FTIR data obtained from these structures may reveal species-specific biochemical profiles reflecting species relationships (Kim et al. 2004; Gorgulu et al. 2007). Recently, we have used this method to classify Camellia section Tuberculata (Lu et al. 2008a), section Thea (Lu et al. 2008b), and sections Oleifera, Paracamellia, Camellia, and Thea (Shen et al. 2008). Therefore, FTIR can be used to discriminate disputed Camellia species with secretory structures.
The goal of the present work is to conduct comparative leaf analysis of Camellia species with secretory structures to: (1) present integrated leaf characters including leaf shape, leaf anatomy, and leaf biochemistry, (2) assess these characters and the taxonomic significance of secretory structures, and (3) revise Camellia sections with secretory structures based on integrated leaf characters.
Materials and methods
Plant materials
Adult leaves fully exposed to sunlight of Camellia plants with secretory structures including 2 species from section Archecamellia Sealy, 2 species from section Stereocarpus (Pierre) Sealy, 11 species from section Furfuracea H.T. Chang, and 12 species from section Chrysantha H.T. Chang were collected from the International Camellia Species Garden of Jinhua City (ICSG, 29°7′N, 119°35′S, altitude 40 m) for this study. Additionally, samples of two species of genus Gordonia Ellis and one species of genus Tutcheria Dunn were included as outgroups. The examined samples had similar habitats to reduce any environmental and geographical effects on leaf anatomy and chemical composition. Vouchers were deposited at the Chemistry and Life Science College of Zhejiang Normal University (ZJNU) (Appendix 1). Leaves from three different individual plants of each species were analyzed.
Principal component analysis of leaf shape
Thirty leaf samples from three different individual plants of each species were scanned using the WinFOLIA system (Regent Instruments Inc., Canada). Average measurements of area, perimeter, width, length, aspect ratio (width/length), and leaf form coefficient were obtained. For PCA, the average values were adjusted according to the formula:
where D j is the transformed value, n is the number of species examined, and x j is the average value of the jth species (1 ≤ j ≤ n). D j is similar to Pearson’s coefficient of variation (Briggs and Walters 1984), which allows comparison of data acquired in different units. Scatterplots were produced using the STATICA statistical package (STATICA for Windows, StatSoft) using PCA coordinates based on the transformed values (D j ). We chose the “correlation (normalized var-covar)” option because the variables were measured in different units, which normalized all variables by division by their standard deviation.
Cluster analysis of leaf anatomic characters
Leaf epidermal scrapings were macerated in 40% NaClO for 10 min at 35°C. After carefully removal of mesophyll tissues, pieces of epidermis were obtained and then dehydrated in a graded alcohol series and stained with safranin and fastgreen. Finally, they were mounted in neutral balsam and examined under an Olympus BX50 light microscope (Olympus Co., Tokyo, Japan). To check the constancy of the epidermal structure, at least five slides were made from five leaves for each species, respectively. Thirty abaxial and 30 adaxial epidermal cells from five slides were randomly selected to estimate their area and perimeter by using Dn-3 Micro-image analysis software (Ningbo Yongxin Optics Co., China). The formula used for estimating the form coefficient (F c) of epidermal cells was
where F c is the form coefficient, S is the area, and P is the perimeter. Pearson correlation analysis (SAS 9.0 package) was conducted to test the relationships between F c and shape (irregular shape = 1, polygonal shape = 2) as well as the pattern [sinuous = 1, repand = 2, arched to straight (arc-str) = 3] of the anticlinal walls of the epidermal cells. It should be noted that the data of C. granthamiana and C. albogigas were not used in the Pearson correlation test, because both of them had various epidermal cells.
The samples for transection examination were cut into pieces and fixed in formalin:acetic acid:ethanol (FAA, 5 ml:5 ml:90 ml) solution. The samples were dehydrated in a graded ethanol series, embedded in paraffin, sectioned, mounted on glass slides, and stained with safranin and fastgreen. The sections were examined and photographed under an Olympus BX50 light microscope, while the thickness of palisade tissues and spongy tissues was estimated using Dn-3 Micro-image analysis software.
For the cluster analysis, 13 characters including various epidermal cells, the pattern of the anticlinal walls of adaxial and abaxial epidermal cells, form coefficient of adaxial epidermal cells, crystals, stomatal clusters, thickness of adaxial and abaxial epidermis, thickness of palisade tissues, thickness of sponge tissues, hypodermic crystals, hypodermis, and stone cells were selected (Tables 3, 4). The values representing character states were as follows: absent = 1, present = 2; sinuous = 1, repand = 2, sinuous & repand = 3, and arched to straight = 4. Additionally, for ready comparison of measurements with different units, the measurements of thickness of adaxial epidermis, abaxial epidermis, palisade tissues, and spongy tissues for cluster analysis were adjusted according to formula 1. Unweighted pair-group average (UPGMA) clustering was performed using the STATICA statistical package (STATICA for Windows, StatSoft) based on Euclidean distance using the transformed values.
Observation of secretory structures
Leaves of six species (two from section Archecamellia Sealy, two from section Furfuracea H.T. Chang, and two species from section Chrysantha H.T. Chang) were selected to study the secretory structures in the genus Camellia using semisections. Shortly after excision from the plants, samples were prefixed in a solution of 2.5% glutaraldehyde at 4°C, adjusted to pH 6.8 using 0.1 M phosphate buffer. Then, they were washed with the same buffer and postfixed in 1% osmium tetroxide. After dehydration through a gradual alcohol series from 30% to 100%, the samples were embedded in Epon812 (hard grade), and desiccated for 3 days in an oven at 60°C. Then, 1-μm semisections were obtained using a Reichert-Jung ultramicrotome (Leica, Bensheim, Germany) and stained with methylene blue-azure. Five sections for each species were observed and photographed under both an Olympus BX50 light microscope and a Leica DM LB2 fluorescent microscope (Hi-Tech Instruments Sdn. Bhd., Malaysia) with ultraviolet (UV)-light excitation.
Cluster analysis based on leaf FTIR data
The FTIR analysis followed a modified method described by Gorgulu et al. (2007) and Lu et al. (2008a, 2008b). Mature leaves were collected, and dried immediately in an oven at 70°C. Then, these samples were pulverized by light grinding in an agate mortar, and stored at 20°C until further use. FTIR analysis of the samples was carried out using a Nicolet FTIR spectrophotometer (Nicolet Nexus 870, American) with Omni-sampler attenuated total reflectance (ATR) accessory. The spectra, which were collected over wavenumbers ranging from 4,000 to 675 cm−1, were displayed in terms of absorbance as calculated from the reflectance–absorbance spectra using the Omnic (Thermo Nicolet Corp.) program (Nicolet version 7.0 package). The spectral resolution was 16 cm−1, and 256 scans of each sample were summed and averaged to improve the signal-to-noise ratio. The collection time for the spectrum for each sample was 60 s. Samples were run in triplicate, all of which were undertaken within a day period. The FTIR raw data were handled with five-point smoothing and 0–1 normalization using the Unscrambler procedure (9.6 package), and then subjected to multivariate analysis. Cluster analysis was performed on the dissimilarity matrix of Euclidean distances with UPGMA as the clustering algorithm using the STATICA statistical package (STATICA for Windows, StatSoft).
Results
PCA based on leaf shape data
Leaf shape data of the Camellia species studied are listed in Table 2. The results of PCA showed that the two most important components, PC1 (eigenvalue 3.72) and PC2 (eigenvalue 1.16) accounted for 72.15% and 22.59% of the total variance, respectively. Other components, whose eigenvalues were much lower than the Jolliffe cutoff value (0.60), were considered insignificant in the light of Jolliffe’s criterion (Jolliffe 1986). We therefore chose the first two components, PC1 and PC2, to display the data in the scatterplot (Fig. 1). Plants of the outgroup genera Gordonia and Tutcheria, usually possessing long-elliptic leaves, were well separated from the Camellia samples, bearing elliptic, oval, obovate leaves. It was further shown that leaf morphology was generally distinct between sections of the genus Camellia, though there was one species in section Chrysantha that clustered with the samples of section Furfuracea. It was interesting that the samples of section Archecamellia were well separated from those of section Chrysantha, though the leaf morphology of section Archecamellia was similar to that of section Chrysantha and thus were difficult to distinguish from each other by naked eye.
PCA scatterplot based on the leaf shape data. Axes represent principle components 1 (PC1) and 2 (PC2). Each number code (see Table 2) represents one species, and related section or genus is indicated by each different symbol
Characters of the leaf epidermis
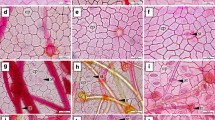
There were two types of epidermal cells in the adaxial epidermis of section Archecamellia (Fig. 2a; Table 3). One was relatively large with a repand anticlinal wall, the other small with a sinuous anticlinal wall. We defined this character as various epidermal cell (VEC). VECs only occurred in section Archecamellia (Fig. 2a; Table 3), which made it distinguishable from the other sections. In section Stereocarpus, the anticlinal walls of the adaxial epidermal cells appeared sinuous (Fig. 2b; Table 3), while in section Furfuracea, all had arched to straight ones (Fig. 2c; Table 3). In section Chrysantha, four species shared sinuous anticlinal walls in the adaxial epidermal cells (Fig. 2d; Table 3), and another nine species bore repand ones (Fig. 2e; Table 3). Furthermore, the species examined in the genus Gordonia and Tutcheria, all had arched to straight anticlinal walls in the adaxial epidermal cells (Fig. 2f, g). It was noticeable that all species examined in section Chrysantha had crystals in the adaxial epidermal cells (Fig. 2d, e), which had rarely been reported in the genus Camellia. In contrast, other sections lacked crystals in the adaxial epidermal cells.
The characters of adaxial epidermis in examined species. a C. granthamiana illustrating the general characters of section Archecamellia: having irregular shape and two distinct types of anticlinal walls, sinuous (arrowhead S) and repand (arrowhead R). b C. liberistyla illustrating the general characters of section Stereocarpus: irregular shape and sinuous anticlinal walls. c C. pubifurfuracea illustrating the general characters of section Furfuracea: polygonal shape and arc-str anticlinal walls. d C. achrysantha illustrating the general characters of section Chrysantha: irregular shape and sinuous anticlinal walls. Arrowheads: C, crystals. e C. parvipetala illustrating the general characters of section Chrysantha: irregular shape and repand anticlinal walls. Arrowheads: C, crystals. f G. acuminate illustrating the general characters of genus Gordonia: polygonal shape and arc-str anticlinal walls. g T. hexalocularia: polygonal shape and arc-str anticlinal walls. Scale bar 50 μm
The abaxial epidermal cells were similar among the sections in genus Camellia, which all had repand anticlinal walls and irregular shapes (Fig. 3a–d; Table 3). However, those of the species examined in genera Gordonia and Tutcheria had arched to straight anticlinal walls and polygonal shape (Fig. 3e, f; Table 3). Furthermore, hairs distributed on the leaf abaxial surface were only seen in C. pubifurfuracea (Fig. 3c), which made it distinct from the other species within section Furfuracea.
The characters of abaxial epidermis in examined species. a–d the abaxial epidermal characters of the Camellia species with secretory structures: repand anticlinal walls and irregular shape of epidermal cells. a C. granthamiana. b C. liberistyla showing secretory structures (SS). c C. pubifurfuracea showing hairs (H). d C. parvipetala showing stomatal cluster (SC). e, f G. acuminate and T. hexalocularia, respectively: polygonal shape and arc-str anticlinal walls. Scale bar 50 μm
All species studied here were hypostomatic, and the stomata were all anisocytic, the guard cells surrounded by three subsidiary cells (Figs. 3a–f, 4a–x, 5a–x, 6a–f). Stomatal clusters, with more than one stoma adjacently arranged with common subsidiary cells, commonly occurred in all sections of genus Camellia (Figs. 3d, 4a–x, 5a–x, 6a–f), while they were rarely seen in Gordonia (Fig. 3e) and Tutcheria (Fig. 3f).
Characteristics of epidermal cells: adaxial epidermis on the left, abaxial epidermis on the right. a, b C. granthamiana. c, d C. albogigas. e, f C. liberistyla. g, h C. yunnanensis. i, j C. pubifurfuracea. k, l C. latipetiolata. m, n C. crapnelliana. o, p C. multibracteata. q, r C. furfuracea. s, t C. oblate. u, v C. gaudichaudii. w, x C. gigantocarpa. Scale bar 100 µm
Characteristics of epidermal cells: adaxial epidermis on the left, abaxial epidermis on the right. a, b C. octopetala. c, d C. parafurfuracea. e, f C. connatistyla. g, h C. euphlebia. i, j C. limonia. k, l C. achrysantha. m, n C. impressinervis. o, p C. grandis. q, r C. parvipetala. s, t C. pingguoensis. u, v C. lunzhouensis. w, x C. huana. Scale bar 100 µm
The secretory structures (Fig. 3b) in genus Camellia shared some identical features: (1) on fresh leaves in surface view, the secretory structures appeared as brown spots; and (2) examined under LM, in surface view, they appeared as black holes, surrounded by two or three layers of small cells, which were more frequently stained red and thus easily differentiated from other epidermal cells.
Leaf characters in transverse section
Discontinuous hypodermis was present in sections Archecamellia and Furfuracea (Fig. 7a, d; Table 4) of genus Camellia, but absent in sections Stereocarpus (Fig. 7b) and Chrysanthae (Fig. 7e, f) and in genera Gordonia and Tutcheria (Fig. 7g, h). Hairs were only seen in C. pubifurfuracea (Fig. 7c).
Characters in transverse view. SS secretory structures (arrowheads). DH discontinuous hypodermis (arrowheads). a C. granthamiana illustrating the general characters of section Archecamellia. Scale bar 76 μm. b C. liberistyla illustrating the general characters of section Stereocarpus. SC, stone cells (arrowhead). Scale bar 76 μm. c C. pubifurfuracea. h hairs (arrowhead). Scale bar 152 μm. d C. oblate illustrating the general characters of section Furfuracea. Scale bar 76 μm. e, f C. liberofilamenta in section Chrysantha. C, crystals. e Scale bar 76 μm. f Scale bar 19 μm. g G. acuminate. Scale bar 152 μm. h T. hexalocularia. Scale bar 76 μm
All species examined had bifacial leaves. The number of layers of palisade cells varied from species to species (Table 4), and, more frequently, it was not easy to distinguish between palisade tissue and spongy tissue because of transition layers.
Stone cells were commonly present in all examined species of genus Camellia (Figs. 7b, 8a–aa), but few were seen in genera Gordonia and Tutcheria. Stone cells frequently occurred in palisade tissue, and some of them penetrated into spongy tissue.
Leaf transverse sections of the examined species. a C. granthamiana. b C. albogigas. c C. liberistyla. d C. yunnanensis. e C. pubifurfuracea. f C. latipetiolata. g C. crapnelliana. h C. multibracteata. i C. furfuracea. j C. oblate. k C. gaudichaudii. l C. gigantocarpa. m C. octopetala. n C. parafurfuracea. o C. connatistyla. p C. euphlebia. q C. limonia. r C. achrysantha. s C. impressinervis. t C. grandis. u C. parvipetala. v C. pingguoensis. w C. lunzhouensis. x C. huana. y C. liberofilamenta. z C. fusuiensis. aa C. multipetala. ab G. acuminata. ac G. longicarpa. ad T. hexalocularia. Scale bar 50 µm
All species examined had crystals in the mesophyll tissues, and only plants of section Chrysantha had crystals in the adaxial epidermal cells (Fig. 7f). Some relatively large crystals were close under the adaxial epidermal cells, which differed from those embedded in the mesophyll tissues and were thus named hypodermic crystals (HC); they were also present only in section Chrysantha (Fig. 7e). These hypodermic crystals (HC) were confirmed in our semisections (Fig. 9e).
Comparative semisection characters under light microscope (left) and fluorescent microscope (right) with UV-light excitation, showing that all secretory structures have similar autofluorescence to stone cells. SS, secretory structures (arrowheads). a, b C. albogigas illustrating the general characters of section Archecamellia. C, crystals (arrowhead). SC, stone cells (arrowhead). c, d C. latipetiolata illustrating the general characters of section Furfuracea. e, f C. huana illustrating the general characters of section Chrysantha. HC, hypodermic crystals. Scale bar 25 μm
In paraffin sections, few diagnostic characters in secretory structures could be identified among the species. All secretory structures usually consisted of several layers of small, compressed cells (Figs. 7a, b, d, e, 8a–aa).
Semisection examinations and fluorescent investigations of secretory structures
The semisections, which had been stained by methylene blue-azure, illustrated that there were few variances in the secretory structures among species. All the secretory structures consisted of loose, suberized tissues, surrounded by several layers of small cells. They were ellipsoidal, somewhat compressed in shape in the species of sections Archecamellia and Furfuracea (Figs. 9a, c), whereas spherical in shape in section Chrysantha (Fig. 9e). When examined under UV light, all emitted blue autofluorescence from the cell walls of the surrounded cells. This autofluorescence was very similar to that from stones cells (Figs. 9b, d, f).
Cluster analysis using data from epidermis and transverse sections
The dendrogram showed five homogeneous groups, conforming to sections Archecamellia, Stereocarpus, Furfuracea, and Chrysanthae and the outgroup genera sampled (Fig. 10). Additionally, the circumscriptions were indicated not only among the sections but also the genera, which was consistent with results of PCA based on leaf morphological data (Fig. 1).
Phenogram obtained from UPGMA cluster analysis using the data of leaf anatomy. Each number code (see Table 2) represents a species, and related section or genus is indicated by each different symbol
Subtraction and cluster analysis based on leaf FTIR data
Comparisons of leaf FTIR spectra indicated that most differences were specific to the carbohydrate fingerprinting region (wavenumbers 1,800–700 cm−1) (Kim et al. 2004). Figure 11a, b illustrates the variances of chemical compositions of the examined species in the four disputed sections. Most of the spectra had some characteristic absorbances that were constant within the same section or genus, but distinct among sections or genera (Fig. 11c). There were significant biomarkers in the spectra at wavenumbers of 1,733, 1,651, 1,601, 1,555, 1,446, 1,415, 1,320, 1,271, 1,235, 1,145, 1,046, and 895 cm−1 (Fig. 11c). The different spectra generated by digital subtraction of the spectra of the disputed sections showed that section C1 (Archecamellia), C2 (Stereocarpus), C3 (Furfuracea), and C4 (Chrysanthae) had marked differences (Fig. 11d). Differences at wavenumbers of 1,733, 1,651, 1,555, 1,446, 1,320, 1,235, 1,046, and 895 cm−1 were found in the digital subtraction of the spectra of C1 (Archecamellia) from the spectra of C4 (Chrysanthae). Accordingly, digital subtractions of the spectra of C3 from C1, C3 from C2, and C2 from C1 also showed differences at wavenumbers of 1,733, 1,651, 1,601, 1,446, 1,320, 1,235, and 1,046 cm−1, of 1,733, 1,651, 1,601, 1,446, 1,415, 1,320, 1,271, 1,235, 1,145, 1,046, and 895 cm−1, and of 1,733, 1,651, 1,446, 1,320, 1,271, 1,046, and 895 cm−1, respectively (Fig. 11d). Furthermore, the clustering dendrogram (Fig. 12), resulting from the FTIR data by 0–1 normalization and five-point smoothing, illustrated the detailed relationships of the taxa examined. As shown in Fig. 12, generally, both genera and sections which were closely related grouped together, except for C. parvipetala, which clustered with section Furfuracea.
Leaf comparative FTIR spectra in the fingerprinting region (1,800–700 cm−1). Each number code (see Table 2) represents a species. a Comparative FTIR spectra of sections Archecamellia and Chrysantha. b Comparative FTIR spectra of sections Archecamellia, Stereocarpus, and Furfuracea. c Average FTIR spectra of section Archecamellia (C1), section Stereocarpus (C2), section Furfuracea (C3), section Chrysantha (C4), genus Gordonia (G), and genus Tutcheria (T). Arrows show different biomarkers in four sections and two outgroups. d Digital subtraction of the spectra of four disputed sections. Arrows indicate different biomarkers in four sections: Archecamellia (C1), Stereocarpus (C2), Furfuracea (C3), and Chrysantha (C4)
Phenogram obtained from UPGMA cluster analysis using the data of leaf FTIR. Each number code (see Table 2) represents a species, and related section or genus is indicated by each different symbol
Discussion
Taxonomic significance of integrated leaf characters in Camellia species with secretory structures
Taxonomic significance of the secretory structures in Camellia plants
Secretory structures have frequently been reported in species of Camellia, but the descriptions of these secretory structures varied and were even conflicting because of few anatomical studies (Keng 1962; Parks and Griffiths 1963; Chang 1996, 1998; Ye 1993; Ming 2000). Originally, Keng (1962) and Parks and Griffiths (1963) identified them as cork warts. Later, Chang (1996, 1998) described them as secretory structures, while Ye (1993) considered them as black glands, whereas Ming (2000) termed them brown glands. Furthermore, although the presence of secretory structures in Camellia species could serve as a diagnostic characteristic, it also brought controversy in the taxonomy of the genus Camellia. Chang (1981) merged C. liberistyla H.T. Chang and C. yunnanensis H.T. Chang into section Stereocarpus because of the presence of secretory structures in both species, while Ming (2000) transferred them from section Stereocarpus into section Heterogenea because the secretory structures were overlooked when observed by naked eye. Ming (2000) also merged section Chrysantha H.T. Chang (1981, 1998) into section Archecamellia Sealy based on comprehensive similarity in morphology, including presence of brown glands, in these two sections. Since it was very difficult to differentiate between the similar colors of the structures by naked eye (for example, dark, black, and brown), a comprehensive anatomical study of the secretory structures was conducted in the present study. Leaf anatomic characters showed that these structures were surrounded by several layers of relatively smaller cells, with ellipsoidal, and somewhat compressed shape in sections Archecamellia and Furfuracea (Fig. 9a, c), but spherical shape in section Chrysantha (Fig. 9e). Furthermore, when cross-sections of secretory structures were examined under fluorescent light, the cell walls had the same autofluorescence as stone cells (Fig. 9). These results indicated that the surrounding cells were different in shape among sections, but all may have thickened lignified walls, which supports well their identification as cork warts by Keng (1962) and Parks and Griffiths (1963).
Leaf shape analysis for classification
Analysis of leaf shape data using multivariate techniques such as PCA (Sneath and Sokal 1973) has been successfully employed for investigation of taxonomic relationships in the past (Premoli 1996; Hodálová and Marhold 1998; Parnell and Needham 1998; Meade and Parnell 2003; Plotze et al. 2005). In the present work, quantitative leaf characters were analyzed by PCA. Our results showed that leaf F c and other quantitative characters could well separate the disputed sections in Camellia with secretory structures. Figure 1 shows that G. acuminate, G. longicarpa, and T. hexalocularia were significantly separated from other species, indicating the difference between the outgroups (genus Gordonia, genus Tutcheria) and the Camellia species with secretory structures. Furthermore, most Chrysantha and Furfuracea species were also separated from one another. Two species from section Archecamellia and two species from section Stereocarpus also formed a cluster in the PCA, respectively. These statistical analyses of leaf shape data thus revealed their potential as powerful tools for taxonomic differentiation in Camellia species with secretory structures.
Leaf anatomic characters for classification
The study reported on herein was carried out to evaluate whether leaf anatomy could be used to provide additional data of taxonomic significance similar to our studies in other Camellia species (Lin et al. 2008; Lu et al. 2008a, 2008b). Leaf epidermal characters have proven to be of great use in identifying species, e.g., in Magnoliaceae (Baranova 1972), Sterculia (Sterculiaceae) (Hussin and Sani 1998), Schisandra (Schisandraceae) (Yang and Lin 2005), and in Camellia sections Oleifera (Lin et al. 2008), Tuberculata (Lu et al. 2008a), and Thea (Lu et al. 2008b). Various epidermal cells and discontinuous hypodermis in the lamina were present in section Archecamellia and absent in section Chrysantha. Hypodermic crystals were only present in section Chrysantha. These characters thus partly support the treatments of Camellia species with secretory structures by Chang (1981, 1998) that section Chrysantha should not be merged into section Archecamellia.
Sinuous anticlinal walls were found in adaxial epidermal cells in section Stereocarpus and arched to straight ones in Furfuracea species, which separated section Stereocarpus from section Furfuracea. This disagrees with Ming’s merging of two Stereocarpus species with Furfuracea species as section Heterogenea. Although qualitative anatomic characters selected in the present work, which successfully classified the disputed sections in Camellia species with secretory structures, could not identify all species, multivariate analysis based on all quantitative and qualitative leaf characters (Tables 3, 4) clearly indicated that the species were different (Fig. 10). Figure 10 shows that four sections of Camellia species with secretory structures were significantly distant from the two outgroups, and these four sections also formed homogeneous groups, indicating their high homogeneity. These results support Chang’s classification (1981, 1996) in which he recognized four sections.
Form coefficient of leaf epidermal cells and its taxonomic value
The shapes of leaf epidermal cells are usually constant within the same species, genus, or higher taxa, and thus are frequently used in taxonomy and classification (Kong 2001; Yang and Lin 2005). Various shapes of epidermal cells can be distinguished in different taxa, for instance, elongated ones for many monocotyledons. However, use of this character in studying relationships between species or genera is still limited, because the shapes of epidermal cells are extremely diverse and thus very difficult to describe. Additionally, much confusion occurs because different taxonomists more often use different terminologies to describe even the same type (Kong 2001). Thus, it is desirable to develop a mathematical method to estimate the various shapes of epidermal cells reliably. In the present study, we propose a new nomenclature, i.e., the form coefficient (F c), to assess the shapes of epidermal cells. Usually, the F c of polygonal cells is larger than that of irregular ones (Table 3). More often, the form coefficient is closely related to the pattern of anticlinal walls: cells with arched to straight walls > cells with repand walls > cells with sinuous walls (Table 3). It is possible to produce some interesting arguments because, to some extent, this value is also related to the ratio of width to length. We overlooked this parameter, because most epidermal cell shapes are irregular and thus an accurate ratio of width to length is very difficult to obtain. Despite this limitation, the form coefficient is still useful to estimate the shapes of epidermal cells, because the Pearson correlation test indicates that there is a significant correlation between F c and epidermal cell shape (r = 0.6517, p < 0.001) and an excellent correlation between F c and the pattern of anticlinal walls of epidermal cells (r = 0.8203, p < 0.001).
Leaf FTIR analysis for classification
Kim et al. (2004) reported that FTIR was an excellent method for determining phylogenetic relationships between flowering plants based on its ease of use and quick results. Gorgulu et al. (2007) used FTIR to characterize and differentiate species in the genera Acantholimon, Ranunculus, and Astragalus and suggested that FTIR could be introduced as a novel method for discrimination of plants based on their chemical structural differences at the level of genus, and even at the level of species living in different environmental conditions. Furthermore, FTIR had been successfully applied to solve the controversy regarding sections Tuberculata (Lu et al. 2008a), Thea (Lu et al. 2008b), Oleifera, Paracamellia, Camellia, and Thea (Shen et al. 2008) in genus Camellia. It was suggested that FTIR analysis combined with comparative leaf anatomical studies could be regarded as a reliable method for taxonomic treatments. In the present study, subtraction of the spectra among the disputed Camellia sections qualitatively illustrated their marked differences (Fig. 11c, d). Cluster analysis of leaf FTIR data quantitatively visualized the degree of affinity among the examined species (Fig. 12). These results were consistent with the leaf shape analysis and cluster analysis of leaf anatomic characters, in support of Chang’s (1981, 1998) classification of Camellia species with secretory structures. This also indicated the usefulness of FTIR for the taxonomic treatment of flowering plants.
Evidence from leaves for the classification of Camellia species with secretory structures
Leaf morphology has always played an important role in traditional plant taxonomy, particularly for identifying taxa in which variation in floral structures is uninformative or in which flowering specimens are infrequent owing to, for example, a limited flowering season (Stace 1989) or dioecy (Möller et al. 2007). However, it is not always possible to characterize plants by leaf morphology, because of various leaf morphologies under different environmental conditions and/or the disputed description of the same character. Therefore, recently, increasing attention has been paid to how to use other leaf characters to discriminate disputed species (Jensen et al. 2002; Meade and Parnell 2003; Neto et al. 2006; Gorgulu et al. 2007). In the present work, integrated leaf characters including leaf shape analyses, leaf anatomical investigations, and cluster analysis of leaf FTIR data succeeded in differentiating disputed species. Therefore, we propose a potential treatment in which integrated leaf characters could be applied in the future as a sensitive and reliable method to distinguish different taxa where only leaves or a small portion of leaves are available rather than the whole plant.
Taxonomic treatment of Camellia species with secretory structures
Taxonomic position of the section Chrysantha H.T. Chang
After section Chrysantha H.T. Chang was published by Chang in 1979, its taxonomic status remained controversial for many years (Ming and Zhang 1993, 1996; Ye 1993; Chang 1981, 1996, 1998; Chang and Bartholomew 1984). According to Chang (1998), most plants of this section are only distributed in a limited area of Guangxi Province in China, and just four of them occur in the north of Vietnam, adjacent to Guangxi Province. The species of this section are characterized by axillary, yellow flowers, distinct, persistent bracts and sepals, and 3–5 cleft styles. Yellow flowers are regarded as a key character of this section, distinguishing it from other Camellia sections (Ye 1993; Chang 1981, 1998). Ming and Zhang (1993, 1996) and Ming (2000) argued that, with the exception of yellow flowers, most features are similar to plants of section Archecamellia Sealy. Thus, he disagreed with Chang on the setup of section Chrysantha and transferred most species of section Chrysantha into section Archecamellia. However, notably, molecular evidence also did not support the merging of section Chrysantha into Sealy’s section Archecamellia (Tang et al. 2001; Tang et al. 2006). In the present study, our investigations showed that many leaf morphological and anatomical features of section Chrysantha differ from those of section Archecamellia Sealy, such as: (1) various epidermal cells and discontinuous hypodermis were present in section Archecamellia Sealy but absent in section Chrysantha; (2) crystals only occurred in adaxial epidermal cells in section Chrysantha but were absent in section Archecamellia; (3) hypodermic crystals were usually seen in the former but not seen in the latter section; and (4) the shapes of the surrounding cells of the secretory structures were ellipsoidal, somewhat compressed in section Archecamellia, while they were spherical in section Chrysantha. Particularly, to date, few publications have described crystals occurring in adaxial epidermal cells in flowering plants, except in Camellia section Chrysantha in the present work (Figs. 2d, e, 7f). Moreover, our multivariate analysis using anatomic and FTIR data also successfully separated section Chrysantha from section Archecamellia (Figs. 10, 12). Therefore, we suggest that section Chrysantha should not be merged into section Archecamellia.
Delimitation of sections Archecamellia Sealy, Stereocarpus (Pierre) Sealy, and Furfuracea H.T. Chang
Although sections Archecamellia and Stereocarpus were retained in Sealy’s (1958), Chang’s (1998), and Ming’s (2000) classifications, conflicts still persisted. The number of species recognized in these two sections varied significantly, with an estimated 7 in Archecamellia and 1 in Stereocarpus by Sealy (1958), 3 in Archecamellia and 5 in Stereocarpus by Chang (1998), and 18 in Archecamellia and 3 in Stereocarpus by Ming (1999, 2000), indicating significant problems with species affinities. More and more new species were recognized after Sealy’s work was published, thus the taxonomic treatments of Chang (1981, 1998) and Ming (1999, 2000) of the genus Camellia were more frequently referred to in the current Camellia literature.
Chang (1981) revised section Archecamellia Sealy. He transferred two species from section Archecamellia Sealy into the new section, Chrysantha H.T. Chang, treating C. nitidissima Chi as its type species, and described this section as characterized by axillary yellow flowers, distinct, persistent bracts and sepals, and 3–5 cleft styles. Three species were recognized in Chang’s Archecamellia, which retained only one species, C. peteloii, from Sealy’s Archecamellia, while another two species, C. granthamiana and C. albogigas, were newly added (Chang 1998). However, Ming disagreed with Chang’s taxonomic treatments of the section. Ming (1999, 2000), highly influenced by Sealy’s (1958) work, reinstated the merger of section Chrysantha H.T. Chang into section Archecamellia. Moreover, Ming (1999, 2000) transferred C. granthamiana and C. albogigas from section Archecamellia in Chang’s classification into section Heterogenea Sealy, which was absent from Chang’s classification (1981, 1998) and comprised section Furfuracea plus several species from subgenus Protocamellia from Chang’s classification (1981, 1998). In our study, C. granthamiana and C. albogigas were assessed based on leaf shape, anatomy, and biochemistry. Although C. peteloii was not examined in the present study due to its absence from China, results showed that these two species were significantly different from other considered Heterogenea species, such as C. liberistyla H.T. Chang, C. yunnanensis H.T. Chang, and Furfuracea species (Figs. 1, 10, 12). Thus, we did not approve of Ming’s transfer of C. granthamiana and C. albogigas from section Archecamellia to section Heterogenea.
The diagnostic characters of section Stereocarpus described by Chang (1998) and Ming (1999, 2000) are terminal solitary flowers, extremely short pedicels, five locules in the ovary, and glands (secretory structures) in the leaf. However, C. liberistyla H.T. Chang and C. yunnanensis H.T. Chang were transferred from section Stereocarpus by Chang (1981, 1998) into section Heterogenea by Ming (1999, 2000), because of their white flowers and absence of brown glands (secretory structure) in the leaves. We examined leaf anatomical characters of the two species by light microscope and found that the two species also had secretory structures (Figs. 7b, 8c, d; Table 4). Furthermore, our multivariate analysis of leaf morphology, anatomy, and FTIR data also supported the separation between the two species and other Heterogenea species (Figs. 1, 10, 12).
Chang (1981) proposed section Furfuracea based on the characters of large leaf, rough capsule, and solitary, large white flower, but Ming (2000) considered that rough capsules also occurred in other sections, and he therefore denied this section and transferred most of the species into section Heterogenea, characterized by flowers without pedicels, calyx persistent until young fruit develop, five to three locules in the ovary, and glands present or absent. In Ming’s classification (1999, 2000), section Heterogenea was recognized with 15 species, including section Furfuracea, 2 species from section Archecamellia Sealy (C. granthamiana and C. albogigas), 2 species from section Stereocarpus (C. liberistyla H.T. Chang and C. yunnanensis H.T. Chang), section Pseudocamellia, and section Protocamellia. In the present study, we employed multivariate analysis of integrated leaf characters data to assess affinities of species and section in the disputed sections. The results illustrated that section Furfuracea, section Archecamellia Sealy, and section Stereocarpus, respectively, formed homogeneous groups, indicating their high homogeneity. Therefore, we agree with Chang (1981, 1998) in considering three groups as separate sections rather than merging them into a single section Heterogenea as done by Ming (1999, 2000).
References
Ao CQ, Ye CX, Zhang HD (2007) A systematic investigation of leaf epidermis in Camellia using light microscopy. Biologia 62(2):157–162
Baranova MA (1972) Systematic anatomy of the leaf epidermis in the Moagnoliaceae and some related families. Taxon 21:447–469
Baranova MA (1987) Historical development of the present classification of morphological type of stomates. Bot Rev 53:53–79
Briggs D, Walters SM (1984) Plant variation and evolution, 2nd edn. Cambridge University Press, Cambridge
Chang HT (1981) Taxonomy of the genus Camellia. Act Sci Nat Univ Sunyats 1:1–180
Chang HT (1996) Diagnosis on the systematic development of Theaceae VII The taxonomic problem of section Glaberrima Chang of Camellia. Act Sci Nat Univ Sunyats 35:87–90
Chang HT (1998) Theaceae. In: Fl. Reip. Pop. Sin. Editorial Committee (ed) Fl. Reip. Pop. Sin., vol 49. Science Press, Beijing
Chang HT, Bartholomew B (1984) Camellias. Timber, Oregon
Gorgulu ST, Dogan M, Severcan F (2007) The characterization and differentiation of higher plants by Fourier transform infrared spectroscopy. Appl Spectrosc 61:300–308
Hodálová I, Marhold K (1998) Morphometric comparison of Senecio germanicus and S. nemorensis (Compositae) with a new species from Romania. Bot J Linn Soc 128:277–290
Hussin KH, Sani ZM (1998) Comparative leaf anatomical studies of some Sterculia L. species (Sterculiaceae). Bot J Linn Soc 127(2):159–174
Jensen RJ, Ciofani KM, Miramontes LC (2002) Lines, outlines, and landmarks: morphometric analyses of Acerrubrum, Acer saccharinum (Aceraceae) and their hybrid. Taxon 51:475–492
Jolliffe IT (1986) Principal component analysis. Springer, New York
Keng H (1962) Comparative morphological studies Theaceae. Univ Calif Publ Bot 33:269–384
Kim SW, Ban SH, Chung H, Cho S, Chung HJ, Choi PS, Yoo OJ, Liu JR (2004) Taxonomic discrimination of flowering plants by multivariate analysis of Fourier transform infrared spectroscopy data. Plant Cell Rep 23:246–250
Kong HZ (2001) Comparative morphology of leaf epidermis in the Chloranthaceae. Bot J Linn Soc 136:279–294
Lin XY, Peng QF, Lu HF, Du YQ, Tang BY (2008) Leaf anatomy of Camellia sect. Oleifera and sect. Paracamellia (Theaceae) with reference to their taxonomic significance. J Syst Evol 46(2):183–193
Lu HF, Jiang B, Shen ZG, Shen JB, Peng QF, Cheng CG (2008a) Comparative leaf anatomy, FTIR discrimination and biogeographical analysis of Camellia section Tuberculata (Theaceae) with a discussion of its taxonomic treatments. Plant Syst Evol 274:223–235
Lu HF, Shen JB, Lin XY, Fu JL (2008b) Relevance of Fourier transform infrared spectroscopy and leaf anatomy for species classification in Camellia (Theaceae). Taxon 57(4):1274–1288
Lubke RA, Phipps JB (1973) Taximetrics of Loudetia (Gramineae) based on leaf anatomy. Can J Bot 51:2127–2146
Meade C, Parnell J (2003) Multivariate analysis of leaf shape patterns in Asian species of the Uvaria group (Annonaceae). Bot J Linn Soc 143:231–242
Ming TL (1999) A systematic synopsis of the genus Camellia. Acta Bot Yunn 21:149–159
Ming TL (2000) Monograph of the Genus Camellia. Yunnan Sci Technol, Kunming
Ming TL, Zhang WJ (1993) On taxonomic problems of sect. Archecamellia Sealy and sect. Chrysantha Chang in the genus Camellia. Act Bot Yunn 15(1):1–15
Ming TL, Zhang WJ (1996) The evolution and distribution of genus Camellia. Act Bot Yunn 18(1):1–13
Möller M, Gao LM, Mill RR, Li DZ, Hollingsworth ML, Gibby M (2007) Morphometric analysis of the Taxus wallichiana complex (Taxaceae) based on herbarium material. Bot J Linn Soc 155(3):307–335
Mouille G, Robin S, Lecomte M, Pagant S, Höfte H (2003) Classification and identification of Arabidopsis cell wall mutants using Fourier-transform infrared (FT-IR) microspectroscopy. Plant J 35:393–404
Naumann D, Helm D, Labischinski H (1991) Microbiological characterizations by FT-IR spectroscopy. Nature 351:81–82
Neto JC, Meyer GE, Jones DD, Samal AK (2006) Plant species identification using elliptic Fourier leaf shape analysis. Comput Electron Agric 50:121–134
Parks CR, Griffiths A (1963) The saluenensis-pitardit-reticulata complex. Camellia Rev 25(2):12–29
Parnell J, Needham M (1998) Morphometric variation in Irish Sorbus L. (Rosaceae). Watsonia 22:153–161
Plotze RO, Falvo M, Pádua JG, Berbacci LC, Vieira MLC, Oliveira GCX, Bruno OM (2005) Leaf shape analysis using the multiscale Minkowski fractal dimension, a new morphometric method: a study with Passiflora (Passifloraceae). Can J Bot 83:287–301
Premoli AC (1996) Leaf architecture of south American Nothofagus (Nothofagaceae) using traditional and new methods in morphometrics. Bot J Linn Soc 121:25–40
Sealy JR (1958) A revision of the genus Camellia. The Royal Horticultural Society, London
Shen JB, Lu HF, Peng QF, Zheng JF, Tian YM (2008) FTIR spectra of Camellia sect. Oleifera, sect. Paracamellia, and sect. Camellia (Theaceae) with reference to their taxonomic significance. J Syst Evol 46(2):194–204
Sneath PHA, Sokal RR (1973) Numerical Taxonomy. Freeman, San Francisco
Stace CA (1966) The use of epidermal characters in phylogenetic considerations. New Phytol 65:304–318
Stace CA (1989) Plant taxonomy and biosystematics, 2nd edn. Cambridge University Press, Cambridge
Tang SQ, Zhong Y, Su SH, Chang HT (2001) Relationship between C. nitidissima Chi and C. petelotii (Merr.) Sealy based on nrDNA ITS. J Wuhan Bot Res 19(6):449–452
Tang SQ, Bin XY, Wang L, Zhong Y (2006) Genetic diversity and population structure of yellow Camellia (Camellia nitidissima) in China as revealed by RAPD and AFLP markers. Biochem Genet 44:444–456
Upchurch GR (1984) Cuticle evolution in early cretaceous angiosperms from the potomac group of Virginia and Maryland. Ann Missouri Bot Gard 71:522–550
Yang ZR, Lin Q (2005) Comparative morphology of the leaf epidermis in Schisandra (Schisandraceae). Bot J Linn Soc 148:39–56
Ye CX (1993) Annotation of phylogeny in the family Theaceae: a review of sect. Chrysantha Chang and evolution of genus Camellia. Guihaia 13(4):306–310
Acknowledgments
We thank the Institute of Physical Chemistry of Zhejiang Normal University for use of the Nicolet FTIR spectrometer. We are grateful to Prof. Cun-Gui Cheng and Ms. Dan-Ting Li for help with FTIR spectrometer operation and FTIR data analysis. This study was supported by grants from the Natural Science Foundation of Jiangsu Province, China (No. BK2006045) and Open the Foundation of the Botany Key Discipline of Changshu Institute of Technology (No. 2008003).
Author information
Authors and Affiliations
Corresponding author
Additional information
B. Jiang and Q. F. Peng contributed equally to this work.
Appendix 1
Appendix 1
Collection localities and vouchers of studied specimens
GenusCamelliaLinn. SectionArchecamelliaSealy. C. granthamiana Sealy, Jinhua International Camellia Species Garden, Zhejiang Province, China, H.B. Dai 200610190201,02,03 (ZJNU); C. albogigas Hu, Jinhua International Camellia Species Garden, Zhejiang Province, China, H.B. Dai 200610190101,02,03 (ZJNU); Section stereocarpus (Pierre) Sealy. C. liberistyla H.T. Chang, Jinhua International Camellia Species Garden, Zhejiang Province, China, Q.F. Peng 200612100301,02,03 (ZJNU); C. yunnanensis (Pitard) Coh. St., Jinhua International Camellia Species Garden, Zhejiang Province, China, Q.F. Peng 200612100401,02,03 (ZJNU); SectionFurfuraceaChang. C. pubifurfuracea Zhong, Jinhua International Camellia Species Garden, Zhejiang Province, China, H.E. Tian 200610170901,02,03 (ZJNU); C. latipetiolata Chi, Jinhua International Camellia Species Garden, Zhejiang Province, China, H.E. Tian 200610171001,02,03 (ZJNU); C. crapnelliana Tutch., Jinhua International Camellia Species Garden, Zhejiang Province, China, H.E. Tian 200610171101,02,03 (ZJNU); C. multibracteata H.T. Chang et Mo ex Mo, Jinhua International Camellia Species Garden, Zhejiang Province, China, H.E. Tian 200610171201,02,03 (ZJNU); C. furfuracea (Merr.) Coh. St., Jinhua International Camellia Species Garden, Zhejiang Province, China, H.E. Tian 200610171301,02,03 (ZJNU); C. oblata H.T. Chang, Jinhua International Camellia Species Garden, Zhejiang Province, China, Q.F. Peng 200610221401,02,03 (ZJNU); C. gaudichaudii (Gagn.) Sealy, Jinhua International Camellia Species Garden, Zhejiang Province, China, H.E. Tian 200610171501,02,03 (ZJNU); C. gigantocarpa Sealy, Jinhua International Camellia Species Garden, Zhejiang Province, China, Q.F. Peng 200610221601,02,03 (ZJNU); C. octopetala Hu, Jinhua International Camellia Species Garden, Zhejiang Province, China, Q.F. Peng 200610221701,02,03 (ZJNU); C. parafurfuracea S. Y. Liang ex H.T. Chang, Jinhua International Camellia Species Garden, Zhejiang Province, China, H.E. Tian 200610171801,02,03 (ZJNU); C. connatistyla Mo et Zhong, Jinhua International Camellia Species Garden, Zhejiang Province, China, H.E. Tian 200610171901,02,03 (ZJNU); SectionChrysanthaH.T. Chang. C. euphlebia Merr. ex Sealy, Jinhua International Camellia Species Garden, Zhejiang Province, China, H.B. Dai 2006031212301,02,03 (ZJNU); C. limonia C. F. Liang et Mo, Jinhua International Camellia Species Garden, Zhejiang Province, China, H.B. Dai 2006031219401,02,03 (ZJNU); C. achrysantha H.T. Chang et S.Y. Liang, Jinhua International Camellia Species Garden, Zhejiang Province, China, X.F. Wu 2006031612401,02,03 (ZJNU); C. impressinervis H.T. Chang et S.Y. Liang, Jinhua International Camellia Species Garden, Zhejiang Province, China, H.B. Dai 2006031212501,02,03 (ZJNU); C. grandis (Liang et Mo) H.T. Chang et S.Y. Liang, Jinhua International Camellia Species Garden, Zhejiang Province, China, X.F. Wu 2006031612601,02,03 (ZJNU); C. parvipetala J.Y. Liang et Su, Jinhua International Camellia Species Garden, Zhejiang Province, China, H.B. Dai 2006031212801,02,03 (ZJNU); C. pingguoensis Fang, Jinhua International Camellia Species Garden, Zhejiang Province, China, H.B. Dai 2006031219301,02,03 (ZJNU); C. lunzhouensis Luo, Jinhua International Camellia Species Garden, Zhejiang Province, China, X.F. Wu 2006031612901,02,03 (ZJNU); C. huana Ming et W.J. Zhang, Jinhua International Camellia Species Garden, Zhejiang Province, China, X.F. Wu 2006031613001,02,03 (ZJNU); C. liberofilamenta H.T. Chang et C.H. Yang, Jinhua International Camellia Species Garden, Zhejiang Province, China, X.F. Wu 2006031613101,02,03 (ZJNU); C. fusuiensis S.Y. Liang et X.J. Dong, Jinhua International Camellia Species Garden, Zhejiang Province, China, Q.F. Peng 2006011413601,02,03 (ZJNU); C. multipetala S.Y. Liang et C.Z. Deng, Jinhua International Camellia Species Garden, Zhejiang Province, China, Q.F. Peng 2006011413701,02,03 (ZJNU); Genus Gordonia Ellis.G. acuminata H.T. Chang, Jinhua International Camellia Species Garden, Zhejiang Province, China, Q.F. Peng 2006111219701,02,03 (ZJNU); G. longicarpa H.T. Chang, Jinhua International Camellia Species Garden, Zhejiang Province, China, Q.F. Peng 2006111219801,02,03 (ZJNU); GenusTutcheria Dunn. T. hexalocularia Hu et Liang ex H.T. Chang, Jinhua International Camellia Species Garden, Zhejiang Province, China, Q.F. Peng 2006111220101,02,03 (ZJNU).
Rights and permissions
About this article
Cite this article
Jiang, B., Peng, Q.F., Shen, Z.G. et al. Taxonomic treatments of Camellia (Theaceae) species with secretory structures based on integrated leaf characters. Plant Syst Evol 290, 1–20 (2010). https://doi.org/10.1007/s00606-010-0342-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-010-0342-x