Abstract
Two novel stationary phases, 1-(4-bromobutyl)-3-methylimidazolium bromide bonded chitosan modified silica and 1-(4-bromobutyl)-3-methylimidazolium bromide bonded chitosan derivatized calix[4]arene modified silica stationary phase, were synthesized using 1-(4-bromobutyl)-3-methylimidazolium bromide bonding chitosan as a polarity regulator solving the limitation of the strong hydrophobicity of calixarene in the application of hydrophilic field. The resulting materials were characterized by solid-state nuclear magnetic resonance, Fourier-transform infrared spectra, scanning electron microscopy, elemental analysis, and thermogravimetric analysis. Based on the hydrophilicity endowed by 1-(4-bromobutyl)-3-methylimidazolium bromide bonded chitosan, the retention mode of ILC-Sil and ILCC4-Sil could be effectively switched from the hydrophilic mode to a hydrophilic/hydrophobic mixed mode and could simultaneously provide various interactions with solutes, including hydrophilic, π-π, ion-exchange, inclusion, hydrophobic, and electrostatic interactions. On the basis of these interactions, successful separation and higher shape selectivity were achieved among compounds that vary in polarity under both reverse-phase and hydrophilic interactive liquid chromatography conditions. Moreover, the ILCC4-Sil was successfully applied to the determination of morphine in actual samples using solid-phase extraction and mass spectrometry. The LOD and LOQ were 15 pg/mL and 54 pg/mL, respectively. This work presents an exceptionally flexible adjustment strategy for the retention and selectivity of a silica stationary phase by tuning the modification group.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polar and nonpolar compounds can be well separated by normal-phase liquid chromatography (NPLC) or reversed-phase liquid chromatography (RPLC). However, with the development of mass spectrometry, the poor solubility of polar analytes in nonaqueous mobile phases limits the application of NPLC. To solve this problem, hydrophilic interaction liquid chromatography (HILIC) was first reported in 1990 by Alpert et al. [1]. Unlike NPLC, HILIC can achieve complete separation of polar compounds with a hydrophilic stationary phase and high concentrations of organic solvents, effectively solving the problems of solubility and compatibility with mass spectrometry [2,3,4,5,6]. HILIC has therefore become a good alternative with high selectivity for polar analytes in many fields, including carbohydrates [7,8,9], glycopeptides [10, 11], peptides [12, 13], amino acids [7, 14, 15], metabolites [16], small drug molecules [17, 18], and polar natural products [19, 20].
Most stationary phases only have one retention mode and can be just used to separate one type of compound, which seriously limits their wide application. Mixed-mode chromatography (MMC) [21], with multiple chromatographic ligands that provide multimodal interactions and excellent separation selectivity, has emerged as an alternative approach to conventional HPLC. Among the different types of mixed-mode stationary phases, ligands containing both hydrophobic moieties and hydrophilic moieties have been employed to create various hydrophilic/hydrophobic (RPLC/HILIC) multiple mode stationary phases (MMSP) [9, 17, 20, 22,23,24,25,26,27,28,29]. In our previous work [30,31,32], we fabricated a series of HILIC and RPLC/HILIC MMSPs by introducing hydrophilic moieties into the hydrophobic moieties of calixarenes [33, 34]. Compared with previous works, our proposed stationary phases have a variety of interaction mechanisms including hydrophobic, π-π, hydrogen bonding, π-electron transfer, and inclusion interactions due to the properties of calixarene [30,31,32, 35]. However, due to the strong hydrophobicity of calixarene, the separation ability of calixarene derivatives with polar groups is still not ideal. Further increasing the bonding amount or the polarity of the modified polar monomer can be an effective strategy to further improve the performance of the calixarene-derived stationary phase in HILIC mode.
Ionic liquids modified chitosan (ILC) is an outstanding candidate for hydrophilic moieties due to its active amino and hydroxyl groups, especially the positively charged imidazole ring endowed by ionic liquids [36,37,38,39]. Hence, we believe that ionic liquids chitosan (ILC) with rich amino, hydroxyl, and positively charged imidazole rings is an effective way to improve the compatibility of calixarene for polar substances separation [40]. Herein, we designed and synthesized two novel kinds of stationary phases: one a silica stationary phase modified with 1-(4-bromobutyl)-3-methylimidazolium bromide bonded chitosan (ILC-Sil), and the second a chitosan derivatized calix[4]arene modified with 1-(4-bromobutyl)-3-methylimidazolium bromide as a silica stationary phase (ILCC4-Sil). Based on the active amino and hydroxyl groups and the imidazole ring of ILC and the hydrophobic skeleton, multipoint recognition sites of calixarene, it is predictably that the two different stationary phases can generate flexible selectivity and application prospects for both hydrophilic and hydrophobic analytes. Nucleosides, nucleobases, monosubstituted benzenes, isomers, sulfa drugs, flavonoids, and sugars were selected as analytes with which to evaluate the chromatographic properties of the synthesized columns. In addition, the separation mechanisms were studied by examination of the effects of mobile phase constitution, buffer salt concentrations, and pH on retention.
Experimental
Chemicals and instruments
Chitosan (2 kDa) was purchased from Energy Chemical (Shanghai, China). Methanol and acetonitrile (all chromatographic grade) were obtained from Merck (Darmstadt, Germany). N-methylimidazole, p-tert-butylphenol lphenol, 1,4-dibromobutane, 1,3-dibromopropane, and glutaraldehyde were bought from Macklin (Shanghai, China). Acetone, chloroform, and other solvents in analytically pure were provided by Henan Chemical Reagents (Henan, China). HPLC-grade water was purified by using Milli-Q purification equipment. γ-Aminopropyl silica (GAPS) (with particle size of 5 μm and pore size of 66.5 Å) was purchased from Baseline (Tianjin, China). Unless otherwise specified, reagents are all analytical grades. The blank urine samples were collected from healthy volunteers, and urine samples from drug addicts were provided by the Institute of Forensic Science and Technology of Henan Provincial Public Security Bureau (men aged from 25 to 40). All experiments were conducted in accordance with the guidelines of the national related biomedical research ethics and approved by the life science ethics review committee of Zhengzhou University. All participants provided their written informed consent for the collection of biological samples. Commercially available HLB cartridges (100 mg/3 mL) were obtained from Waters (Milford, MA, USA). Standard morphine (1.0 mg/mL) was purchased from Cerilliant (Texas, USA). Chromatographic separations were conducted on an Agilent 1260 series (Santa Clara, CA, USA). The homemade ILC-Sil and ILCC4-Sil columns were packed into a stainless steel column (150 mm × 4.6 mm i.d.) using a packing machine (Kerui Tech. Co. Ltd., Dalian, China) at a pressure of 45 MPa. Two commercial chromatographic columns were used for comparative analysis with home-made chromatographic columns, including XbridgeTM HILIC(150 mm × 4.6 mm, 5 μm, Waters, Massachusetts, USA) and Se Quant® ZIC-HILIC (150 mm × 2.1 mm, 3.5 μm, Merck, Darmstadt, Germany).
Elemental analyses (EA) were performed with a Flash EA 1112 elemental analyzer. Solid-state 13C nuclear magnetic resonance (NMR) spectra were carried out with a Bruker 400 MHz spectrometer (AVIII HD 400AVIII HD 400). Scanning electron microscopy-energy dispersive X-ray (SEM-EDS) analysis was recorded with an S-4300 SEM instrument configured with an energy dispersive spectrometer system (Zeiss/Auriga FIB, Germany). Thermal gravimetric analysis (TGA) was performed with a Shimadzu DT-40 thermal analyzer, with the analysis temperature ranging from 20 to 800 °C at a heating rate of 10 °C/min under an argon atmosphere. Fourier-transform infrared (FT-IR) spectra were recorded on a Nicolet 6700 spectrometer (Thermo Fisher, USA).
Synthesis of 1-(4-bromobutyl)-3-methylimidazolium bromide bonded chitosan (ILC)
1-(4-Bromobutyl)-3-methylimidazolium bromide bonded chitosan (ILC) was synthesized according to the following procedure: 1.0 g (0.31 mmol) chitosan (2kDa), 1-(4-bromobutyl)-3-methylimidazolium bromide (ionic liquid, IL) 8.32 mmol, deionized water (2 mL), and isopropyl alcohol (15 mL) were added to a 50 mL 3-necked boiling flask. The mixture was magnetically stirred and heated to reflux under a nitrogen atmosphere for 12 h at 75 °C. The resulting opaque solution was precipitated with ethanol and filtered to obtain the solid material. Then, ionic liquids were completely extracted from the solid material by Soxhlet extraction using anhydrous ethanol, and finally, the product was vacuum dried for 24 h at 40 °C.
Synthesis of 5,11,17,23-tetra-tert-butyl-25,27-bis(3-bromo-propoxy)-26,28-dihydroxy-alix[4]arene (BrC4)
BrC4 was synthesized according to our previous work with a minor modification [41], and the detail was described in the Electronic Supplementary Material (ESM).
1-(4-bromobutyl)-3-methylimidazolium bromide bonded chitosan modified silica stationary phase (ILC-Sil)
ILC-Sil was prepared by covalently bonding ILC onto glutaraldehyde-modified γ-aminopropyl silica (GAPS) according to published methods with a minor modification [42]. The detail was described in the ESM.
Preparation of ILCC4-Sil
To obtain 3-(4-bromobutyl)-1-methyl imidazole bromide bonded chitosan derivatized calix[4]arene bonded silica stationary phase (ILCC4-Sil), 0.6 g BrC4, 3 g ILC-Sil, and 0.25 g K2CO3 were added into anhydrous acetonitrile. The mixture was stirred and refluxed for 48 h under a nitrogen atmosphere. The product was washed thoroughly with acetone, distilled water, and methylene chloride, and then dried at 60 °C under vacuum for 8 h.
Solid-phase extraction (SPE) pretreatment of urine
The sample was loaded onto the HLB SPE cartridge, which had been conditioned sequentially with 1 mL water, 1 mL of MeOH, and 2 mL water. Washing was conducted with 1 mL of water to remove the matrix and 2 mL of acetonitrile to elute the analytes. The eluant was collected and dried with a gentle stream of nitrogen at room temperature (rt), then the residue was redissolved in 100 μL acetonitrile and water (8:2, v/v). Finally, 20 μL of the extraction solution was filtered and loaded in the autosampler for HILIC-MS/MS analysis. All tests were performed in triplicate.
Determination of morphine in human urine using SPE-HILIC-MS-MS based on ILCC4-Sil column
The HILIC-MS/MS analysis was conducted on an AB Sciex QTRAP 6500 tandem mass spectrometer (Milwaukee, Wisconsin, American) in positive ion mode. The homemade HILIC column was used for chromatography separation in this work (ILCC4-Sil, 150 x 4.6 mm i.d.). The flow rate and injection volume were 0.5 ml/min and 20 μL, respectively. The ionspray voltage was maintained at 5.5 kV and the temperature at 550 °C. Ion source gas 1, curtain gas, and ion source gas 1, collision gas flows were 50, 30, 50, and medium, respectively. The declustering potential and entrance potential were set at 40 V and 10 V, respectively. The mass spectrometer was operated under multiple reaction monitoring (MRM) mode with a collision energy of 33.7 V. The dwell time was 150 ms. The transitions (precursor to product) monitored were 286, 201, and 152 m/z for morphine.
Recovery study
A recovery investigation was performed to validate the accuracy and precision of the results from the developed SPE-HILIC-MS/MS method. Spiked samples at five concentration levels (1 ng/mL, 10 ng/mL, 50 ng/mL, 500 ng/mL, and 5 μg/mL) were selected as test standards. All tests were performed in triplicate.
Results and discussion
Preparation and characterization of synthesized ILC-Sil and ILCC4-Sil
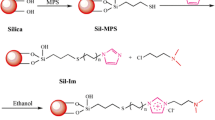
As shown in Fig.1, ILC-Sil and ILCC4-Sil were prepared using a chemical bonding strategy, with ILC and calix[4]arene as hydrophilic moieties and hydrophobic moieties, respectively. First, ILC was successfully grafted to the surface of aminopropyl silica gel through a Schiff base reaction using glutaraldehyde as the linking molecule. Then, BrC4 was grafted onto the surface of ILC-Sil through a Williamson synthesis. The obtained ILC-Sil and ILCC4-Sil were characterized and packed into the column for the following experiments.
The morphology and elementary composition of the synthesized microspheres were confirmed by SEM-EDS, as shown in Fig. 2. From the SEM image of ILC-Sil and ILCC4-Sil, one can see that both ILC-Sil and ILCC4-Sil possess mono-dispersed spherical shaped particles whose average size is ~5 μm. The EDS spectrum confirmed the existence and distribution of C, O, Si, N, and Br, which was consistent with the fabrication of ILC and ILCC4 onto silica cores.
FT-IR spectra were used to characterize the microspheres of CHO-NH2-Sil, ILC-Sil, and ILCC4-Sil to further verify the cladding of ILC and ILCC4, as shown in Fig. 3a. In comparison to pristine CHO-NH2-Sil, the FT-IR spectra of ILC-Sil and ILCC4-Sil show characteristic bands at 3400 cm−1 corresponding to -OH and -NH groups. The peaks at 1450 cm−1, 1500 cm−1, and 1600 cm−1 in the ILC-Sil spectrum indicate the presence of an imidazole ring. The intensity increase in the ILCC4-Sil spectrum of the peaks at 1450 cm−1, 1500 cm−1, and 1600 cm−1 can be attributed to the introduction of aromatic units, which indicates that the calix[4]arene was successfully grafted on ILC-Sil.
The structures of ILC-Sil and ILCC4-Sil conjugation were further characterized by their solid-state 13C NMR spectra, and a typical 13C NMR spectrum of ILC and ILCC4 is shown in Fig. 3b. The peaks at δ = 15 ppm and 20 ppm are attributed to the methyl and methylene groups; the peak at 60 ppm is produced by the shift of carbon, which may be affected by the parent oxygen, bromine, and nitrogen; the peaks at 30 ppm come from the quaternary carbon and bridged methylene; the imidazole ring or aromatic carbons give the peak at 128 ppm, and the increase of the peak intensity in ILCC4-Sil is attributed to the introduction of calix[4]arene. These results conform well with the structure of ILC-Sil and ILCC4-Sil.
The thermal stability of ILC-Sil and ILCC4-Sil was investigated by TGA (Fig. 3c). The results showed that the weight loss for ILC-Sil and ILCC4-Sil in the range of 44.7–200 °C was negligible, which indicates that both ILC-Sil and ILCC4-Sil possess high thermal. Obvious weight loss occured in the range of 200–800 °C and can be attributed to the loss of organic moieties, which further proved that ILC and calix[4]arene had been successfully grafted on the silica particles.
The quantitative composition of the prepared materials was confirmed by elemental analysis. As shown in Fig. 3d, the carbon and hydrogen content of NH2-Sil, CHO-NH2-Sil, ILC-Sil, and ILCC4-Sil increase in turn, confirming that ILC and calix[4]arene were successfully covalently bonded onto the surface of NH2-Sil. The bonded amounts of ILC-Sil and ILCC4-Sil were 390.48 and 104.43 μmol g−1, which were calculated based on the change of the carbon content.
Chromatographic retention properties
The overall chromatographic characteristics of four columns including NH2-Sil, CHO-NH2-Sil, ILC-Sil, and ILCC4-Sil were evaluated using five nucleosides. Firstly, a comparison among NH2-Sil, CHO-NH2-Sil, ILC-Sil, and ILCC4-Sil was conducted to verify the separation selectivity of ILC-Sil and ILCC4-Sil endowed by ILC and calix[4]arene. Fig. 4a shows higher selection factors of ILC-Sil and ILCC4-Sil than that of NH2-Sil and CHO-NH2-Sil. Especially, the shorter retention of five nucleosides demonstrated that the hydrophilic retention capacity of ILCC4-Sil is weaker than ILC-Sil, which may be due to the activity of calix[4]arene.
a Separation of 5 nucleosides on NH2-Sil, CHO-NH2-Sil, ILC-Sil, and ILCC4-Sil in HILIC mode. b Separation of 5 nucleosides on ILC-Sil, ILCC4-Sil, ZIC, and XBridgeTM in HILIC mode. Peaks: 1. 5-methyluridine; 2. uridine; 3. adenosine; 4. 2-aminoadenosine; 5. cytidine. c Effect of water content in the mobile phase for the k of 5 nucleosides on ILC-Sil. d Effect of water content in the mobile phase for the k of 5 nucleosides on ILCC4-Sil. Other chromatographic conditions as shown in ESM
Additionally, two commercial chromatographic columns ZIC and XBbridgeTM were used to further describe the chromatographic performance of ILC-Sil and ILCC4-Sil. As shown in Fig. 4b, the elution order of five nucleosides on ILC-Sil and ILCC4-Sil is consistent with that on ZIC and XbridgeTM in HILIC mode, and the retention factors of tested compounds demonstrated a decreased trend when the volume fraction of water increased (Fig. 4c, d). All these results indicated that the two columns had a typical HILIC retention characteristic. It is worth noting that both ILC-Sil and ILCC4-Sil show obvious separation advantages over XbridgeTM in the separation of 5-methyluridine and uridine, which is probably due to the CH-π interaction between the methyl group and the conjugated system in ILC-Sil and ILCC4-Sil (Table S1). While compared with the ZIC column, tailings were observed in the chromatographic peaks of the five nucleosides on ILC-Sil and ILCC4-Sil, which may be ascribed to the formation of strong hydrogen bonds between the bases and hydroxyl groups in the nucleosides and the amino groups and hydroxyl groups in ILC.
Chromatographic performance in HILIC mode
Four sulfanilamides, 3 flavones, and 5 sugars were selected as probes to further evaluate the hydrophilic performance of ILC on the ILC-Sil column. In Fig. 5a, the separation is shown of four sulfanilamides on NH2-Sil, CHO-NH2-Sil, ZIC, XbridgeTM, C18, and ILC-Sil columns. Baseline separation was achieved on ILC-Sil with better resolution and symmetrical peak shape, indicating the higher selectivity of the ILC-Sil column than NH2-Sil, CHO-NH2-Sil, ZIC, XbridgeTM, and C18 columns. The probable reason for this is that the rich functional groups existed in the ionic liquids bonded chitosan endowed ILC-Sil with abundant interaction forces, such as strong hydrogen-bonding interaction between the N-heterocycle, or the amino groups in sulfanilamides and the imino or hydroxyl groups in ILC-Sil, and the π-π interaction between the N-heterocycle and the imidazole ring. Simultaneously, we found that the retention time of sulfamethazine, sulfamerazine, and sulfadiazine increased with the increase of their polarity, which conforms to the retention characteristics of hydrophilic chromatography separation. Therefore, the retention profile of sulfanilamides can be explained by a synergistic effect induced by hydrophilic, π–π, and hydrogen-bonding interaction.
a The separation of 3 flavonoids on NH2-Sil, CHO-NH2-Sil, ILC-Sil, ZIC, and XBridgeTM in HILIC mode. Peaks: 1. tangeretin; 2. icariin; 3. genistein. b The separation of 4 sulfonamides on NH2-Sil, CHO-NH2-Sil, ILC-Sil, C18, ZIC, and XBridgeTM in HILIC mode. Peaks: 1 sulfamethazine; 2. sulfamerazine; 3. sulfadiazine; 4. sulfamethoxazole. c The separation of 5 sugars on NH2-Sil, CHO-NH2-Sil, ILC-Sil, ZIC, and XBridgeTM in HILIC mode. Peaks: 1. L-rhamnose; 2. D-xylose; 3. D-fructose; 4. D-glucose; 5. D-maltose. Other chromatographic conditions as shown in ESM
Other different types of hydroxyl-rich compounds such as the flavones and sugars were also chosen to evaluate the separation performance of the developed column (Fig. 5b, c and Table S2). Compared with the separation capability of the CHO-NH2-Sil, ZIC, and XbridgeTM columns, ILC-Sil and NH2-Sil columns display enhanced separation of the analytes with a better resolution and a different elution order. Hesperetin and Icaritin coelute on the ZIC column but are well resolved on NH2-Sil and ILC-Sil columns. Especially, the ILC-Sil column shows an excellent ability to separate three flavones and five sugars with better resolution and symmetric peak shape in a shorter time than the NH2-Sil column. Interestingly, we also found that the retention capacity of flavonoids and carbohydrates gradually increased with the increase in the number of hydroxyl groups. These results suggest that hydrogen bonding interactions are involved and enhance both the separation selectivity on the ILC-Sil column and the hydrophilic interaction.
Chromatographic performance in RPLC mode
According to the literature [43,44,45,46], the Tanaka test was also conducted to evaluate the hydrophobicity, hydrogen bonding, and stereoselectivity of ILCC4-Sil in RPLC mode. Table 1 shows the Tanaka test results of C18 and ILCC4-Sil. Both KPB value and ɑCH2 value of the ILCC4-Sil column being lower than that of C18 can be attributed to the lower carbon load of ILCC4-Sil. The ɑTR/O value of the ILCC4-Sil column is significantly higher than that of C18, indicating that there may exist a stronger spatial configuration on ILCC4-Sil. The ɑC/P value of the ILCC4-Sil column is also obviously higher than that of C18, which demonstrates the existence of stronger hydrogen bonding interaction in ILCC4-Sil.
To further verify the excellent regulation property of hydrophobic calix[4]arene, the RPLC mode separation capacity of ILCC4-Sil was investigated using six monosubstituted benzenes, four sulfonamides, and three nitrophenol isomers as molecular probes (Table S2–3). Compared with NH2-Sil columns and CHO-NH2-Sil, the better resolution and symmetric peak of all the probes demonstrated the excellent separation capacity of ILCC4-Sil derived from the functional groups. Additionally, the elution sequence of the six monosubstituted benzenes on ILCC4-Sil and C18 is the same, and their retention factors decrease with the increase in the organic proportion (Fig. 6a), which implies that the separation mode on ILCC4-Sil features a typical reversed-phase mechanism. More interestingly, when the four sulfonamides are separated (Fig. 6b), their retention order on ILCC4-Sil is the same as that on ILC-Sil, but is basically the opposite of what is observed on C18. However, its retention factor decreased with the increase in the proportion of the organic phase. Therefore, we infer that the separation of sulfonamides is not only dominated by hydrophobic or hydrophilic mode, but the results from the RPLC/HILIC mixed mode. In addition, as shown in Fig. 6b and Table S4, compared with ILC-Sil, C18, and published works, ILCC4-Sil can achieve higher separation efficiency for sulfonamides. This can be attributed to the spatial recognition ability and large conjugate system of calix[4]arene, which endowed ILCC4-Sil with higher separation selectivity. To further prove our hypothesis, three isomers of nitrophenol were separated on NH2-Sil, CHO-NH2-Sil, C18, and ILCC4-Sil. As shown in Fig. 6c, three nitrophenol isomers were well separated with a symmetric peak within the shortest time, which may be due to the combined effect of hydrophilicity of ILC and steric selectivity of calix[4]arene. Besides, the effect of acetonitrile content in the mobile phase on retention was studied. As shown in Fig. 6d, the retention time decreased as the acetonitrile content increased from 60 to 80% and increased when the acetonitrile content increased further from 80 to 90%, demonstrating a combination of RPLC and HILIC retention mechanisms with ILCC4-Sil.
a The separation of 6 monosubstituted benzenes on NH2-Sil, CHO-NH2-Sil, ILCC4-Sil, and C18 in RPLC mode. Peaks: 1. aniline; 2. phenol; 3. anisole; 4. nitrobenzene; 5. chlorobenzene; 6. iodobenzene. b The separation of 4 sulfonamides on NH2-Sil, CHO-NH2-Sil, ILC-Sil, ILCC4-Sil, and C18 in RPLC mode. Peaks: 1. sulfamethazine; 2. sulfamerazine; 3. sulfadiazine; 4. sulfamethoxazole. c The separation of 3 nitrophenols on NH2-Sil, CHO-NH2-Sil, ILCC4-Sil, and C18 in RPLC mode; Peaks: 1. m-nitrophenol; 2. o-nitrophenol; 3. p-nitrophenol. d Separation chromatogram of nitrophenol isomers at different acetonitrile contents. Other chromatographic conditions as shown in ESM
Separation mechanism of ILCC4-Sil
The exploration of the separation mechanism is an important part of the evaluation of new chromatographic columns. Firstly, the separation mechanism of ILCC4-Sil was evaluated using partitioning and adsorption interaction, which can be described in Eqs. (1) and (2), respectively. Firstly, six sulfanilamides were selected as probes and separated in HILIC mode. As shown in Fig. 7a, most curves do not have good linearities between the CB of 15% and 20%, such as the curves of sulfisomidin and sulfadimethoxypyrimidine. This phenomenon illustrated that in addition to the partitioning interaction, more interactions (e.g., adsorption, electrostatic interaction, ion exchange, and inclusion) may govern the retention mechanisms of the sulfanilamides. For the log-log curve plotted in Fig. 7b, the six substances are also not completely linearly related in the range of 15–30% water. For example, at 25% and 23% water content, sulfisomidin and sulfadimethoxypyrimidine have inflection points, respectively. Therefore, we believe that the retention mechanism of sulfanilamides is also not completely controlled by either adsorption or partitioning. Therefore, we boldly infer that its retention mechanism should be a mixed effect of partitioning mechanism and adsorption phenomena or a more complicated mixing mechanism.
Effect of a, b water content, c buffer salt concentration, and d pH of the mobile phase on the retention of analytes. a and b Mobile phase: ACN/water (v/v, %), flow rate: 1 mL/min; detection wavelength: 270 nm; c and d Mobile phase: ACN/water (90/10, v/v) mobile phase containing the corresponding concentration of ammonium acetate for c and ACN/water (90/10, v/v) mobile phase with different pH for d; flow rate: 1 mL/min
where kB is the solute retention factor with pure B (water) as the eluent, AS and nB are the cross-sectional areas occupied by the solute molecule on the surface and the B (water) molecules, respectively, and CB is the percentage of the stronger member B (water) in the eluent.
Subsequently, polar compounds with different ionization characteristics, including alkaloids, nucleosides, and organic acids, were used to explore the effect of buffer solutions in mobile phases on the chromatographic separation of ILCC4-Sil. Due to the high solubility of ammonium salts at high organic levels, different concentrations (5, 10, and 15 mM) of ammonium acetate (NH4OAc) were selected as the buffer solution. As shown in Fig. 7c, as the salt concentration in the mobile phase increases, the retention time of cytidine and 2-amino-6-chloropurine nucleoside increases slightly. This result was consistent with the partitioning mechanism described in the previous reference, indicating that the partitioning mechanism dominates the retention behavior of these types of compounds. At the same time, the retention time of organic acids such as cinnamic acid and benzoic acid decreases as the salt concentration increases. The possible reason is that the electrostatic interaction of attractive force weakens with the increase of buffer salt concentration.
Finally, the pH of the mobile phase, an important factor in HILIC separation, was studied using ammonium acetate buffer. Simply, aqueous solutions of ammonium acetate with different pH values (pH = 6.0, 5.0, 4.3, 3.6, and 3.2) were prepared before mixing with ACN and used for separation and analysis. As we can see from Fig. 7d, the retention time of cytosine or 2-amino-6-chloropurine was almost unaffected by the pH of the buffer, which may be because they are neutral compounds, and their ionization is not affected by pH. The retention time of cinnamic acid (pKa = 4.4) and benzoic acid (pKa = 4.2) was almost unchanged at pH values between 6.3 and 4.3, while their retention times decreased as the pH of the buffer decreased from 4.3 to 3.2. This may be because the electrostatic interactions between the imidazole and the organic acids are weakened by decreasing the pH since the organic acid exists in a molecular, un-ionized state at low pH.
Stability and reproducibility
Considering the flexibility of the chitosan network, the chemical stability of ILC-Sil was verified by immersing it in methanol and acetonitrile for 1 week. The FT-IR spectra (Fig. S1) were almost unchanged, indicating the robustness of ILC-Sil. What is more, the swelling degree of ILC-Sil soaked by methanol and acetonitrile was also characterized by SEM, and the results showed that the swelling property of ILC-Sil was also very good (Fig. S2). Additionally, the retention time of five sugars on the ILC-Sil column remained constant during five consecutive injections, demonstrating the high repeatability of the ILC-Sil (Fig. S3). The above results demonstrated that the developed columns were relatively steady and repeatable.
Determination of morphine in human urine using SPE-HILIC-MS/MS based on ILCC4-Sil column
Morphine, a potent narcotic analgesic and the active metabolite derived from heroin (3,6-diacetylmorphine), has been reported to influence various immune functions. It has been used extensively throughout the world for many years to relieve moderate to severe pain. According to the program of the WHO’s Cancer Pain Relief Program, morphine is a primary drug utilized in treating cancer pain. However, illegal abuse and overdose of morphine can be fatal. The toxic effects of morphine usage include many severe symptoms. Therefore, the determination of morphine concentration in urine is required for clinical medicine to prevent illegal abuse and overdose-induced toxicity.
In this paper, we present a rapid and efficient method comprising SPE and analysis with HPLC–MS/MS for the determination of morphine in human urine (Fig. S4). It can be seen from Table 2 that the recoveries ranged from 80.6 to 92.1%, and the RSDs (n = 3) were 3.2, 4.1, and 5.6% indicating that the developed SPE-HILIC-MS/MS method based on the ILCC4-Sil column possesses high accuracy. As shown in Table 3, the contents of morphine in urine samples ranged from 227.3 to 340.1 ng/mL; the linear dynamic ranges of the matrix standard curve for morphine were 1.5–1000 ng/mL with good coefficient of determinations (y = 4714.53330x-1.24533e5, R2 = 0.9995); The limits of detection (LOD) and quantification (LOQ) were determined by a signal/noise ratio (S/N) equal to 3 and 10, respectively. The LOD was 15 pg/mL, and the LOQ was 54 pg/mL, which are much lower than in previous reports [33]. This method is promising for its application to the preconcentration and determination of morphine in human bodily fluids. From the results above, it is concluded that the present SPE-HILIC-MS/MS method based on the ILCC4-Sil column is more suitable for the determination of morphine in real samples.
Conclusions
In this work, two new stationary phases modified with different polar and hydrophobic moieties were successfully prepared and characterized. Upon adjustment of the proportions of hydrophilic and hydrophobic groups, ILC-Sil and ILCC4-Sil can behave in the hydrophilic or RPLC/HILIC mixed mode, respectively. The multiple specific interactions, including hydrogen-bonding interactions, electrostatic interactions, ion exchange, π-π and π-electron transfer interactions, and inclusion interactions, induced by calix[4]arene or ionic-liquids bonded to chitosan promoting efficient separation of complex polar and nonpolar compounds with different physicochemical properties. The multiple interactions endow it with excellent potential application to the highly selective separation of complex compounds in complex samples. Subsequently, numerical analyses were conducted to investigate the retention mechanism of ILCC4-Sil and found that the synergistic effect of the partitioning mechanism and adsorption phenomena or more complicated mixing mechanism ensures the efficient separation performance of the prepared chromatographic column. Moreover, the proposed method for determining morphine in human urine utilizing ILCC4-Sil as the analytical column is faster and more sensitive than the previously published work. All these results demonstrated the broad application prospects of these chromatographic stationary phases in the separation and analysis of polar and nonpolar compounds in a complex matrix.
References
Alpert AJ (1990) Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J Chromatogr A 499:177–196
Qing G, Yan J, He X et al (2020) Recent advances in hydrophilic interaction liquid interaction chromatography materials for glycopeptide enrichment and glycan separation. TrAC, Trends Anal Chem 124:115570. https://doi.org/10.1016/j.trac.2019.06.020
McCalley DV (2017) Understanding and manipulating the separation in hydrophilic interaction liquid chromatography. J Chromatogr A 1523:49–71. https://doi.org/10.1016/j.chroma.2017.06.026
Salas D, Borrull F, Fontanals N, Marcé RM (2017) Hydrophilic interaction liquid chromatography coupled to mass spectrometry-based detection to determine emerging organic contaminants in environmental samples. TrAC, Trends Anal Chem 94:141–149. https://doi.org/10.1016/j.trac.2017.07.017
Hemström P, Irgum K (2006) Hydrophilic interaction chromatography. J Sep Sci 29:1784–1821. https://doi.org/10.1002/jssc.200600199
Karatapanis AE, Fiamegos YC, Stalikas CD (2011) A revisit to the retention mechanism of hydrophilic interaction liquid chromatography using model organic compounds. J Chromatogr A 1218:2871–2879. https://doi.org/10.1016/j.chroma.2011.02.069
Yuan N, Chen J, Cai T et al (2020) Glucose-based carbon dots-modified silica stationary phase for hydrophilic interaction chromatography. J Chromatogr A 1619:460930. https://doi.org/10.1016/j.chroma.2020.460930
Pazourek J (2014) Fast separation and determination of free myo-inositol by hydrophilic liquid chromatography. Carbohydr Res 391:55–60. https://doi.org/10.1016/j.carres.2014.03.010
Fan C, Chen J, Li H et al (2022) Preparation and evaluation of two silica-based hydrophilic-hydrophobic and acid-base balanced stationary phases via in-situ surface polymerization. J Chromatogr A 1667:462912. https://doi.org/10.1016/j.chroma.2022.462912
Guan F, You Y, Li X, Robinson MA (2019) A comprehensive approach to detecting multitudinous bioactive peptides in equine plasma and urine using hydrophilic interaction liquid chromatography coupled to high resolution mass spectrometry. Drug Test Anal 11:1308–1325. https://doi.org/10.1002/dta.2671
Dedvisitsakul P, Jacobsen S, Svensson B, Bunkenborg J, Christine Finnie PH (2014) Glycopeptide enrichment using a combination of ZIC-HILIC and cotton wool for exploring the glycoproteome of wheat flour albumins. J Proteome Res 13:2696–2703
Yang Y, Boysen RI, Hearn MTW (2009) Hydrophilic interaction chromatography coupled to electrospray mass spectrometry for the separation of peptides and protein digests. J Chromatogr A 1216:5518–5524. https://doi.org/10.1016/j.chroma.2009.05.085
Zhou W, Wang PG, Ogunsola OA, Kraeling MEK (2013) Rapid determination of hexapeptides by hydrophilic interaction LC-MS/MS for in vitro skin-penetration studies. Bioanalysis 5:1353–1362. https://doi.org/10.4155/bio.13.60
Douša M, Srbek J, Stránský Z et al (2014) Retention behavior of a homologous series and positional isomers of aliphatic amino acids in hydrophilic interaction chromatography. J Sep Sci 37:739–747. https://doi.org/10.1002/jssc.201301348
Noga S, Jandera P, Buszewski B (2013) Retention mechanism studies of selected amino acids and vitamin b6 on HILIC columns with evaporative light scattering detection. Chromatographia 76:929–937. https://doi.org/10.1007/s10337-013-2502-y
Iturrospe E, Da Silva KM, Talavera Andújar B et al (2021) An exploratory approach for an oriented development of an untargeted hydrophilic interaction liquid chromatography-mass spectrometry platform for polar metabolites in biological matrices. J Chromatogr A 1637:461807. https://doi.org/10.1016/j.chroma.2020.461807
Zuo H, Guo Y, Zhao W et al (2019) Controlled fabrication of silica@covalent triazine polymer core-shell spheres as a reversed-phase/hydrophilic interaction mixed-mode chromatographic stationary phase. ACS Appl Mater Interfaces 11:46149–46156. https://doi.org/10.1021/acsami.9b16438
Yaroshenko DV, Grigoriev AV, Yaroshenko IS et al (2021) Hydrophilic interaction liquid chromatography method for eremomycin determination in pre-clinical study. J Chromatogr A 1637:461750. https://doi.org/10.1016/j.chroma.2020.461750
Guo X, Zhang X, Guo Z et al (2014) Hydrophilic interaction chromatography for selective separation of isomeric saponins. J Chromatogr A 1325:121–128. https://doi.org/10.1016/j.chroma.2013.12.006
Fu Q, Guo Z, Zhang X et al (2012) Comprehensive characterization of Stevia Rebaudiana using two-dimensional reversed-phase liquid chromatography/hydrophilic interaction liquid chromatography. J Sep Sci 35:1821–1827. https://doi.org/10.1002/jssc.201101103
Wang L, Wei W, Xia Z et al (2016) Recent advances in materials for stationary phases of mixed-mode high-performance liquid chromatography. TrAC, Trends Anal Chem 80:495–506. https://doi.org/10.1016/j.trac.2016.04.001
Zhao W, Wang X, Guo J et al (2020) Evaluation of sulfonic acid functionalized covalent triazine framework as a hydrophilic-lipophilic balance/cation-exchange mixed-mode sorbent for extraction of benzimidazole fungicides in vegetables, fruits and juices. J Chromatogr A 1618:460847. https://doi.org/10.1016/j.chroma.2019.460847
Bo C, Jia Z, Dai X, Wei Y (2020) Facile preparation of polymer-brush reverse-phase/hydrophilic interaction/ion-exchange tri-mode chromatographic stationary phases by controlled polymerization of three functional monomers. J Chromatogr A 1619:460966. https://doi.org/10.1016/j.chroma.2020.460966
Liang T, Fu Q, Shen A et al (2015) Preparation and chromatographic evaluation of a newly designed steviol glycoside modified-silica stationary phase in hydrophilic interaction liquid chromatography and reversed phase liquid chromatography. J Chromatogr A 1388:110–118. https://doi.org/10.1016/j.chroma.2015.02.019
Wu Q, Hou X, Zhang X et al (2021) Amphipathic carbon quantum dots-functionalized silica stationary phase for reversed phase/hydrophilic interaction chromatography. Talanta 226:122148. https://doi.org/10.1016/j.talanta.2021.122148
Hosseini ES, Heydar KT (2021) Silica modification with 9-methylacridine and 9-undecylacridine as mixed-mode stationary phases in HPLC. Talanta 221:121445. https://doi.org/10.1016/j.talanta.2020.121445
Wu Q, Hou X, Lv H et al (2021) Synthesis of octadecylamine-derived carbon dots and application in reversed phase/hydrophilic interaction liquid chromatography. J Chromatogr A 1656:462548. https://doi.org/10.1016/j.chroma.2021.462548
Sun HF, Cui YY, Zhen CQ, Yang CX (2023) Monomer-mediated fabrication of microporous organic network@silica microsphere for reversed-phase/hydrophilic interaction mixed-mode chromatography. Talanta 251:123763. https://doi.org/10.1016/j.talanta.2022.123763
Zhang YP, Li K, Xiong LX et al (2022) “Click” preparation of a chiral macrocycle-based stationary phase for both normal-phase and reversed-phase high performance liquid chromatography enantioseparation. J Chromatogr A 1683:463551. https://doi.org/10.1016/j.chroma.2022.463551
Zhang W, Zhang Y, Zhang Y et al (2019) Tetra-proline modified calix[4]arene bonded silica gel: a novel stationary phase for hydrophilic interaction liquid chromatography. Talanta 193:56–63. https://doi.org/10.1016/j.talanta.2018.09.083
Zhang W, Zhang Y, Zhang G et al (2019) Tetra-proline modified calix[4]arene bonded silica stationary phase for simultaneous reversed-phase/hydrophilic interaction mixed-mode chromatography. J Sep Sci 1–10:1374. https://doi.org/10.1002/jssc.201800967
Lu J, Zhang W, Zhang Y et al (2014) A new stationary phase for high performance liquid chromatography: calix[4]arene derivatized chitosan bonded silica gel. J Chromatogr A 1350:61–67. https://doi.org/10.1016/j.chroma.2014.05.021
Zhao W, Hu K, Wang C et al (2012) New oxo-bridged calix[2]arene[2] triazine stationary phase for high performance liquid chromatography. J Chromatogr A 1223:72–78. https://doi.org/10.1016/j.chroma.2011.12.031
Zhao W, Wang W, Chang H et al (2012) Tetraazacalix[2]arene[2] triazine modified silica gel: a novel multi-interaction stationary phase for mixed-mode chromatography. J Chromatogr A 1251:74–81. https://doi.org/10.1016/j.chroma.2012.06.030
Zhang W, Wang F, Zhang Y et al (2019) 1,3,5-Triazine tetrad-aza cyclophanes coated silica as a novel stationary phase for high performance liquid chromatography. Sep Purif Technol 211:227–232. https://doi.org/10.1016/j.seppur.2018.09.082
Chen H-Y, Guo D, Gan Z-F et al (2019) A phenylboronate-based SERS nanoprobe for detection and imaging of intracellular peroxynitrite. Microchim Acta 186:11. https://doi.org/10.1007/s00604-018-3129-3
Ostovan A, Arabi M, Wang Y et al (2022) Greenificated molecularly imprinted materials for advanced applications. Adv Mater 34:2203154. https://doi.org/10.1002/adma.202203154
Ostovan A, Ghaedi M, Arabi M et al (2018) Hydrophilic multitemplate molecularly imprinted biopolymers based on a green synthesis strategy for determination of B-family vitamins. ACS Appl Mater Interfaces 10:4140–4150. https://doi.org/10.1021/acsami.7b17500
Arabi M, Ostovan A, Li J et al (2021) Molecular imprinting: green perspectives and strategies. Adv Mater 33:2100543. https://doi.org/10.1002/adma.202100543
Zhang L, Shen J, Zuo W, Okamoto Y (2014) Synthesis of chitosan 3,6-diphenylcarbamate-2-urea derivatives and their applications as chiral stationary phases for high-performance liquid chromatography. J Chromatogr A 1365:86–93. https://doi.org/10.1016/j.chroma.2014.09.002
Hu K, Liu J, Tang C et al (2012) Preparation, characterization and application of a new 25,27-bis-[2-(5- methylthiadiazole)thioethoxyl]-26,28-dihydroxy-para-tert-butyl calix[4]arene stationary phase for HPLC. J Sep Sci 35:239–247. https://doi.org/10.1002/jssc.201100733
Liu Y, Zou H, Haginaka J (2006) Preparation and evaluation of a novel chiral stationary phase based on covalently bonded chitosan for ligand-exchange chromatography. J Sep Sci 29:1440–1446. https://doi.org/10.1002/jssc.200600015
Deng Z, Liu J, Hu C et al (2014) Liquid chromatographic behavior of two alanine-substituted calix[4]arene-bonded silica gel stationary phases. J Sep Sci 37:3268–3275. https://doi.org/10.1002/jssc.201400366
Tanaka N, Tokuda Y, Iwaguchi K, Araki M (1982) Effect of stationary phase structure on retention and selectivity in reversed-phase liquid chromatography. J Chromatogr A 239:761–772. https://doi.org/10.1016/S0021-9673(00)82036-1
Zhao W, Chu J, Xie F et al (2017) Preparation and evaluation of pillararene bonded silica gel stationary phases for high performance liquid chromatography. J Chromatogr A 1485:44–51. https://doi.org/10.1016/j.chroma.2016.12.019
Ohyama K, Inoue Y, Kishikawa N, Kuroda N (2014) Preparation and characterization of surfactin-modified silica stationary phase for reversed-phase and hydrophilic interaction liquid chromatography. J Chromatogr A 1371:257–260. https://doi.org/10.1016/j.chroma.2014.10.073
Acknowledgements
We acknowledge financial support from the projects of the National Natural Science Foundation of China (22004109, 22276177, and 21974124) and the China Postdoctoral Science Foundation (2022M710167).
Author information
Authors and Affiliations
Contributions
Wenfen Zhang: conceptualization, methodology, supervision, writing—original draft, funding acquisition, and project administration. Yumin Feng: validation and investigation. Long Pan: methodology and experiment supplement. Guangrui Zhang: methodology and experiment supplement. Yanhao Zhang: experiment supplement. Wuduo Zhao: experiment supplement. Zhengkun Xie: visualization. Shusheng Zhang: project administration, writing—review and editing, supervision, and funding acquisition
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, W., Feng, Y., Pan, L. et al. Silica microparticles modified with ionic liquid bonded chitosan as hydrophilic moieties for preparation of high-performance liquid chromatographic stationary phases. Microchim Acta 190, 176 (2023). https://doi.org/10.1007/s00604-023-05755-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05755-6