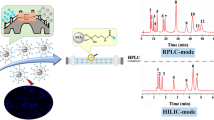
Abstract
Carbon dots derivatized from N-(β-aminoethyl)-γ-aminopropyl-methyldimethoxysilane (AEAPMS) were coated onto silica microparticles. These particles (Sil-CDs) are shown to be an excellent stationary phase for use in hydrophilic interaction chromatography. Analytes including sulfonamides, nucleosides and bases, flavones and amino acids can be well separated on this stationary phase. Compared to a silica stationary phase functionalized with AEAPMS only, the Sil-CDs show enhanced separation performance. The selectivity factors of three nucleosides and bases (1.02–1.09) and four sulfonamides (1.04–1.11) on AEAPMS functionalized silica stationary phase were improved to 1.10–1.20 and 1.13–1.15 respectively on Sil-CDs stationary phase. This is attributed to the higher number of surface functional groups due to the introduction of carbon dots. The successful application of the Sil-CDs stationary phase highlights the potential of carbon dots as a modified material in chromatography.

Schematic presentation of the preparation of silanized carbon dots coated onto silica microparticles. The material represents a new stationary phase for hydrophilic interaction chromatography. It shows improved separation performance compared to a silane-only functionalized silica stationary phase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The improvement of the separation capability of chromatographic columns is a subject of ongoing research. Even reversed-phase liquid chromatography (RPLC), especially C18 column, which is applicable to most of the analytes, has been fully developed and commercialized [1, 2], but it is not suitable for the very polar and hydrophilic samples [3]. With the in-depth research of proteomic and metabolomics, a more effective separation method for the polar biological sample is desperately needed. As an alternative to normal phase liquid chromatography (NPLC), hydrophilic interaction chromatography (HILIC) use aqueous-organic mobile phase which is more compatible to hydrophilic molecules [4]. Besides, HILIC is a better choice to couple with mass spectrometry (MS), for organic-rich mobile phase remarkably improved ESI-MS sensitivity [5].
Since HILIC was proposed by Alpert in 1990 [6], various functional groups were employed as stationary phases for HILIC. The NPLC stationary phases, such as amino, cyano, diol, and bare silica, can be used directly into HILIC mode. Besides, stationary phase designed especially for HILIC were also prepared by researchers, including saccharides [7, 8], amide [9], zwitterionic molecules [10] and hydrophilic polymers [11, 12]. As the scientific applications of carbon nanomaterials (CNMs) proliferated, serials of CNMs were found out to be excellent materials for analytical research. Thanks to their outstanding properties like large surface areas, high adsorption capacities and high thermal and mechanical stabilities, CNMs has been successfully used in sample pretreatment [13,14,15], analytical electrochemistry [16] and liquid chromatography. Among them, some carbon nanomaterial with hydrophilic functional groups, like graphene oxide (GO) [17], oxidized nanodiamond [18], carbon nanoparticle (CNP) [19], were selected to prepare HILIC stationary phases, which showed acceptable separation performance [20].
Because of the good water solubility, excellent biocompatibility [21], neglectable cytotoxicity [22], outstanding photostability and high quantum yield (QY) [23, 24], carbon dots (CDs) were widely used in bioimaging [25], chemical sensor [26], electronic sensor [27], photocatalysis [28], optoelectronics [29] and etc. And almost every application are related to their fluorescence property [30]. The tunable surface functionalization of CDs is also a hot research point, which meant to broaden their applications with enhanced properties to meet specific requirements [31], such as easier conjugation to specific materials and higher selectivity and sensitivity to specific targets [32].
Our lab has presented a carbon dot-decorated silica stationary phase in deep eutectic solvents. Carbon dots were prepared via a solid-phase synthesis approach using tryptophan and aconitic acid in a molar ratio of 2:1. This phase gives a typical example of using carbon dots as stationary-phase component for HILIC [33]. On the other hand, silanized carbon dots, in particular, were developed with easy conjugation to silica-based matrix, which has been used in modification of silica nanoparticles [34, 35] and glasses [36] etc. Inspired by this, we extended the application of silanized CDs to liquid chromatography stationary phase, in which properties like easy stabilization and tunable surface functional groups were treated as major concerns.
To accomplish this, our lab used a method by pyrolysis of anhydrous citric acid in hot N-(β-aminoethyl)-γ-aminopropyl methyldimethoxy silane (AEAPMS) to synthesis amino-modified silanized carbon dots, and then grafted the CDs onto the spherical porous silica. The resulting silanized carbon dots-grafted silica (Sil-CDs) was then used as stationary phase in HILIC, and satisfactory separation performance for sulfonamides, flavones, amino acids, nucleosides and bases were obtained.
Experimental
Materials and reagents
All chemicals and reagents were used without further purification. Spherical porous silica (diameter: 5 μm, pore size: 90 Å, surface area: 306 m2 g−1) were obtained from Fuji Silysia Chemical Ltd. (Aichi, Japan, http://www.fuji-silysia.co.jp/). N-(β-Aminoethyl)-γ-aminopropyl methyldimethoxy silane (98%) and anhydrous citric acid (99%) were purchased from Energy Chemical (Shanghai, China, http://www.energy-chemical.com.cn/). Sulfonamide drugs were obtained from Aladdin (analytical standard: sulfadimoxine, sulfamerazine, sulfapyridine, sulfanilamide, sulfadimethoxine, sulfamethazine, sulfisoxazole) (Shanghai, China, http://www.aladdin-e.com/) and Energy Chemical (analytical standard: sulfadiazine, sulfathiazole) (Shanghai, China, http://www.energy-chemical.com.cn/). Nucleosides and bases including thymine, uridine, thymidine, uridine, adenosine, adenine, cytosine, hypoxanthine, cytidine and inosine were all purchased from Aladdin (Shanghai, China, http://www.aladdin-e.com/). Flavones were supplied by Chengdu MUST Bio-technology CO., TLD (Chengdu, China, http://chengdumust.en.china.cn/). The others chemicals and solvents were gotten from Energy Chemical (Shanghai, China, http://www.aladdin-e.com/).
Instruments
Inspire 5 μm HILIC column (150 × 4.6 mm i.d.) was supplied by Dikma Technologies Inc. The test of analytes was carried out with a Shimadzu-GL LC-15C system including two high-pressure pumps, a SPD-15C UV/vis detector, a CTO-15C column oven and a 50 μL Shimadzu-GL microsyringe, the UV/vis detector was set at 254 nm wavelength. The test of four saccharides was carried out with Agilent 1260 Infinity Series modular system with quaternary pumps (Agilent Technologies, USA), a Alltech 3300 evaporative light scattering detector (Grace, USA) with GCK3302 air generator (BCHP Analytical Technology Institute, China). The ELSD was set as follows: gas flow, 1.5 L min-1; evaporative temperature, 45 °C; photomultiplier, 1; gas pressure, 0.50 Mpa. The FT-IR spectra were collected from IFS 120HR Fourier transform infrared spectrometer (Bruker, Germany). Elemental analysis results were determined by Vario EL III elemental analyzer (Hanau, Germany). Transmission electron microscopies (TEM) imaging were obtained from Tecnai G2 TF20 transmission electron microscope (FEI, USA). Laser scanning confocal microscope (LSCM) imaging were received from Laser Scanning Confocal Microscope FV1200 (Olympus, Japan). X-ray photoelectron spectroscopy (XPS) results were gotten from ESCALAB250Xi (Thermo Scientific). N2 adsorption surface areas were measured by BET technique on a Micrometritics ASPS 2010 analyzer (USA).
Preparation of Sil-CDs and Sil-AEAPMS
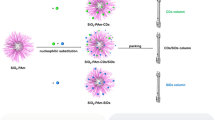
First, CDs were prepared according to the reference [37]. Briefly, 10 mL AEAPMS was degassed with nitrogen for 5 min, and then heated to 240 °C. Subsequently, 0.5 g anhydrous citric acid was added to the solution with vigorous stirring. After reacted for 1 min, the products were purified by washing with hexane five times, about 2 g silanized CDs were obtained.
These silanized CDs (1.2 g) were dispersed into 20 mL toluene, followed by the addition of 3.0 g spherical porous silica, the mixture was sonicated till a homogeneous dispersion was obtained. Then, the dispersion was heated to 110 °C and mechanically stirred for 24 h. After that, the resulted material was washed successively with toluene and ethanol and dried at 60 °C in vacuum oven. (Fig. 1).
Because the obtained CDs were surface passivated with AEAPMS [37], so AEAPMS-modified silica was also prepared to process comparative experiment. Thus, 3.0 g spherical porous silica was dispersed in 20 mL toluene by sonication, 6 mmol AEAPMS was added to the dispersion, and then the mixture was heated at 100 °C for 24 h. Finally, the AEAPMS modified silica (Sil-AEAPMS) was washed and dried with the same procedure as above.
Column packing
The Sil-CDs and Sil-AEAPMS stationary phases were slurry-packed into stainless steel columns (150 mm × 4.6 mm i.d.) with carbon tetrachloride as slurry solvent and acetonitrile as propulsion solvent, separately.
Results and discussion
Characterization
The morphology of CDs and Sil-CDs were characterized by transmission electron microscopy (TEM) and laser scanning confocal microscope (LSCM). From TEM image, the particle size of CDs can be identified as around 3 nm (Figs. 2a, and S1), and thin layer of CDs was coated on silica substrate (Fig. 2b). Because the fluorescence of CDs, it is convenient to monitor the coverage of CDs on the silica with LSCM (Fig. 2c), in which homogeneous fluorescence was observed on the surface of silica spheres. From the overlay of confocal fluorescence and bright-field images of Sil-CDs (Fig. 2d), one can see every silica sphere were emitting fluorescence, which indicated uniform bonding reaction of CDs with Silica. Fluorescent performance of silanized CDs (a), Sil-CDs (b) and bare silica (c) (λex = 360 nm) were shown in Fig. S2 (ESI). The materials were also characterized by elemental analysis, Fourier transform infrared spectroscopy (FTIR). From the elemental analysis results of Sil-CDs and Sil-AEAPMS (Table 1), one can see much higher of C content and a little higher of N, H contents are obtained in Sil-CD packing material than in Sil-AEAPMS. This result indicates that more interaction sites exist on the packing material, which would enable the Sil-CD stationary phase to have better separation performance. This proposition can also get positive evidence from FT-IR and XPS analysis results. In FT-IR results (Fig. S3, ESI), a typical signal arising at 1654 cm−1 represent the existence of C = ONR, that means the successful surface passivation reaction between amine groups of AEAPMS and carboxyl groups originate from the pyrolyzed species. And the combination between pyrolyzed CD core and AEAPMS allow higher density of amine groups on the matrix. Because despite the C = ONR vibration peaks, similar peaks are observed from Sil-CDs and Sil-AEAPMS, but Sil-CDs show more remarkable peaks. From the results of XPS analysis (Table S1, ESI), the N atomic percentage of Sil-CDs is higher than that of Sil-AEAPMS, which also confirm that higher density of amine groups existed on Sil-CDs. Besides, the higher percentage of O atom on Sil-CDs implies the successful silanization of CDs by AEAPMS.
Moreover, the BET surface area measurements were performed with N2 adsorption-desorption isotherms at −196 °C. The BET surface areas of Sil-CDs and Sil-AEAPMS particles were tested to be 205 and 313 m2 g−1, respectively, and their pore volumes are 0.50 and 0.83 m3 g−1, respectively. Furthermore, estimation of the pore size distribution by the density functional theory (BJH) showed a maximum at 8.31 and 11.02 nm for Sil-CDs and Sil-AEAPMS particles, respectively. After comparison, the BET surface areas and pore sizes of Sil-CDs were found to be lower than Sil-AEAPMS, implying that CDs were not only grafted on the surface of silica spheres, but partly imbedded into the pores of the silica spheres.
Chromatographic performance under HILIC mode
Since the synthesized CDs were surface passivated by AEAPMS which allowed Sil-CDs and Sil-AEAPMS to have similar surface functional groups, it is an interesting subject to study the differences of chromatographic behavior of both stationary phases. So middle polar molecules (sulfonamides and flavones), and hydrophilic molecules (amino acids, nucleosides and bases) were selected as probes to analyze the retention performance of the columns which were also compared with a commercial HILIC column (DIKMA Inspire 5 μm Hilic). Besides, the influences of buffer concentration, buffer pH and organic solvent concentration in mobile phase were tested to get an insight into the retention mechanism of the Sil-CD stationary phase.
Fig. 3 shows the separation of ten nucleosides and bases, and Fig. 4 the separation of nine sulfonamides by using the above columns. One can see that Sil-CDs and Sil-AEAPMS show similar retention behavior, and that most analytes exhibit similar retention time and elution order. However, several analytes with close retention time become fully separated on Sil-CDs (as opposed to Sil-AEAPMS).
Separation of ten nucleosides and bases with Sil-CDs,Sil-AEAPMS and Inspire 5 μm Hilic columns: thymine (1), uridine (2), thymidine (3), uridine (4), adenosine (5), adenine (6), cytosine (7), hypoxanthine (8), cytidine (9), inosine (10); mobile phase: 93% acetonitrile: 7% 20 mM ammonium acetate, pH = 6.62, flow rate = 1.0 mL min−1, T = 35 °C, UV detection: 254 nm
Separation of nine sulfonamides with Sil-CDs, Sil-AEAPMS and Inspire 5 μm Hilic columns: sulfanilamide (1), sulfapyridine (2), sulfamethazine (3), sulfamerazine (4), sulfadoxine (5), sulfadiazine (6), sulfadimethoxine (7), sulfathiazole (8), sulfisoxazole (9); mobile phase:85% acetonitrile: 15% 10 mM ammonium acetate, pH = 6.62, flow rate = 1.0 mL min−1, T = 35 °C, UV detection: 254 nm
For nucleosides and bases, uridine, adenosine and adenine were nicely separated on Sil-CDs, which were inseparable on Sil-AEAPMS. The selectivity factors of these three analytes are 1.09 and 1.02 on Sil-AEAPMS, which were enhanced to 1.10 and 1.20 on Sil-CDs (Table S2). As shown in the elemental analysis results, the introduction of CDs allows more amino groups exist on Sil-CDs than Sil-AEAPMS, which ensure Sil-CDs have stronger electrostatic interaction with the analytes than Sil-AEAPMS. So the retention of adenosine (pKaadenosine = 4.99) and adenine (pKaadenine = 4.15), which exhibit negative charge under the experimental condition, get considerably enhanced on Sil-CDs compared with Sil-AEAPMS, because of the stronger electrostatic attraction with Sil-CDs. Besides, the retention of uridine (pKauridine = 9.2) shows almost no difference on Sil-CDs and Sil-AEAPMS, because there are no electrostatic attraction exists between the positive-charged uridine and protonated amino groups on both stationary phase under the experimental condition.
For sulfonamides, the retentions of sulfadoxine, sulfadiazine, sulfadimethoxine, sulfathiazole all get enhanced on Sil-CDs when compared with Sil-AEAPMS. The selectivity factors of these five analytes range from 1.04 to 1.11 on Sil-AEAPMS, which were enhanced to 1.13–1.15 (Table S3). And the resolutions of these four analytes are also better on Sil-CDs. These results can be explained by the higher density of interaction sites on Sil-CDs than Sil-AEAPMS, which allow higher separation efficiency under the identical chromatographic condition.
Compared to a commercial HILIC column, the use of a Sil-CD based column results in stronger retention for both medium-polar molecules (sulfonamides) and hydrophilic molecules (nucleosides and bases) under the same conditions. Besides, seven flavones and seven amino acids also get satisfactory separation results (Fig. S4a , b in ESI). An overview on recently reported nanomaterial-based stationary phases for separation in hydrophilic interaction chromatography were also shown in Table S4 (ESI).
Investigation of retention behavior for Sil-CD stationary phase
The effect of various chromatographic factors, including organic solvent concentration in mobile phase, mobile phase buffer concentration and buffer pH, were studied to get an in-depth understanding of the retention behavior for Sil-CD stationary phase.
Firstly, the influence of organic solvent concentration to nucleosides and bases (Fig. 5a) and sulfonamides (Fig. 5b) showed diverse results: as the acetonitrile concentration increase, the retention factors of all nucleosides and bases increase gradually, which obey the typical HILIC mechanism; however, the retention of sulfonamides decreases firstly and then increases, which means Sil-CD stationary phase switch from RPLC mode to HILIC mode. And this can be explained by the short alkyl chains on the CDs enabled hydrophobic interaction between stationary phase and hydrophobic ends of middle polar analytes. As the organic solvent concentration increase, the hydrophilic interaction between amine groups on CDs and hydrophilic ends of the middle polar analytes became dominant interaction, which correspondingly exhibit HILIC behavior. Because of this mixed retention mechanism, good retention on Sil-CDs in a wide range of organic solvent concentration is obtained.
Effect of organic solvent concentration on the retention factor (k) of nucleosides and bases (a), sulfonamides (b) with Sil-CDs column. Mobile phase: a acetonitrile and 20 mM ammonium acetate aqueous solution, pH = 6.62; b acetonitrile and 10 mM ammonium acetate aqueous solution, pH = 6.62, flow rate = 1.0 mL min−1, T = 35 °C, UV detection: 254 nm
Secondly, the retention of ionizable analytes on Sil-CD stationary phase in HILIC mode mainly realize by the partition of analytes into water-rich layer on stationary phase and electrostatic interaction between the analytes and amine groups of CDs [38, 39]. As the buffer concentration of mobile phase increased, water-rich layer became thicker and electrostatic interaction got shielded by high concentration of salt. That means when electrostatic interaction play the primary role, the retention of the analytes would getting weaker as buffer concentration increase; when partitioning dominate the retention, the analytes tend to better retain on the stationary phase as buffer concentration increase. According to this principle, electrostatic interaction shows significant effect on the retention of sulfonamides, while partitioning shows feeble effect; nucleosides and bases, however, shows diverse results. Then, the rationality of this deduction is confirmed by experimental results (Fig. S5a, b, ESI). Specifically, most of the selected nucleosides and bases obey the partitioning mechanism, while adenine, hypoxanthine and inosine get more effected by electrostatic interaction mechanism, but decrease rate is getting small when buffer concentration increase, which means partitioning shows non-ignorable influence on the retention of these three analytes.
Furthermore, the ionizable analytes suffer less electrostatic attraction to Sil-CDs when analytes and amine group on the stationary phase are all protonated at low pH, so if electrostatic attraction play critical role for the retention of analytes, the analytes would getting easier to be eluted when buffer pH getting lower. However, ionized state shows negligible influence for the analytes dominated by partitioning mechanism. So the above mentioned adenine, hypoxanthine, inosine and sulfonamides would decrease their retention as the buffer pH becoming lower, while the retention of rest of nucleosides and bases would remain steady. All these statements are accordant with the experimental results (Fig. S5c, d, ESI), except some of the nucleosides and bases showed slightly increased retention trend as pH decrease, which resulted from increased ionic strength when pH is getting lower.
Reproducibility of Sil-CD stationary phase
Even only two steps were employed for the synthesis of Sil-CD stationary phase, the column exhibited decent stability during chromatographic utilization. The reproducibility test was proceeded by continuous injection of 9 sulfonamides for 10 times (Fig. 6). The intraday RSD values (n = 10) of the retention factors of the analytes were calculated within 0.44–1.02%. Moreover, the intraday and interday precision were tested using four nucleosides and bases as model analytes, and the retention time of the four model analytes were monitored every 8 h for three days. The chromatographic conditions were same as Fig. 3. The intraday and interday RSD of the retention factor were less than 0.81% and 0.61% (Table 2).
The reproducibility test of Sil-CDs column with sulfanilamide (1), sulfapyridine (2), sulfamethazine (3), sulfamerazine (4), sulfadoxine (5), sulfadiazine (6), sulfadimethoxine (7), sulfathiazole (8), sulfisoxazole (9); Mobile phase: 85% acetonitrile: 15% 10 mM ammonium acetate solution, pH = 6.62, flow rate = 1.0 mL min−1, T = 35 °C, UV detection: 254 nm
Conclusion
In summary, a new amino-modified silanized carbon dots functionalized silica stationary phase was prepared, which exhibited excellent separation performance for both middle polar and hydrophilic analytes in HILIC mode. The electrostatic interaction and partitioning act as the two major factors for the retention behavior of Sil-CDs, which are inherited from the passivator of CD (AEAPMS). But interestingly, Sil-CDs show enhanced separation ability for samples which cannot be separated on Sil-AEAPMS. And the reason is confirmed to be the higher density of amine groups on Sil-CDs than Sil-AEAPMS due to the formation of carbon dot. The proposed Sil-CDs nicely overcame the serious peak tailing and low column efficiency which usually shown by the other carbon nanomaterials stationary phases. For another, this work revealed a new way to enhance the chromatographic selectivity by forming carbon dots which increase the density of the interaction sites on stationary phase. Thus, it is reasonable to believe CDs are promising materials for chromatographic application, and more researches will be conducted for CDs-based stationary phase with better separation performance and further understanding of the chromatographic behavior of CDs. Also more and more CDs can be coated on silica and other supports lead to broad applications, including solid phase extraction, solid electrochemistry, etc.
References
Mallik AK, Qiu H, Kuwahara Y, Takafuji M, Ihara H (2015) A remarkable enhancement of selectivity towards versatile analytes by a strategically integrated H-bonding site containing phase. Chem Commun 51(75):14243–14246
Mallik AK, Qiu H, Oishi T, Kuwahara Y, Takafuji M, Ihara H (2015) Design of C18 organic phases with multiple embedded polar groups for Ultraversatile applications with ultrahigh selectivity. Anal Chem 87(13):6614–6621
Zhang M, Mai W, Zhao L, Guo Y, Qiu H (2015) A polar-embedded C30 stationary phase: preparation and evaluation. J Chromatogr A 1388:133–140
Qiao L, Shi X, Xu G (2016) Recent advances in development and characterization of stationary phases for hydrophilic interaction chromatography. Trends Anal Chem 81:23–33
Nguyen HP, Schug KA (2008) The advantages of ESI-MS detection in conjunction with HILIC mode separations: fundamentals and applications. J Sep Sci 31(9):1465–1480
Alpert AJ (1990) Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J Chromatogr 499(2):177–196
Guo Z, Lei A, Zhang Y, Xu Q, Xue X, Zhang F, Liang X (2007) "click saccharides": novel separation materials for hydrophilic interaction liquid chromatography. Chem Commun 24:2491–2493
Liang T, Fu Q, Shen A, Wang H, Jin Y, Xin H, Ke Y, Guo Z, Liang X (2015) Preparation and chromatographic evaluation of a newly designed steviol glycoside modified-silica stationary phase in hydrophilic interaction liquid chromatography and reversed phase liquid chromatography. J Chromatogr A 1388:110–118
Shen A, Guo Z, Yu L, Cao L, Liang X (2011) A novel zwitterionic HILIC stationary phase based on "thiol-ene" click chemistry between cysteine and vinyl silica. Chem Commun 47(15):4550–4552
Jandera P (2011) Stationary and mobile phases in hydrophilic interaction chromatography: a review. Anal Chim Acta 692(1–2):1–25
Yu D, Shen A, Guo Z, Yan Y, Yan J, Jin G, Liang X (2015) A controlled thiol-initiated surface polymerization strategy for the preparation of hydrophilic polymer stationary phases. Chem Commun 51(79):14778–14780
Qiu H, Mallik AK, Takafuji M, Jiang S, Ihara H (2012) New poly(ionic liquid)-grafted silica multi-mode stationary phase for anion-exchange/reversed-phase/hydrophilic interaction liquid chromatography. Analyst 137(11):2553–2555
Behbahani M, Najafi M, Amini MM, Sadeghi O, Bagheri A, Salarian M (2013) Dithizone-modified nanoporous fructose as a novel sorbent for solid-phase extraction of ultra-trace levels of heavy metals. Microchim Acta 180(9–10):911–920
Behbahani M, Bagheri A, Amini MM, Sadeghi O, Salarian M, Najafi F, Taghizadeh M (2013) Application of multiwalled carbon nanotubes modified by diphenylcarbazide for selective solid phase extraction of ultra traces cd(II) in water samples and food products. Food Chem 141(1):48–53
Tabani H, Fakhari AR, Shahsavani A, Behbahani M, Salarian M, Bagheri A, Nojavan S (2013) Combination of graphene oxide-based solid phase extraction and electro membrane extraction for the preconcentration of chlorophenoxy acid herbicides in environmental samples. J Chromatogr A 1300:227–235
Hosseini H, Behbahani M, Mahyari M, Kazerooni H, Bagheri A, Shaabani A (2014) Ordered carbohydrate-derived porous carbons immobilized gold nanoparticles as a new electrode material for electrocatalytical oxidation and determination of nicotinamide adenine dinucleotide. Biosens Bioelectron 59:412–417
Jiang B, Liang Y, Wu Q, Jiang H, Yang K, Zhang L, Liang Z, Peng X, Zhang Y (2014) New GO-PEI-au-L-Cys ZIC-HILIC composites: synthesis and selective enrichment of glycopeptides. Nano 6(11):5616–5619
Cai T, Zhang H, Li Z, Rahman AFMM, Qiu H (2016) A new nano-on-micro stationary phase based on nanodiamond bonded on silica for hydrophilic interaction chromatography. RSC Adv 6(39):32757–32760
Li Y, Xu L, Chen T, Liu X, Xu Z, Zhang H (2012) Carbon nanoparticles from corn stalk soot and its novel application as stationary phase of hydrophilic interaction chromatography and per aqueous liquid chromatography. Anal Chim Acta 726:102–108
Zhang M, Qiu H (2015) Progress in stationary phases modified with carbonaceous nanomaterials for high-performance liquid chromatography. Trends Anal Chem 65:107–121
Su Y, Zhang M, Zhou N, Shao M, Chi C, Yuan P, Zhao C (2016) Preparation of fluorescent N, P-doped carbon dots derived from adenosine 5′-monophosphate for use in multicolor bioimaging of adenocarcinomic human alveolar basal epithelial cells. Microchim Acta:1–8
Qu Q, Zhu A, Shao X, Shi G, Tian Y (2012) Development of a carbon quantum dots-based fluorescent Cu2+ probe suitable for living cell imaging. Chem Commun 48(44):5473–5475
Liu W, Li C, Ren Y, Sun X, Pan W, Li Y, Wang J, Wang W (2016) Carbon dots: surface engineering and applications. J Mater Chem B 4(35):5772–5788
Zhu S, Meng Q, Wang L, Zhang J, Song Y, Jin H, Zhang K, Sun H, Wang H, Yang B (2013) Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew Chem Int Ed 52(14):3953–3957
Zhou J, Zhou H, Tang J, Deng S, Yan F, Li W, Qu M (2016) Carbon dots doped with heteroatoms for fluorescent bioimaging: a review. Microchim Acta. doi:10.1007/s00604-016-2043-9
Guo Y, Yang L, Li W, Wang X, Shang Y, Li B (2016) Carbon dots doped with nitrogen and sulfur and loaded with copper (II) as a “turn-on” fluorescent probe for cystein, glutathione and homocysteine. Microchim Acta 183(4):1409–1416
Zhuang Z, Lin H, Zhang X, Qiu F, Yang H (2016) A glassy carbon electrode modified with carbon dots and gold nanoparticles for enhanced electrocatalytic oxidation and detection of nitrite. Microchim Acta 183(10):2807–2814
Fernando KA, Sahu S, Liu Y, Lewis WK, Guliants EA, Jafariyan A, Wang P, Bunker CE, Sun YP (2015) Carbon quantum dots and applications in photocatalytic energy conversion. ACS Appl Mater Inter 7(16):8363–8376
Wang Y, Hu A (2014) Carbon quantum dots: synthesis, properties and applications. J Mater Chem C 2(34):6921
Zuo P, Lu X, Sun Z, Guo Y, He H (2015) A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim Acta 183(2):519–542
Wang B, Song A, Feng L, Ruan H, Li H, Dong S, Hao J (2015) Tunable amphiphilicity and multifunctional applications of ionic-liquid-modified carbon quantum dots. ACS Appl Mater Inter 7(12):6919–6925
Gupta A, Chaudhary A, Mehta P, Dwivedi C, Khan S, Verma NC, Nandi CK (2015) Nitrogen-doped, thiol-functionalized carbon dots for ultrasensitive hg(II) detection. Chem Commun 51(53):10750–10753
Zhang H, Qiao X, Cai T, Chen J, Li Z, Qiu H (2017) Preparation and characterization of carbon dot-decorated silica stationary phase in deep eutectic solvents for hydrophilic interaction chromatography. Anal Bioanal Chem 409(9):2401–2410
Liu X, Zhang N, Bing T, Shangguan D (2014) Carbon dots based dual-emission silica nanoparticles as a ratiometric nanosensor for cu(2+). Anal Chem 86(5):2289–2296
Wang C, Xu Z, Lin H, Huang Y, Zhang C (2015) Large scale synthesis of Highly stable fluorescent carbon dots using silica spheres as carriers for targeted Bioimaging of cancer cells. Part Part Syst Charact 32(10):944–951
Xie Z, Wang F, Liu CY (2012) Organic-inorganic hybrid functional carbon dot gel glasses. Adv Mater 24(13):1716–1721
Wang F, Xie Z, Zhang H, C-y L, Zhang Y (2011) Highly luminescent Organosilane-functionalized carbon dots. Adv Funct Mater 21(6):1027–1031
Chirita RI, West C, Zubrzycki S, Finaru AL, Elfakir C (2011) Investigations on the chromatographic behaviour of zwitterionic stationary phases used in hydrophilic interaction chromatography. J Chromatogr A 1218(35):5939–5963
Wang J, Guo Z, Shen A, Yu L, Xiao Y, Xue X, Zhang X, Liang X (2015) Hydrophilic-subtraction model for the characterization and comparison of hydrophilic interaction liquid chromatography columns. J Chromatogr A 1398:29–46
Acknowledgements
Financial supports from the National Natural Science Foundation of China (No. 21475142, 21611140105), CAS President’s International Fellowship Initiative (SL: 191), the funds for Distinguished Young Scientists of Gansu (1506RJDA281) and the top priority program of “One-Three-Five” Strategic Planning of Chinese Academy of Sciences are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 2075 kb)
Rights and permissions
About this article
Cite this article
Cai, T., Zhang, H., Rahman, A.F.M.M. et al. Silica grafted with silanized carbon dots as a nano-on-micro packing material with enhanced hydrophilic selectivity. Microchim Acta 184, 2629–2636 (2017). https://doi.org/10.1007/s00604-017-2277-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2277-1