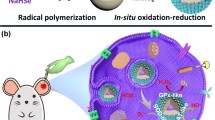
Abstract
Lysosome-targetable selenium-doped carbon nanodots (Lyso-Se-CDs) that can efficiently scavenge lysosomal •OH in living cells and mice were designed in this research. Se-CDs with redox-responsive fluorescence (λex = 379 nm, λem = 471 nm, quantum yield = 7.1%) were initially synthesized from selenocystine by a facile hydrothermal method, followed by the surface modification with morpholine, a lysosome targeting moiety. The as-synthesized Lyso-Se-CDs exhibited excellent colloidal stability, efficient scavenging abilities towards •OH, low biotoxicity, as well as good biocompatibility and lysosome targetability. Due to these desirable properties, Lyso-Se-CDs had been successfully utilized for rescuing cells from elevated lysosomal •OH levels. More importantly, Lyso-Se-CDs efficiently relieved phorbol 12-myristate 13-acetate (PMA) triggered ear inflammation in live mice. These findings reveal that Lyso-Se-CDs are potent candidates for treating •OH-related inflammation.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reactive oxygen species (ROS) comprising superoxide anions (O2•−), hydrogen peroxide (H2O2), and hydroxyl radicals (•OH) are a series of highly reactive oxygen-containing species [1,2,3,4,5]. Normally, the production and elimination of ROS are in a delicate balance, and the generated ROS are beneficial to many physiological processes [6]. However, due to some exogenous and/or endogenous stimuli, overproduction and accumulation of ROS may happen [7]. This can overwhelm endogenous protective mechanisms and trigger ROS-mediated injury to biomolecules including proteins, DNAs and lipids, leading to a variety of severe diseases, such as stroke, diabetes, inflammatory disorders, neurodegenerative diseases, and cancer [8, 9].
Over the past few years, numerous nanomaterial-based antioxidants including ceria nanoparticles (CeO2) [10, 11], manganese oxide nanoparticles (Mn3O4, MnO2) [12], polydopamine nanoparticles [13], copper-based nanoparticles[14], metal-organic frameworks [15], graphene oxide [16], etc. [17] have been developed for their therapeutic effects to relieve injury and treat ROS-related diseases. For instance, Qu’s group synthesized selenium nanoparticle-graphene oxide hybrid nanomaterials to protect cell components against oxidative stress [16]. Wei and coworkers fabricated Mn3O4 nanoparticles for in vivo eliminating cellular •OH and O2•− with extraordinary efficiency [12]. Wu et al. fabricated ROS eliminating tissue adhesive nanocomposite based on ceria nanocrystal-modified mesoporous silica nanoparticles for restricting ROS-mediated harmful effects and accelerating wound healing [18]. These antioxidants are useful for preventing oxidative damages from cellular ROS. Considering the extremely high reactivity and short half-life of ROS, it is important to prepare targetable antioxidants for in situ scavenging ROS. Lysosomes as cellular acidic organelles (pH 4.0–6.0) contain more than 50 degradative enzymes and play crucial roles in cellular homeostasis and the regulation of varieties of physiological processes [19]. In fact, lysosomes are prone to be affected by •OH, as it is the most abundant ROS inside lysosomes, where its formation is favored by the richness of labile iron ions and the internal acidic microenvironments [20]. To our knowledge, no antioxidants with lysosome-targetable capability have been reported. Thus, to maintain normal status and function of lysosome, it is of great importance to design efficient methods for precisely clearing overproduced •OH in the lysosome region.
Carbon nanodots (CDs), a novel type of fluorescent carbonaceous nanomaterials, have received tremendous attention in recent years for their water solubility, high resistance to photobleaching, low biotoxicity, good biocompatibility, and wide applications in biological fields [21,22,23,24]. In this work, we demonstrate the design and construction of lysosome-targetable Se-doped carbon nanodots (Lyso-Se-CDs) that significantly scavenge lysosomal •OH in living cells and mice (Fig. 1). Se-CDs covered with redox-responsive unit (–C–Se) were initially synthesized from selenocystine by a facile hydrothermal method, followed by the surface modification with morpholine, a lysosome targeting moiety. The as-synthesized Lyso-Se-CDs exhibited excellent colloidal stability, robust scavenging abilities towards •OH, low biotoxicity, as well as good biocompatibility and lysosome targetability. Due to these desirable properties, Lyso-Se-CDs had been successfully utilized for rescuing cells from elevated lysosomal •OH levels. More impressively, Lyso-Se-CDs efficiently relieved PMA triggered ear inflammation in live mice.
Experimental section
Preparation of Se-CDs
Se-CDs were prepared from selenocystine through a one-pot, facile hydrothermal carbonization method. In brief, 0.1 g selenocystine was dispersed in 40 mL of redistilled water with a pH value of ca. 10 (adjusted by 1 M NaOH solution) under rapid stirring. The alkaline solution was used for facilitating the dissolution of selenocystine. Then, the aqueous dispersion was heated at a controlled temperature of 200 °C for 4 h in a 50-mL Teflon-lined autoclave. The resulting solution with a brown color was sequentially purified by filtration with a 0.22 μm filter membrane, centrifugation at 12,000 g for 15 min, and dialysis through a dialysis membrane (cutoff: 1000 Da). After freeze-drying of the as-purified solution, Se-CD powder can be obtained. CDs without Se atom doping were prepared in a same way.
Preparation of Lyso-Se-CDs
Morpholine was immobilized on the Se-CD surface through covalently EDC/NHS activated process. Typically, 5.0 mL of morpholine solution (2.5 mM) was placed into a flask, followed by the injection of 100 mg EDC and 100 mg NHS, and activated at room temperature for 4 h. Afterwards, 2.0 mL of Se-CD solution (1.0 mg mL−1) was added, reacted for 12 h to give morpholine-modified Se-CDs, namely, Lyso-Se-CDs.
Cytotoxicity evaluations
HeLa cells were fostered at a density of ~1×104 cells per well in 96-well plates and grown in DMEM containing 10% fetal bovine serum, 80 U mL−1 penicillin, and 80 μg mL−1 streptomycin in a humid incubator with 5% CO2/95% air. After 12 h, the culture media were deserted, and fresh media with serial amounts of Lyso-Se-CDs (0–100 μg mL−1) was introduced, cultured for 48 h. As to each amount, five replicate experiments were performed in parallel. Thereafter, MTT solution (1.0 mg mL–1, 20 μL) was injected into each well, waiting for 4 h to allow the generation of formazan crystals. Subsequently, DMSO with a volume of 150 μL was introduced into the wells. Absorbance (A) of the resulting hybrid was determined. The cellular viability values were evaluated by the following equation: cellular viability (%) = Atest/Acontrol × 100%, where Acontrol represents the absorbance recorded from the control group, and Atest represents the absorbance obtained in the existence of Lyso-Se-CDs.
Fluorescent imaging
Before conducting confocal imaging experiments, HeLa cells were detached, replanted onto confocal dishes (diameter: 35 mm), and adhered for ca. 12 h. Then, the medium in the dish was changed by new one containing Lyso-Se-CDs (20 μg mL−1) and cultured for 4 h. Subsequently, the Lyso-Se-CDs labeled HeLa cells were further labeled with LysoTracker Deep Red (100 nM) for 0.5 h. After finishing the above experiments, the labeled cells were rinsed three times with PBS. Under the excitation of 405 nm, the fluorescence image of Lyso-Se-CDs was acquired within 430–510 nm wavelength region, whereas for LysoTracker Deep Red, a 638-nm semiconductor laser was used, and the fluorescence signal was obtained in the wavelength range of 640–720 nm.
All the animal experiments were conducted with the approvement of the Animals Ethics Committee of East China Normal University. Kunming mice with a body weight about 15 g were randomly divided into four groups (three mice in each group). The mice in group one, acting as the control group, were subcutaneously injected with 2′,7′-dichlorofluorescein diacetate (DCFH-DA, 1 mM, 50 μL) on the right ear. While for the other three groups, PMA acetone solution (100 μg mL−1, 50 μL) was topically applied on the right ear to establish an ear inflammation model. After 6 h, the mice were anesthetized using chloral hydrate solution and subcutaneously administered with DCFH-DA (1 mM, 50 μL) or Lyso-Se-CDs (20 μg mL−1, 100 μL; 40 μg mL−1, 100 μL) and DCFH-DA (1 mM, 50 μL). After another 0.5 h, fluorescent images of the whole body were acquired on an in vivo imaging system (PerkinElmer) with appropriate wavelength (λex = 488 nm; λem = 520 nm).
Results and discussion
Preparation and characterization of Se-CDs
Inspired by the pioneering work reported by Xu and coworkers [25], Se-CDs were initially prepared in our research through a facile hydrothermal method. Briefly, selenocystine was dissolved in an alkaline solution, treated at 200 °C for 4 h, and water soluble Se-CDs can thus be formed. Morphological characterizations of the synthesized Se-CDs were conducted. TEM image in Fig. 2A demonstrates that the Se-CDs are randomly and uniformly distributed, having a finite size distribution in 2.4–3.1 nm range with a mean value of 2.7 nm, as judged from ca. 100 individual particles (Fig. 2B). This size is much smaller in comparison with the Se-CDs reported by Xu [25] and Huang [26]. Moreover, no assembled larger nanoparticles were observed from our Se-CDs, which suggests that the developed protocol had a good control on size. From AFM image in Fig. 2C, we can find that the Se-CDs were mono-dispersed, showing a height of 2.0 nm (Fig. 2D). This result matched well with TEM characterization.
(A) TEM image of the obtained Se-CDs. (B) Size distribution of the Se-CDs. (C) Typical AFM image of the Se-CDs. (D) Height distribution of the Se-CDs along the pink line in Fig. 1C
Then, XPS experiments were performed to analyze the surface states of the Se-CDs. As depicted in Fig. S1A, four predominant peaks were found at 529.6 eV (O1s), 400.9 eV (N1s), 284.5 eV (C1s), along with 55.2 eV (Se3d), testifying the Se-CDs were made up of oxygen, nitrogen, carbon, and selenium, with the atomic ratio of 10.48%, 21.23%, 63.07%, and 5.22%, respectively [27,28,29]. Specifically, peak deconvolution of the C1s spectrum generates three peaks at 287.9, 286.1, and 284.7 eV, which are corresponded to C=O, C–O/C–N/C–Se, and C–C groups, respectively (Fig. S1B) [30]. The high-resolution XPS spectrum of N1s can be deconvoluted to two peaks at 401.2 and 399.6 eV, attributed to amino N and C–N–C, respectively (Fig. S1C) [31]. The high-resolution XPS spectrum of Se3d (55.2 eV) demonstrates the existence of –C–Se group (Fig. S1D) [32]. In addition, the FTIR spectrum was also determined to investigate the functional groups existed on the surface of Se-CDs (Fig. S2, curve a). The intense absorption band appeared at 3347 cm−1 is attributed to the characteristic stretching vibrations of O–H and N–H [33]. And the absorption peaks at 1570 and 1693 cm−1 is respectively assigned to the C=O stretching vibration of the carbonyl group and N–H [34]. The FTIR data indicated the abundance of –COOH, –OH, and NH2 groups on Se-CDs surface, which is beneficial to its dispersion in aqueous media and the following surface functionalization.
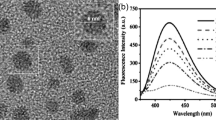
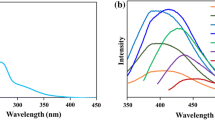
The fluorescent properties of the Se-CDs were subsequently measured. As depicted, under the excitation with a maximum wavelength at 379 nm (Fig. S3A, curve a), an intense emission peak at 471 nm is observed (Fig. S3A, curve b). The emission peak undergoes a hypsochromic shift compared to that of the Se-CDs reported by Xu [25], with a peak position at 490 nm. The differences between the emission position possibly result from the variation in their sizes [35, 36]. The quantum yield of the Se-CDs was determined to be 7.1%. Similar to most of previously reported CDs, the Se-CDs presented here exhibited excitation-energy-dependent fluorescence (Fig. S3B) [37, 38].
As the surface of Se-CDs was functionalized with –C–Se, a unit that can be easily oxidized by oxidant, and the surface states of CDs are correlated with their fluorescence feature, we expected that the Se-CDs would give off redox-responsive fluorescence. To verify our thoughts, H2O2 was added into Se-CD solution, and a dramatical enhancement of its fluorescence was witnessed (Fig. S4, curve b). Concurrently, XPS spectroscopy was utilized to study the states of Se atom in Se-CDs in the presence of H2O2. The oxidation state of Se had been changed and oxidized to selenic acid as expected (Fig. S5A). When glutathione was included in the mixed solution of Se-CDs and H2O2, selenic acid could be reduced to –C–Se again (Fig. S5B), associated with the decrease of the fluorescence intensity (Fig. S4, curve c). In parallel, control experiments were also conducted, in which CDs without Se atom doping were prepared, using cystine as the precursor. In this context, no response of the fluorescence of CDs (λem = 463 nm) to H2O2 was found (Fig. S6). Overall, these results demonstrated that the existence of Se atom and its oxidation state had a quite large impact on the fluorescence emission of Se-CDs.
Synthesis and characterization of Lyso-Se-CDs
For achieving Se-CDs that primarily retained in lysosome, morpholine, an acidotropic group that helps the probe specifically reside in acidic lysosome via the protonation of the amine unit in acidic milieu [39, 40], was decorated on the Se-CD surface. The decoration of morpholine was implemented through the amidation between –COOH group of 3-morpholinopropanoic acid and –NH2 group of Se-CDs. The covalent conjugation process, activated by EDC/NHS, was characterized by FTIR spectroscopy. 3-morpholinopropanoic acid (Fig. S2, curve b) shows the typical absorption bands of carboxyl group at 3347 cm−1 (VO−H) and 1693 cm−1 (VC=O). The newly generated absorption band at 1612 cm−1 in Lyso-Se-CDs spectrum suggests the formation of amide group (Fig. S2, curve c) and the successful anchor of morpholine on the Se-CD surface. Besides, the dynamic light scattering results showed that Lyso-Se-CDs had a larger hydrodynamic size compared to that of Se-CDs, which again proved the attachment of morpholine on the Se-CD surface (Fig. S7).
It is worthy to state that the conjugation of morpholine on the surface of Se-CDs showed no discernible effect on its shape, size (Fig. S8), and fluorescence (Fig. S9). Following, the stability of Lyso-Se-CDs in PBS was analyzed over a period of 7 days. During this period, both the hydrodynamic diameter and fluorescence intensity kept almost constant (Fig. S10), indicative of the high colloidal stability of Lyso-Se-CDs.
ROS scavenging activity of Lyso-Se-CDs
The presence of redox-responsive unit –C–Se on the Lyso-Se-CDs surface suggested its potential usage for eliminating •OH, the most reactive ROS. For analyzing its scavenging activity towards •OH, EPR experiments, a direct method to detect ROS, were conducted, choosing 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as the spin trap agent. In the following experiments, Fe2+/H2O2, the classical Fenton reaction system, was served as the source to afford •OH. As seen in Fig. 3A (curve a), DMPO molecule showed no EPR signal. Once •OH was injected to the DMPO solution, a characteristic EPR signal of DMPO-OH adduct appeared, with a 1:2:2:1 quarter pattern (Fig. 3A, curve b) [41]. Gladly, as anticipated, the introduction of Lyso-Se-CDs weakened the EPR intensity in a dose-dependent manner (Fig. 3A, curve c–f; Fig. S11A). The intensity of EPR signal decreased to 40.7%, with the existence of Lyso-Se-CDs at concentration of 40 μg mL−1, reflecting the strong •OH scavenging power of Lyso-Se-CDs. Fluorescence assays further manifested a sensitive response of Lyso-Se-CDs to •OH (Fig. S12). Moreover, Lyso-Se-CDs also showed an excellent scavenging capacity towards O2•− (Fig. 3B; Fig. S11B). Notably, the scavenging behavior of Lyso-Se-CDs is very similar to that of Se-CDs (Fig. S13), disclosing that conjugation of morpholine on the Se-CD surface had no recognizable influence on its antioxidant capacity.
(A) EPR spectra of DMPO under different experimental conditions. Curve a: DMPO alone; curve b: DMPO + •OH (10 μM); curve c: DMPO + •OH (10 μM) + Lyso-Se-CDs (10 μg mL−1); curve d: DMPO + •OH (10 μM) + Lyso-Se-CDs (20 μg mL−1) ; curve e: DMPO + •OH (10 μM) + Lyso-Se-CDs (30 μg mL−1); curve f: DMPO + •OH (10 μM) + Lyso-Se-CDs (40 μg mL−1). The concentration of DMPO was 50 mM. (B) Fluorescence spectra of hydroethidine under various treatments. Curve a: hydroethidine alone; curve b: hydroethidine + O2•− (10 μM); curve c: hydroethidine + O2•− (10 μM) + Lyso-Se-CDs (10 μg mL−1); curve d: hydroethidine + O2•− (10 μM) + Lyso-Se-CDs (20 μg mL−1); curve e: hydroethidine + O2•− (10 μM) + Lyso-Se-CDs (30 μg mL−1); curve f: hydroethidine + O2•− (10 μM) + Lyso-Se-CDs (40 μg mL−1). The concentration of hydroethidine was 2 μM
Intracellular •OH scavenging activity of Lyso-Se-CDs
After in vitro affirming the scavenging ability of Lyso-Se-CDs against •OH, then, its feasibility to scavenging intracellular •OH was tested. As such, the biotoxicity of Lyso-Se-CDs was checked by MTT assay, in which HeLa cells were treated for 48 h with varied concentrations of Lyso-Se-CDs. As illustrated in Fig. 4A, Lyso-Se-CDs still exerted feeble side-effects on cellular viabilities even at a concentration up to 100 μg mL−1, with a value over 90%, denoting low biotoxicity of the Lyso-Se-CDs. Additionally, flow cytometry experiments were also performed (Fig. S14). And the data showed that Lyso-Se-CDs had a good biocompatibility.
(A) Viabilities of HeLa cells incubated for 48 h with series amounts of Lyso-Se-CDs. (B) Subcellular localization investigations in HeLa cells that were counterstained with Lyso-Se-CDs and LysoTracker Deep Red: a: blue fluorescence from Lyso-Se-CDs (λem = 430–510 nm); b: red fluorescence from LysoTracker Deep Red (λem = 640–720 nm); c: the merged image of a with b; d: bright-field image; e: the merged image of c with d; f: the emission correlation plot of the blue channel with red channel. (C) Protective power of Lyso-Se-CDs to HeLa cells against oxidative injury induced by •OH (100 μΜ). Data shown are average values, and standard deviations (error bars) are obtained from five independent experiments
Due to Lyso-Se-CDs were engineered with morpholine, its intracellular localization to lysosome was then explored by confocal microscopy experiments. To do so, HeLa cells pretreated with Lyso-Se-CDs were counterstained with LysoTracker Deep Red, a commercial lysosome marker. Figure 4B shows the corresponding fluorescence images of HeLa cells counterstained with Lyso-Se-CDs and LysoTracker Deep Red. The subcellular location of the blue fluorescence signal from Lyso-Se-CDs (Fig. 4Ba) and the deep red emission from LysoTracker Deep Red (Fig. 4Bb) were compared. We can easily find that a large degree of overlap exists between the two images, as visualized from the bright pink signals in Fig. 4Bc, the merged image. Moreover, the intensity correlation plot analysis shows Pearson’s coefficient with a high value of 0.91 (Fig. 4Bf). These observations, together, solidly demonstrate that Lyso-Se-CDs, with morpholine grafted on surface, specifically reside in lysosome.
In biosystems, oxidative stress happens when the production of ROS surpasses the antioxidant capability of cellular antioxidants, resulting in severe damage to lipids, proteins, and nucleic acids. Since Lyso-Se-CDs exhibited remarkable •OH-scavenging ability, low biotoxicity, as well as good biocompatibility and lysosome targetability, we investigated if Lyso-Se-CDs could suppress oxidative damage from •OH within the lysosome matrix. Here, the Fe2+/H2O2 system was selected as an injury model to evaluate the protection capability of Lyso-Se-CDs from oxidative stress. The results are presented in Fig. 4C. It was evident that Lyso-Se-CDs, the first antioxidant with lysosome targeting capacity (Table S1), could dose-dependently inhibit •OH-induced decline of cell viabilities.
In vivo anti-inflammation
We further studied the in vivo anti-inflammation behavior of Lyso-Se-CDs. In the experiments, an ear inflammation model was established via topical application of PMA, an apoptotic drug that initiates cell and/or tissue inflammation [42]. As shown in the control mouse (treated with DCFH-DA alone), no obvious fluorescence was observed from the right ear (Fig. 5A), suggesting a low level of ROS. By contrast, an intense
(A) In vivo fluorescence imaging of mice treated with DCFH-DA (1 mM, 50 μL) alone in the right ear region. (B) In vivo fluorescence imaging of mice sequentially treated with PMA (100 μg mL−1, 50 μL) and DCFH-DA (1 mM, 50 μL). (C) In vivo fluorescence imaging of mice treated with PMA (100 μg mL−1, 50 μL), Lyso-Se-CDs (20 μg mL−1, 100 μL), and DCFH-DA (1 mM, 50 μL) in order. (D) In vivo fluorescence imaging of mice treated with PMA (100 μg mL−1, 50 μL), Lyso-Se-CDs (40 μg mL−1, 100 μL), and DCFH-DA (1 mM, 50 μL) in order. (E) Relative fluorescence intensity in panel A–D. All the fluorescence intensities were compared to panel B, which was normalized to 1.0. (F–I) Corresponding HE stained images in panel A–D
fluorescence was observed from the ear treated with DCFH-DA and PMA, revealing the elevated level of ROS in this process (Fig. 5B). After subcutaneous treatment with Lyso-Se-CDs, the fluorescence in the inflamed ear drastically decreased (Fig. 5C, D, and E). Hematoxylin and eosin (HE) stained images of ears were also captured. As displayed in Fig. 5G, lymphocyte infiltration was obviously observed from the slice obtained from PMA-treated mouse, in sharp contrast to the control group (the healthy mouse, Fig. 5F). When further treated with Lyso-Se-CDs, the symptom was attenuated (Fig. 5H, I). All these results suggested that the Lyso-Se-CDs with low biotoxicity and good biocompatibility possessed efficient ROS scavenging ability against ear inflammation.
Conclusions
In conclusion, in this work, we reported the design and construction of Lyso-Se-CDs that can significantly diminish lysosomal •OH in living cells and mice. Further studies revealed that the Lyso-Se-CDs exhibited excellent colloidal stability, robust scavenging abilities towards •OH, low biotoxicity, as well as good biocompatibility and lysosome targetability. Due to these desirable properties, Lyso-Se-CDs had been applied to protect cells from elevated lysosomal •OH levels. More impressively, Lyso-Se-CDs efficiently relieved PMA triggered ear inflammation in live mice. These findings reveal Lyso-Se-CDs are potent candidates for treating •OH-related inflammation and their potential applications in the fields of biology and medicine.
References
Hou J-T, Yu K-K, Sunwoo K, Kim WY, Koo S, Wang J, Ren WX, Wang S, Yu X-Q, Kim JS (2020) Fluorescent imaging of reactive oxygen and nitrogen species associated with pathophysiological processes. Chem 6:832–866
Li H, Ma H (2018) New progress in spectroscopic probes for reactive oxygen species. J Anal Test 2:2–19
Zhen X, Pu K (2018) Development of optical nanoprobes for molecular imaging of reactive oxygen and nitrogen species. Nano Res 11:5258–5280
Huang H, Tian Y (2018) A ratiometric fluorescent probe for bioimaging and biosensing of HBrO in mitochondria upon oxidative stress. Chem Commun 54:12198–12201
Liu Z, Tian Y (2021) Recent advances in development of devices and probes for sensing and imaging in the brain. Sci China Chem. https://doi.org/10.1007/s11426
Kowaltowski AJ, De Souza-Pinto NC, Castilho RF, Vercesi AE (2009) Mitochondria and reactive oxygen species. Free Radic Biol Med 47:333–343
Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J (2004) Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem 266:37–56
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Markesbery WR, Carney JM (1999) Oxidative alterations in Alzheimer's disease. Brain Pathol 9:133
Kwon HJ, Cha M-Y, Kim D, Kim DK, Soh M, Shin K, Hyeon T, Mook-Jung I (2016) Mitochondria-targeting ceria nanoparticles as antioxidants for Alzheimer’s disease. ACS Nano 10:2860–2870
Kwon HJ, Kim D, Seo K, Kim YG, Han SI, Kang T, Soh M, Hyeon T (2018) Ceria nanoparticle systems for selective scavenging of mitochondrial, intracellular, and extracellular reactive oxygen species in Parkinson's disease. Angew Chem Int Ed 57:9408–9412
Yao J, Cheng Y, Zhou M, Zhao S, Lin S, Wang X, Wu J, Li S, Wei H (2018) ROS scavenging Mn3O4 nanozymes for in vivo anti-inflammation. Chem Sci 9:2927–2933
Bao X, Zhao J, Sun J, Hu M, Yang X (2018) Polydopamine nanoparticles as efficient scavengers for reactive oxygen species in periodontal disease. ACS Nano 12:8882–8892
Liu T, Xiao B, Xiang F, Tan J, Chen Z, Zhang X, Wu C, Mao Z, Luo G, Chen X, Deng J (2020) Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat Comomun 11:2788
Li H, Cao X, Fei X, Zhang S, Xian Y (2019) Nanoscaled luminescent terbium metal–organic frameworks for measuring and scavenging reactive oxygen species in living cells. J Mater Chem B 7:3027–3033
Huang Y, Liu C, Pu F, Liu Z, Ren J, Qu X (2017) A GO-Se nanocomposite as an antioxidant nanozyme for cytoprotection. Chem Commun 53:3082–3085
Liu Y, Cheng Y, Zhang H, Zhou M, Yu Y, Lin S, Jiang B, Zhao X, Miao L, Wei C-W, Liu Q, Lin Y-W, Du Y, Butch CJ, Wei H (2020) Integrated cascade nanozyme catalyzes in vivo ROS scavenging for anti-inflammatory therapy. Sci Adv 6:eabb2695
Wu H, Li F, Wang S, Lu J, Li J, Du Y, Sun X, Chen X, Gao J, Ling D (2018) Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 151:66–77
Wu Y, Huang W, Peng D, Huang X-A, Gu J, Wu S, Deng T, Liu F (2021) Synthesis of dihydroquinolines as scaffolds for fluorescence sensing of hydroxyl radical. Org Lett 23:135–139
Benitez-Martin C, Guadix JA, Pearson JR, Najera F, Perez-Pomares JM, Perez-Inestrosa E (2019) A turn-on two-photon fluorescent probe for detecting lysosomal hydroxyl radicals in living cells. Sensors Actuators B Chem 284:744–750
Li F, Yang D, Xu H (2019) Non-metal-heteroatom-doped carbon dots: synthesis and properties. Chem Eur J 25:1165–1176
Zhou J, Zhou H, Tang J, Deng S, Yan F, Li W, Qu M (2017) Carbon dots doped with heteroatoms for fluorescent bioimaging: a review. Microchim Acta 184:343–368
Zhou D, Huang H, Wang Y, Wang Y, Hu Z, Li X (2019) A yellow-emissive carbon nanodot-based ratiometric fluorescent nanosensor for visualization of exogenous and endogenous hydroxyl radicals in the mitochondria of live cells. J Mater Chem B 7:3737–3744
Liu Z, Pei H, Zhang L, Tian Y (2018) Mitochondria-targeted DNA nanoprobe for real-time imaging and simultaneous quantification of Ca2+ and pH in neurons. ACS Nano 12:12357–12368
Li F, Li T, Sun C, Xia J, Jiao Y, Xu H (2017) Selenium-doped carbon quantum dots for free-radical scavenging. Angew Chem Int Ed 56:9910–9914
Rosenkrans ZT, Sun T, Jiang D, Chen W, Barnhart TE, Zhang Z, Ferreira CA, Wang X, Engle JW, Huang P, Cai W (2020) Selenium-doped carbon quantum dots act as broad-spectrum antioxidants for acute kidney injury management. Adv Sci 7:2000420
Su R, Shi J, Pu Y, Wang J-X, Wang D, Chen J-F (2020) Synthesis of ultrasmall and monodisperse selenium-doped carbon dots from amino acids for free radical scavenging. Ind Eng Chem Res 59:16876–16883
Zhu J-H, Feng Y-G, Wang A-J, Mei L-P, Luo X, Feng J-J (2021) A signal-on photoelectrochemical aptasensor for chloramphenicol assay based on 3D self-supporting AgI/Ag/BiOI Z-scheme heterojunction arrays. Biosens Bioelectron 181:113158
Shen Z, Cai Q, Yin C, Xia Q, Cheng J, Li X, Wang Y (2020) Facile synthesis of silica nanosheets with hierarchical pore structure and their amine-functionalized composite for enhanced CO2 capture. Chem Eng Sci 217:115528
Luo W, Wang Y, Lin F, Liu Y, Gu R, Liu W, Xiao C (2020) Selenium-doped carbon quantum dots efficiently ameliorate secondary spinal cord injury via scavenging reactive oxygen species. Int J Nanomedicine 15:10113–10125
Ye X, Xiang Y, Wang Q, Li Z, Liu Z (2019) A red emissive two-photon fluorescence probe based on carbon dots for intracellular pH detection. Small 15:1901673
Huang H, Shen Z, Chen B, Wang X, Xia Q, Ge Z, Wang Y, Li X (2020) Selenium-doped two-photon fluorescent carbon nanodots for in-situ free radical scavenging in mitochondria. J Colloid Interface Sci 567:402–409
Simões EFC, Leitão JMM, Esteves Da Silva JCG (2017) Sulfur and nitrogen co-doped carbon dots sensors for nitric oxide fluorescence quantification. Anal Chim Acta 960:117–122
Bauer C, Jacques P, Kalt A (1999) Investigation of the interaction between a sulfonated azo dye (AO7) and a TiO2 surface. Chem Phys Lett 307:397–406
Wang Y, Hu A (2014) Carbon quantum dots: synthesis, properties and applications. J Mater Chem C 2:6921
Li H, He X, Kang Z, Huang H, Liu Y, Liu J, Lian S, Tsang CHA, Yang X, Lee ST (2010) Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew Chem 122:4532–4536
Lu S, Sui L, Liu J, Zhu S, Chen A, Jin M, Yang B (2017) Near-infrared photoluminescent polymer–carbon nanodots with two-photon fluorescence. Adv Mater 29:1603443
Dam BV, Nie H, Ju B, Marino E, Paulusse JMJ, Schall P, Li M, Dohnalová K (2017) Excitation-dependent photoluminescence from single-carbon dots. Small 13:1702098
Liu H, Sun Y, Li Z, Yang J, Aryee AA, Qu L, Du D, Lin Y (2019) Lysosome-targeted carbon dots for ratiometric imaging of formaldehyde in living cells. Nanoscale 11:8458–8463
Wang Q, Zhou L, Qiu L, Lu D, Wu Y, Zhang X-B (2015) An efficient ratiometric fluorescent probe for tracking dynamic changes in lysosomal pH. Analyst 140:5563–5569
Huang H, Lou X, Hu B, Zhou Z, Chen J, Tian Y (2019) A comprehensive study on the generation of reactive oxygen species in Cu-Aβ-catalyzed redox processes. Free Radic Biol Med 135:125–131
Wu J, Yu Y, Cheng Y, Cheng C, Zhang Y, Jiang B, Zhao X, Miao L, Wei H (2021) Ligand-dependent activity engineering of glutathione peroxidase-mimicking MIL-47(V) metal–organic framework nanozyme for therapy. Angew Chem Int Ed 60:1227–1234
Funding
The authors gratefully thank the Zhejiang Provincial Natural Science Foundation of China (Grant No. LQ20B050003) and Grant from the Scientific Research Start-up Project Foundation of Jiaxing University (CD70519012).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Nanomaterials for biomedical imaging and targeting
Supplementary Information
ESM 1
(DOCX 2128 kb)
Rights and permissions
About this article
Cite this article
Zhou, D., Huang, H., Yu, J. et al. Lysosome-targetable selenium-doped carbon nanodots for in situ scavenging free radicals in living cells and mice. Microchim Acta 188, 223 (2021). https://doi.org/10.1007/s00604-021-04883-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04883-1