Abstract
This work describes an anti-ovalbumin antibody-based lateral flow immunoassay (LFI) for T-2 toxin. The antibody uses a coating antigen as a bifunctional element for universality and introduces preincubation to improve the detection limits of the method. T-2 toxin and ovalbumin-modified T-2 toxin competitively binds on the anti-T-2 toxin monoclonal antibody modified on CdSe/ZnS quantum dot beads during preincubation. The modified T-2 toxin acts as a bifunctional element that forms immuno complexes during preincubation and combines with anti-ovalbumin antibody coated in the test line through the ovalbumin terminal. Fluorescence is detected at 610 nm on the test zone following photoexcitation at 365 nm. It has a reverse dose-effect relationship with the amount of T-2 toxin. The calibration plot is linear in the 20–110 fg mL−1 T-2 toxin concentration range, and the limit of detection (LOD) is 10 fg mL−1, which is lower by 8-fold than that of the traditional LFI system (LOD 80 fg mL−1) and one order of magnitude than those of LFIs with labels of colloidal gold nanoparticles (LOD 150 fg mL−1) or fluorophores (LOD 190 ng mL−1). Universality was verified through aflatoxin B1 detection using the established ovalbumin antibody-based LFI system (LOD 10 fg mL−1). The performance of the method was compared with that of established systems and a commercial ELISA kit (LOD 360 fg mL−1).

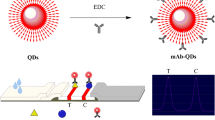
Schematic representation of ovalbumin antibody-based immunochromatographic lateral flow assay for T-2 toxin. Preincubation is introduced for high sensitivity. T-2- anti-ovalbumin acts as a bi-functional element for universality. CdSe/ZnS quantum dot beads act as label. Fluorometric signal is detected at 610 nm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In lateral flow immunochromatographic (LFI) systems, a specific recognition element is confined onto the surface of the porous membrane and the flow of the sample and reagents are sustained by capillarity force. LFI technology has the inherent advantages of rapidity, simplicity, cost-effectiveness, no requirement of technical expertise, and application in diverse fields, such as food safety, environmental analysis, and veterinary.
LFI is traditionally developed for the detection of a single compound per assay. LFI has been modified, such as by improving the sensitivity through novel labels and the throughput via multiplexing, to enhance its detection efficiency [1,2,3,4,5,6,7,8,9,10,11,12]. A recent work established carbon nanotube-based LFI for ultrasensitive detection [13]. The sensitivity of LFI was increased by 30-fold with the use of spherical core-shell gold-silica nanoparticles (AuNP@SiO2NPs) [14]. High sensitivity was also achieved by applying a “turn on” mode [15, 16]. However, one ubiquitous characteristics of the existing LFI systems is that the target-specific antigen or antibody immobilized on the testing zone hinders the development of universality, which is another crucial property. LFI systems with sensitivity and universality have not been reported. The immune recognition activated by the flow of the sample and reagents mixed by capillarity force on the testing zone is transient and largely uncontrolled, leading to insufficient reaction and thus limited sensitivity, especially in competitive immunoassay.
A LFI system with combined sensitivity and universality was established for T-2 detection using quantum dot beads (QBs) as label. T-2 toxin is a heat-stable trichothecene produced particularly by the Fusarium species, is abundant in crops, and poses a health risk through the ingestion of milk, meat, and eggs from poultry and livestock animals fed with trichothecenes-contaminated feed [17, 18]. T-2 toxin is one of the most dangerous contaminants according to the European Food Safety Authority [19]. This substance is nearly 100 times more toxic than mustard in absolute amount in dermal exposure and can be used as biological warfare agent (BWA) in various forms [20]. After the 9/11 attack, the possibility of bioterrorism, including the release of T-2 toxin, has become credible in peacetime. Thus, developing effective methods for T-2 detection, especially for the on-site classification of “supposed released BWA”, is urgently needed.
Traditional ELISA and chromatographic methods are usually highly selective and very accurate, but these methods are sophisticated, tedious, and expensive, which are disadvantageous for on-site fast detection [21,22,23]. On the contrary, LFI was developed as a rapid, specific, and reliable detection method which usually uses luminescence materials and colored nanoparticles as label [24, 25]. Quantun dots (QDs) has attracted interest as an ideal fluorescent label due to its unique property of broad adsorption, narrow emission spectra, high quantum yield, and high photo-stability [26]. Quantum dot beads (QBs) is prepared by doping numerous QDs inside polybeads to further improve the sensitivity of LFI [27]. Ultrasensitive detection using QBs as label has been established [28]. However, a LFI system that is based on QB and has sensitivity and universality for the detection of T-2 toxin has not been reported.
A QB-LFI system with combined sensitivity and universality for T-2 toxin detection was established. Anti-ovalbumin (anti-OVA) antibody acted as the coating element on the testing zone, and traditional coating antigen T-2-OVA was used as the bi-functional element. T-2-OVA competed with T-2 toxin for QB-mAb during preincubation before LFI process. Immune complexes were formed during preincubation and named as QB-mAb-T-2 and QB-mAb-T-2-OVA. The latter combined with anti-OVA antibody through the OVA terminal in the testing zone. After optimization, the limit of detection (LOD) was used to compare the sensitivity of the anti-OVA antibody-based LFI system with that of LFIs that are based on the traditional structure and uses QB, colloidal gold nanoparticles (GNPs), or luminescence materials as signal probes. A similar LFI system using anti-bovine albumin antibody has been investigated, but that using anti-OVA antibody has not been discussed [29]. The result shows that the LOD of the proposed method was improved more than eight times than that based on the novel structure. Furthermore, its universality was validated through aflatoxin B1 detection. Comparative study showed that QB-LFI is more sensitive than conventional ELISA methods. Hence, the anti-OVA antibody-based QB-LFI system has great potential for the rapid, sensitive, quantitative, and universal detection of analytes in biosafety monitoring and on-site classification of the “supposed released BWA.”
Experimental
Materials and instruments
T-2 toxin standard and its metabolites HT-2, T-2 triol and fumonisin B1 (FMB1), zearalenone (ZEN), deoxynivalenol (DON) and Tween-20 were purchased from J&K Scientific Ltd. (Shanghai, China, www.jkchemical.com/Company-inf.aspx?language=ch). T-2 − OVA conjugates, anti-T-2 antibody, anti-OVA antibody, goat anti-mouse IgG antibodies, and the commercial T-2 ELISA kit were supplied by Shandong Landu Bio-Science & Technology Co., Ltd. (Shandong, China, www.11467.com/qiye/45274356.htm). Carboxylate-modified QBs with excitation and emission wavelength of 365 nm and 610 nm, respectively (Cat: FM610C), were supplied by Beijing Najing Biological Technology Co., Ltd. (Beijing, China, www.najingbio.com/) and used as label in LFI system. Nitrocellulose (NC) membrane, glass fiber, absorbent pad, and polyvinylchloride (PVC) backing card were obtained from Kinbio Tech. Co., Ltd. (Shanghai, China, www.goldbio.cn/article-item-288.html). All other reagents (analytical grade) were supplied by the National Pharmaceutical Group Co., Ltd. (Shanghai, China, www.sinopharm.com/1156.html).
Ultrapure water was obtained from a Milli-Q purification system (Millipore Co., Bedford, MA, USA, http://www.well-honor.com/goods1-202.html) for the preparation of buffers. BioJet Quanti 3000™ which was used for antibody immobilization on NC membrane and an automatic cutter were supplied by Kinbio Tech. Co., Ltd. (Shanghai, China, www.goldbio.cn/article-item-288.html). The employed QB-LFI strip reader which was supplied by Beijing Najing Biological Technology Co., Ltd. (Beijing, China, www.najingbio.com/) excited QB at 365 nm and the signal was collected at 610 nm.
Preparation of the QB-mAbs
QB surface was modified with anti-T-2 mAbs using active ester method. Briefly, carboxyl groups on QB were activated by adding 0.96 μL of 10 mg·mL−1 EDC·HCl and 1.64 μL of 10 mg·mL−1 NHS to 50 μL of QB solution. The solution was then incubated under 37 °C for 15 min. After centrifugation and re-suspension by MES buffer (pH 6.0, 10 mmol·L−1), anti-T-2 mAbs was added to the solution under gentle stirring (800 rpm) and incubated under 37 °C for 1 h. Then, the QB-mAbs were further blocked by glycine solution (100 mmol·L−1, containing 10% skimmed milk powder) under 37 °C for 30 min. The mixture was then centrifuged, the supernatant was discarded, and the precipitates were re-suspended and stored in protein stabilizer solution(Cat: PR-SS-002, Huzhou Yingchuang Biological Technology Co., Ltd., Huzhou, China) at 4 °C until use.
Fabrication of the QB-LFI system
The principle of QB-LFI system is shown in Fig. 1. Similar to traditional LFI structure, the QB-LFI system also contains three parts: sample pad (using glass fiber instead), NC membrane, and absorbent pad. In the PVC backing card, NC membrane was positioned in the middle, and the glass fiber and absorption pads overlapped for nearly 2 mm on both ends. The anti-OVA antibody (0.25 mg·mL−1) and goat anti-mouse IgG antibodies (0.5 mg·mL−1) were immobilized (densities of 3 μL cm−1) in the NC membrane as test and control lines with distance of 5 mm. The formed LFI system was then dried at 37 °C for 2 h, cut into strips with width of 3.5 mm using an automatic cutter, and stored in a plastic bag containing desiccant gel at 4 °C until use.
Schematic of OVA antibody-based LFI assay using CdSe/ZnS QBs as label for T-2 toxin detection. The T-2 toxin and T-2-OVA competitively bind for anti-T-2-mAb modified on QB during preincubation. T-2-OVA was a bi-functional element, which formed immune complexes during preincubation and combined with anti-OVA antibody coated in the test line through the OVA terminal. Complexes formed by T-2 toxin and QB-mAb were captured by anti-IgG antibody in the control line
Quantitative procedure of the QB-LFI system
QB-LFI system introduced preincubation and anti-OVA antibody. Coating antigen (T-2-OVA), which was traditionally immobilized in the testing line on NC membrane, was replaced by anti-OVA antibody. Instead, T-2-OVA competed with the analyte (T-2) for QB-mAb in the introduced preincubation process. In preincubation stage, the parameters of the mixed solution such as incubation time, ion strength, pH value, and optimal amount of reagent involved in competitive recognition, was optimized, thus forming the two complexes, QB-mAb-T-2-OVA and QB-mAb-T-2. The former was captured by anti-OVA antibody in the testing line through the OVA terminal, and fluorescent intensity (FIT) was detected. The latter was captured by goat anti-mice antibody in the control line, of which the fluorescent intensity was marked as FIC. As T-2 toxin concentration increased, QB-mAb became more prone to conjugation, leading to fewer QB-mAb-T-2-OVA and ultimately weaker FIT. QB-mAbs probed at a dilution ratio of 1:800 and 0.1 μg of T-2-OVA were premixed in 100 μL of the target solution in a microplate at 37 °C for 11 min. Then, QB-LFI strip was dipped into the mixture and collected FIT after a 9-min immunochromatographic period. FIT at 610 nm was inversely proportional to T-2 toxin content. A calibration plot was established by plotting the B/B0 × 100% against the T-2 toxin content in logarithm, where B and B0were FIT of the standard solutions with and without T-2 toxin, respectively. The standard T-2 toxin solutions were made by serial dilutions of 100 μg mL−1 T-2 toxin solution in PB buffer (pH 5.6, 0.05 mol·L−1) to a final concentration of 0 (as negative control), 0.01, 0.02, 0.04, 0.08, 0.16, 0.32, and 0.64 ng·mL−1.
Spiked tap water samples and pretreatment
Water, which is commonly used in agriculture, is one of the most possibly contaminated resources and is a potential biological warfare agent, which would pose risks to human health. Tap water samples were collected in our lab, filtered using a 0.22-μm PTFE filter (Phenomenex, Torrance, CA), and mixed with equal volume of PB (0.1 mol·L−1, pH 5.6, containing 10% sucrose, 4% fructose, 2% PEG 20000, and 2% Tween-20) prior to T-2 toxin LFI detection. Accuracy and precision analysis were performed by detecting T-2 toxin-spiked standard solution with concentrations of 0.15, 0.30, and 0.60 ng·mL−1. All LFI tests were carried out in triplicates. LFI results were also compared with QB-LFI based on traditional structure and reported T-2 toxin-detecting LFI using GNPs. The assay using fluorescent material were summarized in Table 1.
Comparative evaluation
Comparative evaluation was carried out between the results obtained by QB-LFI and commercial ELISA Kit with spiked T-2 toxin concentrations over the range of 0.30–1.20 ng·mL − 1. Sample pretreatment for the commercial ELISA kit was in accordance with the manufacturer’s instructions.
Results and discussion
The target on the T line coated target-modifying protein in traditional LFI is not fully exposed, which leads to weaker competitiveness against free target and low sensitivity. The modified LFI system overcame the abovementioned limitation. QB was selected as label for the modified LFI system The schematic diagram of the QB-LFI system is shown in Fig. 1. Preincubation, which was introduced to improve sensitivity, included the competitive binding of T-2 toxin and T-2-OVA for QB-mAb in the mixture solution under optimal conditions. T-2 toxin-specific mAb modified on QB determined the specificity of the binding and formed the immune complex, QB-mAb-T-2-OVA, which was captured through the OVA terminal by the anti-OVA antibody in T line for signaling. The universality of the QB-LFI strip was due to the specific recognition of the OVA terminal of the formed immune complexes by the anti-OVA antibody in T line. Thus, any target that is modified with OVA and has formed OVA terminal-containing immune complexes would be captured by the QB-LFI strip.
The FIT detected at 610 nm on the test line was stimulated at 365 nm and had a reverse dose-effect relationship with T-2 toxin concentration. With high T-2 concentration in the solution, many QB-mAb-T-2 complexes were formed. This immune complex was not captured by the anti-OVA antibody in the T line because it does not contain an OVA terminal, thus weaker FIT was reported. The novel LFI system was optimized in a competitive mode and the parameter which resulted to highest inhibition was chosen.
Optimization
The following parameters were optimized to achieve the best performance of the LFI system: (a) labeling amount of anti-T-2-mAb on QB, (b) T-2-OVA concentration in preincubation, (c) sample pH value, (d) ionic strength, (e) anti-OVA antibody concentration, (f) Tween-20 concentration, (g) incubation time, and (h) LFI time. Respective texts and figures on the optimization are given in the Electronic Supporting Material (Fig. S1 and S2). The following experimental conditions produce the best results: (a) 0.3 mg of anti-T-2-mAb per 1 mL of commercial QB solution, (b) 4 μL of 0.025 mg.mL−1 T-2-OVA, (c) sample pH of 5.6, (d) ionic strength of 0.05 mol·L−1, (e) 0.25 mg·mL−1 anti-OVA antibody in T line, (f) tween-20 concentration of 1% (v/v), (g) incubation time of 11 min, and (h) LFI time of 11 min.
Analytical performance
Figure 2a shows that FIT decreased with increasing T-2 toxin concentration (0, 0.04, 0.08, 0.16, and 0.32 ng·mL−1 in sequence). A calibration plot for the spiked T-2 toxin concentration from 0 ng·mL−1 to 0.625 ng·mL−1 against the relative intensity was established. A linear range from 0.02 ng·mL−1 to 0.11 ng·mL−1 was observed in Fig. 2b. LFI detection was conducted in triplicates, and the results from the novel structure were compared with those of traditional LFI systems with labels of QB, GNPs, and fluorescent materials as shown in Table 1.
LOD was defined as the concentration of T-2 toxin, at which 10% inhibition (IC10) of the total FIT difference was reached. Under optimal conditions, the LOD of the constructed calibration plot was 0.01 ng·mL−1, which is about 8 and 15 times lower than those of the traditional LFI system using QB (LOD = 0.08 ng·mL−1) and GNPs (LOD = 0.15 ng·mL−1) as labels, respectively, and about 19 times lower than those of assay using fluorescent material (LOD = 0.19 ng·mL−1) as label. The specificity of the QB-LFI system was investigated using cross-reaction (CR) value among structural analogs such as HT-2, T-2 triol, and other common toxins such as ZEN, DON, and FMB1. CR value was calculated using the equation [31]:
Figure 3 showed that only the structural analogues HT-2 and T-2 triol with CR values of 6.49% and 17.26%, respectively, showed notable CR values while for other toxins such as DON, ZEN, and FMB1 CR values were lower than 0.01, which suggests the LFI is specific.
The recovery of the intra- and inter-assay was evaluated to characterize the accuracy and precision of QB-LFI system. Accuracy was calculated in three replicates of the spiked standard solutions with concentrations of 0.04, 0.08, and 0.16 ng·mL−1, and precision was investigated in three replicates of intra-assay. As shown in Table 3, the average recoveries ranged from 84.15% to 111.89%, which is acceptable for LFI quantitative analysis [32, 33].
The universality of the LFI system was validated by the detection of aflatoxin B1 (AFB1) using the same anti-OVA antibody coated LFI strip and the abovementioned screened optimal parameters. The only difference for this experimental set-up was that the antibody modified on QB surface was AFB1-specific and AFB1-OVA was used for competitive recognition in preincubation. Results showed LOD of 0.01 ng·mL−1, a linear range (IC20 – IC80) of 0.016 to 0.068, and IC50 of 0.033 ng·mL−1, which clearly demonstrated that different targets could be successfully detected using the same LFI strip (Table 2).
Determination of T-2 toxin in tap water
The performance of QB-LFI system was compared with that of a commercial ELISA kit by analyzing three T-2 toxin spiked tap water samples. As shown in Table 3, the two methods verified the results. QB-LFI took nearly 20 min (preincubation and LFI were 11 min 9 min, respectively) to complete one sample analysis, whereas traditional ELISA required approximately 90 min. The introduced preincubation increased the complexity, but QB-LFI was still suitable for on-site use. The LFI results also validated the applicability of the established QB-LFI method.
The QB showed contrast to standard fluorophores by broadband absorption spectrum, narrow fluorescent emission spectra, large stokes shifts, high quantum yield, high photochemical stability, and higher fluorescence intensity than traditional quantum dot [27]. Combining with a spectral demixing algorithm of the strip reader, a Background-free fluorescence signal can be collected in combination with spectral demixing algorithm of the strip reader [34]. Quantitative interpretation is specific for the data collected at a specific time. Preincubation increased the complexity of the method and is a disadvantage for the new LIF system. Thus, fixed timing for every step should be strictly applied.
Conclusion
Anti-OVA antibody-based sensitivity and universality combined QB-LFI system for T-2 toxin was successfully established. QB-LFI system was systematically optimized and the LOD for T-2 toxin in standard solutions was 0.01 ng·mL−1, which was improved by approximately one order of magnitude compared with traditional LFI systems using QB and GNPs as labels, and was much better than that of bioassay using fluorescent material. The CVs for intra- and inter-assay representing the accuracy and precision were below 10%, which is acceptable for LFI system. The recovery performance of QB-LFI system on detecting spiked tap water samples were comparable with those based on a commercial ELISA kit. High sensitivity was established by the introduced preincubation, in which T-2 toxin and T-2-OVA competed for QB-mAb under optimal conditions. Universal detection of the sensitive LFI system was realized by coating anti-OVA antibody in the T line of the LFI strip, which captured the OVA-terminal-containing immune complexes formed in preincubation. Any OVA-modified target competes with other targets for the anti-target antibody modified QB in preincubation, forms OVA-terminal-containing complexes, and is captured by the anti-OVA antibody coated T line. Thus, the new LFI system has combined sensitivity and universality. Universality was further validated by the detection of T-2 toxin and aflatoxin B1 using the same LFI strip. The universal LFI system was beneficial for quantitative detection, comparative studies, and construction of more reliable big data system. In conclusion, the QB-LFI system could be a reference for the development of LFI assays with combined sensitivity and universality using other labels. Furthermore, QB-LFI system can be an alternative on-site quantitative detection of contaminants for biosafety monitoring or even susceptibility detection of “supposed released BWA”.
References
Anfossi L, Di Nardo F, Cavalera S, Giovannoli C, Baggiani C (2019) Multiplex lateral flow immunoassay: an overview of strategies towards high-throughput point-of-need testing. Biosensors 9:2–14. https://doi.org/10.3390/bios9010002
Zhao S, Wang S, Zhang S, Liu J, Dong Y (2018) State of the art: lateral flow assay (LFA) biosensor for on-site rapid detection. Chin Chem Lett 29:1567–1577. https://doi.org/10.1016/j.cclet.2017.12.008
Wu C, Hu L, Xia J, Xu G, Luo K, Liu D, Duan H, Cheng S, Xiong Y, Lai W (2017) Comparison of immunoassays based on fluorescent microsphere and quantum-dot submicrobead for quantitative detection of aflatoxin M1 in milk. J Dairy Sci 100:2501–2511. https://doi.org/10.3168/jds.2016-12065
Sheng W, Li S, Liu Y, Wang J, Zhang Y, Wang S (2017) Visual and rapid lateral flow immunoassay for enrofloxacin using dyed polymer microspheres and quantum dots. Mikrochim Acta 184:4313–4321. https://doi.org/10.1007/s00604-017-2474-y
Luo K, Hu L, Guo Q, Wu C, Wu S, Liu D, Xiong Y, Lai W (2017) Comparison of 4 label-based immunoassays for the detection of Escherichia coli O157:H7 in milk. J Dairy Sci 100:5176–5187. https://doi.org/10.3168/jds.2017-12554
Xing KY, Peng J, Liu DF, Hu LM, Wang C, Li GQ, Zhang GG, Huang Z, Cheng S, Zhu FF, Liu NM, Lai WH (2018) Novel immunoassay based on Eu (III)-doped polystyrene nanoparticle-linker-monoclonal antibody for sensitive detection of Escherichia coli O157:H7. Anal Chim Acta 998:52–59. https://doi.org/10.1016/j.aca.2017.10.027
Yan L, Dou L, Bu T, Huang Q, Wang R, Yang Q, Huang L, Wang J, Zhang D (2018) Highly sensitive furazolidone monitoring in milk by a signal amplified lateral flow assay based on magnetite nanoparticles labeled dual-probe. Food Chem 261:131–138. https://doi.org/10.1016/j.foodchem.2018.04.016
Han J, Zhang L, Hu L, Xing K, Lu X, Huang Y, Zhang J, Lai W, Chen T (2018) Nanozyme-based lateral flow assay for the sensitive detection of Escherichia coli O157:H7 in milk. J Dairy Sci 101:5770–5779. https://doi.org/10.3168/jds.2018-14429
Bu T, Huang Q, Yan L, Huang L, Zhang M, Yang Q, Yang B, Wang J, Zhang D (2018) Ultra technically-simple and sensitive detection for Salmonella Enteritidis by immunoassay based on gold growth. Food Control 84:536–543. https://doi.org/10.1016/j.foodcont.2017.08.036
Zhou J, Nie W, Chen Y, Yang C, Gong L, Zhang C, Chen Q, He L, Feng X (2018) Quadruplex gold immunoassay for four families of antibiotic residues in milk. Food Chem 256:304–310. https://doi.org/10.1016/j.foodchem.2018.02.002
Cheng N, Song Y, Zeinhom MMA, Chang YC, Sheng L, Li H, Du D, Li L, Zhu MJ, Luo Y, Xu W, Lin Y (2017) Nanozyme-mediated dual immunoassay integrated with smartphone for use in simultaneous detection of pathogens. ACS Appl Mater Interfaces 9:40671–40680. https://doi.org/10.1021/acsami.7b12734
Zhong YH, Chen YJ, Yao L, Zhao DP, Zheng L, Liu GD, Ye Y, Chen WW (2016) Gold nanoparticles based lateral flow immunoassay with largely amplified sensitivity for rapid melamine screening. Mikrochim Acta 183:1989–1994. https://doi.org/10.1007/s00604-016-1812-9
Qiu WW, Baryeh K, Takalkar S, Chen W, Liu GD (2019) Carbon nanotube-based lateral flow immunoassay for ultrasensitive detection of proteins: application to the determination of IgG. Microchim Acta 186(7):436–444. https://doi.org/10.1007/s00604-019-3508-4
Lu XW, Mei T, Guo Q, Zhou WJ, Li XM, Chen JT, Zhou XK, Sun N, Fang ZY (2019) Improved performance of lateral flow immunoassays for alpha-fetoprotein and vanillin by using silica shell-stabilized gold nanoparticles. Microchim Acta 186(1):2–9. https://doi.org/10.1007/s00604-018-3107-9
Anfossi L, Di Nardo F, Cavalera S, Giovannoli C, Spano G, Speranskaya ES, Goryacheva IY, Baggiani C (2018) A lateral flow immunoassay for straightforward determination of fumonisin mycotoxins based on the quenching of the fluorescence of CdSe/ZnS quantum dots by gold and silver nanoparticles. Microchim Acta 185(2):94–104. https://doi.org/10.1007/s00604-017-2642-0
Sheng W, Chang Q, Shi YJ, Duan WX, Zhang Y, Wang S (2018) Visual and fluorometric lateral flow immunoassay combined with a dual-functional test mode for rapid determination of tetracycline antibiotics. Microchim Acta 185(9):404–414. https://doi.org/10.1007/s00604-018-2945-9
Chen L, Tian Y, Sun B, Wang J, Tong Q, Jin Z (2017) Rapid, accurate, and simultaneous measurement of water and oil contents in the fried starchy system using low-field NMR. Food Chem 233:525–529. https://doi.org/10.1016/j.foodchem.2017.04.147
Zou Z, He Z, Li H, Han P, Tang J, Xi C, Li Y, Zhang L, Li X (2012) Development and application of a method for the analysis of two trichothecenes: Deoxynivalenol and T-2 toxin in meat in China by HPLC-MS/MS. Meat Sci 90:613–617. https://doi.org/10.1016/j.meatsci.2011.10.002
Sun Y, Zhang G, Zhao H, Zheng J, Hu F, Fang B (2014) Liquid chromatography–tandem mass spectrometry method for toxicokinetics, tissue distribution, and excretion studies of T-2 toxin and its major metabolites in pigs. J Chromatogr B 958:75–82. https://doi.org/10.1016/j.jchromb.2014.03.010
Ler S, Lee F, Gopalakrishnakone P (2006) Trends in detection of warfare agents: detection methods for ricin, staphylococcal entertoxin B and T-2 toxin. J Chromatogr A 1133:1–12. https://doi.org/10.1016/j.chroma.2006.08.078
Deng Q, Qiu M, Wang Y, Lv P, Wu C, Sun L, Ye R, Xu D, Liu Y, Gooneratne R (2017) A sensitive and validated immunomagnetic- bead based enzyme-linked immunosorbent assay for analyzing total T-2 (free and modified) toxins in shrimp tissues. Ecotoxicol Environ Saf 142:441–447. https://doi.org/10.1016/j.ecoenv.2017.04.037
Khan IM, Zhao S, Niazi S, Mohsin A, Shoaib M, Duan N, Wu S, Wang Z (2018) Silver nanoclusters based FRET aptasensor for sensitive and selective fluorescent detection of T-2 toxin. Sensors Actuators B Chem 277:328–335. https://doi.org/10.1016/j.snb.2018.09.021
Porricelli ACR, Lippolis V, Valenzano S, Cortese M, Suman M, Zanardi S, Pascale M (2016) Optimization and validation of a fluorescence polarization immunoassay for rapid detection of T-2 and HT-2 toxins in cereals and cereal-based products. Food Anal Methods 9:3310–3318. https://doi.org/10.1007/s12161-016-0527-1
Wang C, Li X, Peng T, Wang Z, Wen K, Jiang H (2017) Latex bead and colloidal gold applied in a multiplex immunoassay for high-throughput detection of three classes of antibiotic residues in milk. Food Control 77:1–7. https://doi.org/10.1016/j.foodcont.2017.01.016
Kong D, Liu L, Song S, Suryoprabowo S, Li A, Kuang H, Wang L, Xu C (2016) A gold nanoparticle-based semi-quantitative and quantitative ultrasensitive paper sensor for the detection of twenty mycotoxins. Nanoscale 8:5245–5253. https://doi.org/10.1039/C5NR09171C
Bilan R, Fleury F, Nabiev I, Sukhanova A (2015) Quantum dot surface chemistry and functionalization for cell targeting and imaging. Bioconjug Chem 26:609–624. https://doi.org/10.1021/acs.bioconjchem.5b00069
Ren M, Xu H, Huang X, Kuang M, Xiong Y, Xu H, Xu Y, Chen H, Wang A (2014) Immunoassay for ultrasensitive detection of Aflatoxin B1 in Maizi by highly luminescent quantum dot beads. ACS Appl Mater Interfaces 6:14215–14222. https://doi.org/10.1021/am503517s
Zhang P, Lu H, Chen J, Han H, Ma W (2014) Simple and sensitive detection of HBsAg by using a quantum dots Nanobeads based dot-blot immunoassay. Theranostics 4:307–315. https://doi.org/10.7150/thno.8007
Qie ZW, Liu QQ, Yan WL, Gao ZC, Meng W, Xiao R, Wang SQ (2019) Universal and ultrasensitive Immunochromatographic assay by using an antigen as a Bifunctional element and Antialbumin antibody on a test line. Anal Chem 91(15):9530–9537. https://doi.org/10.1021/acs.analchem.9b00673
He D, Wu Z, Cui B, Xu E, Jin Z (2019) Building a fluorescent Aptasensor based on exonuclease-assisted target recycling strategy for one-step detection of T-2 toxin. Food Anal Methods 12:625–632. https://doi.org/10.1007/s12161-018-1392-x
Li C, Luo W, Xu H, Zhang Q, Xu H, Aguilar ZP, Lai W, Wei H, Xiong Y (2013) Development of an immunoassay for rapid and quantitative detection of Clenbuterol in swine urine. Food Control 34:725–732. https://doi.org/10.1016/j.foodcont.2013.06.021
Semenova V, Schiffer J, Steward-Clark E, Soroka S, Schmidt D, Brawner M, Lyde F, Thompson R, Brown N, Foster L (2012) Validation and long term performance characteristics of a quantitative enzyme linked Immunosorbent assay (ELISA) for human anti-PA IgG. J Immunol Methods 376:97–107. https://doi.org/10.1016/j.jim.2011.12.002
Bai Y, Liu Z, Bi Y, Wang X, Jin Y, Sun L, Wang H, Zhang C, Xu S (2012) Preparation of polyclonal antibodies and development of a direct competitive enzyme-linked Immunosorbent assay to detect residues of Phenylethanolamine a in urine samples. J Agric Food Chem 60:11618–11624. https://doi.org/10.1021/jf3036066
Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S (2005) Quantum dots for live cells, in vivo imaging, and diagnostic. Science. 307(5709):538–544. https://doi.org/10.1126/science.1104274
Acknowledgments
We thank colleagues for making great efforts to this manuscript. This work was supported by Major Infectious Diseases such as AIDS and Viral Hepatitis Prevention and Control Technology Major Projects (2018ZX10712-001) and the Major Projects (No. AWS16J020).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supporting Material
ESM 1
An Electronic Supporting Material about parameters optimization of the LFI system: (a) labeling amount of anti-T-2-mAb on QB, (b) T-2-OVA concentration in preincubation, (c) sample pH value, (d) ionic strength, (e) anti-OVA antibody concentration, (f) Tween-20 concentration, (g) incubation time, and (h) LFI time. (DOCX 806 kb)
Rights and permissions
About this article
Cite this article
Qie, Z., Yan, W., Gao, Z. et al. Ovalbumin antibody-based fluorometric immunochromatographic lateral flow assay using CdSe/ZnS quantum dot beads as label for determination of T-2 toxin . Microchim Acta 186, 816 (2019). https://doi.org/10.1007/s00604-019-3964-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3964-x