Abstract
A glassy carbon (GC) electrode was modified with poly(1,8-diaminonaphthalene) (p-1,8-DAN) that was coated with silver nanoparticles (Ag NPs) (size: 10.0–60.0 nm by TEM) by electrodeposition process using cyclic voltammetry (CV) technique. The resulting nanocomposite was characterized by FE-SEM, AFM, EDX, XPS, TEM and XRD. The surface area and the electrochemical characteristics of the electrode were investigated by CV and square wave voltammetry (SWV) techniques, and the probe preparation conditions were optimized. The electrode was used for individual and simultaneous determination of the heavy metal ions cadmium(II) (Cd2+), lead(II) (Pb2+) and copper(II) (Cu2+) in water samples by square wave anodic stripping voltammetry (ASV) using scan rate 0.005 V. s−1. The probe showed well separated anodic stripping peaks for Cd2+, Pb2+, and Cu2+. Attractive features of the method include (a) peak voltages of −1.02, −0.78 and − 0.32 V (vs. Ag/AgCl) for the three ions, and (b) low limits of detection (19, 30 and 6 ng.L−1, respectively. The electrode can also detect zinc(II) (Zn2+) and mercury(II) (Hg2+), typically at −1.36 V and + 0.9, respectively.

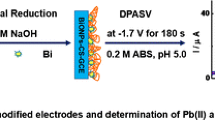
Schematic presentation of simultaneous electrochemical determination of Pb2+, Cd2+, and Cu2+ at a poly(1,8-diaminonaphthalene)-modified glassy carbon electrode coated with silver nanoparticles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the major involvement of the environmental pollution is heavy metals such as cadmium (Cd), lead (Pb) and Copper (Cu) owing to their contribution in different natural and industrial activates, and playing a vital role in numerous biological activities in organisms [1]. Different analytical techniques such as atomic absorption developed for determination of metal traces [2]. On the other hand, electrochemical techniques, specifically electrochemical stripping technique were extensively proved to be suitable for determination of heavy metals because of its good selectivity, cost effectiveness, compactness and the ability for accurate simultaneous determination elements at trace levels [3]. Modified electrodes have noticeable advantages in the water heavy metals determinations. Introducing specific binding groups as thiol or cyano groups accelerate the rate of electron transfer rate at the surface of the electrode and increase the efficiency of preconcentration [4].
Silver (Ag) is regarded as the most inexpensive element among noble metals. Its unique optoelectronic properties and stability, make it has a versatile candidate to be utilized for the fabrication of polymer nanocomposites [5]. Ag shows excellent characteristics for detection of Cd and Pb with high repeatability and stability without any requirement for pretreatment, in addition to lower limits of detection (LOD) [6].
In this field of environmental monitoring, the application of nanoparticles (NPs) as a functional probe for analyzing inorganic and organic pollutants in water has been extensively studied [7, 8]. NPs usually exhibit unique characteristics rather than bulk-sized materials, principally due to the electron confinement of the NPs [9]. From a sensing standpoint, the smaller size of them results in large aspect ratio, surface-to-volume, which lead to quick responses with high sensitivity. In addition, the optoelectronic and magnetic properties of NPs can be adjusted via manage its particle size, composition, morphology and the surface chemistry, to produce highly functional molecular probes [10].
Ag NP modified surfaces are substantial to improve optical and electrochemical characteristics and applications of potential such as catalytic and electro catalytic materials [11]. Therefore, the means of Ag NPs deposition have been widely investigated [12]. Besides the chemical methods, the electrochemical double-pulse method has previously disclosed the successful dispersion of Ag NPs on an ITO surface [13]. Though, the particle size and the distributed density of Ag NPs are highly reliant on the methods of preparations [14].
In the present work, Ag NPs were successfully deposited onto poly1,8-diaminonaphthalene/glassy carbon (p-1,8-DAN/GC) modified electrode by cyclic voltammetry (CV) technique. The modified silver nanoparticles/p-1,8-DAN/GC (AgNPs@p-1,8-DAN/GC) electrode was assessed towards individual and simultaneous electrochemical determination of heavy metal ions such as lead (II) (Pb2+), cadmium (II) (Cd2+), and copper (II) (Cu2+) in 0.1 M acetate buffer solution (ABS) adjusted at pH value of 4.6 using anodic stripping voltammetry (ASV) technique. To the best of our knowledge, this is the first nanoprobe composed of an organic polymer and Ag NPs as a new green electrode material.
Experimental
Materials
1,8-Diaminonaphthalene (1,8-DAN) (Aldrich, https://www.sigmaaldrich.com), sulphuric acid (H2SO4) (Merck, https://www.merckmillipore.com) (98%), acetonitrile (CH3CN) (ACN) (99.9%) HPLC (LAB-SCAN, http://www.hurstscientific.com.au/chemicals/labscan.html), ethanol (CH3CH2OH) (ADWIC, https://www.nasrpharma.com/index.html) (96%) and diamond paste (Presi) were used as received. Sodium acetate (CH3COONa), acetic acid (CH3COOH), silver nitrate (AgNO3), potassium nitrate (KNO3), lead nitrate (Pb(NO3)2), cadmium chloride (CdCl2) and copper chloride (CuCl2) were of analytical grade.
0.1 M ABS (pH = 4.6) was performed by addition of 5.4 g CH3COONa to 2.4 mL glacial CH3COOH (0.1 M) and diluted with distilled H2O until 100.0 mL.
Instruments
A potentiostat voltammetric analyzer (BioAnalytical System (BAS)) was utilized for electrochemical measurements using CV-50w software. All obtained voltammograms were performed in a conventional three-electrode cell composed of 3.0 mm GC (working), a platinum wire (auxiliary) and Ag/AgCl (reference) electrodes. Working electrode cleaning was accomplished by its polishing with diamond paste, rinsing in ethanol followed by used solvents.
Morphology and composition of the modified electrode surface were studied using field emission scanning electron microscopy (FE-SEM) and energy dispersive x-ray (EDX) techniques by a JEOL JSM-7001F operating at 120 kV. An Agilent 5420 atomic force microscope (AFM) was used to study the film hardness and its topography. The measurements of transmission electron microscopy (TEM) were achieved via Joel JEM 1230 with CCD camera (USC1000, Gatan Inc.) operating at 100kv. X-ray diffraction (XRD) measurements were carried on a Bruker, D8 ADVANCE X-ray powder diffractometer (range: 20–80°). X-ray photoelectron spectroscopy (XPS) was performed by using model thermo ESCA Lab 250xi. For its calibration, the binding energy was calibrated to the C 1 s line at 284.6 eV as a reference.
River samples were analyzed by inductively coupled plasma optical emission spectrometry (ICP-OES).
Electrochemical preparation of the p-1,8-DAN-modified glassy carbon electrode
P-1,8-DAN/GC modified electrode was prepared by electro polymerization of 1.0 mM 1,8-DAN in a mixed solvent of 4.5 M H2SO4 in ACN with a volume ratio of (1: 3) at GC electrode, for which 2.50 mL of H2SO4 was added to 7.50 mL of ACN to complete a total volume of 10.0 mL. This system was subjected to CV technique with potential range of +0.2 to +1.2 V at scan rate of 0.1 V. s−1 for 20 cycles as described in our previous work [15].
Electrodeposition of Ag NPs onto p-1,8-DAN film
Different techniques for electrodeposition of Ag NPs onto p-1,8-DAN film was conducted such as pulse voltammetry (PV), double pulse voltammetry (DPV), in situ procedure (Ag+ ions in situ), and CV protocol. Firstly, PV was performed at p-1,8-DAN/GC modified electrode in 25.0 mL of aqueous solution of 0.01 M potassium nitrate (KNO3) containing 0.01 M AgNO3 for which a potential of −0.8 was applied with accumulation time of 300 s for Ag deposition. DPV technique was conducted in two steps at p-1,8-DAN/GC modified electrode in the previously mentioned solution (in the last point). The first step was nucleation pulse at an applied potential of +0.13 V for 50 s, and the second step was growth pulse at an applied potential of +0.24 V for 120 s. Secondly, Ag NPs electrodeposition was achieved in situ (Ag+ ions in situ) for which 0.01 M AgNO3 (0.0169 g) was added to 10.0 mL of the polymerization mixed solvent (4.5 M H2SO4 in ACN) system during the electro polymerization process of 1.0 mM 1,8-DAN monomer using CV technique with scan rate of 0.1 V. s−1 at potential range of +0.2 to +1.2 V for 20 cycles. Final technique was performed by CV protocol by cycling a freshly prepared p-1,8-DAN/GC electrode in a solution of 0.01 M potassium nitrate (KNO3) containing 0.01 M AgNO3 in a potential range from −0.9 to +0.6 V at a scan rate 0.05 V. s−1 for 2 cycles [16]. Based on anodic peaks currents of electrochemical responses (using CV technique at scan rate 0.05 V. s−1) of different AgNPs@p-1,8-DAN/GC modified electrodes prepared by different techniques in 0.1 M ABS, CV technique was chosen as it showed the highest and favorable current response (figure not shown).
Results and discussion
Analytical parameters for Ag NP electrodeposition
P-1,8-DAN film thickness was controlled using different number of polymerization cycles from 5 to 25 cycles. Film thickness performed using 20 cycles arises to give the favorable electrochemical response in ABS using CV technique at scan rate 0.05 V. s−1 in the potential range − 0.9 to +0.6 V (vs. Ag/AgCl) (Fig. S1 A). For studying the effect of Ag+ ions concentration, p-1,8-DAN/GC electrode was immersed in different AgNO3 concentrations (0.1, 0.5, 1.0, 5.0, and 10.0 mM) for which the corresponding current responses were measured. Fig. S1 B indicated that the current response of AgNPs@ p-1,8-DAN/GC increases gradually as Ag+ concentration raised from 0.1 to 10.0 mM. Number of cycles of Ag electrodeposition was also studied by scanning p-1,8-DAN/GC electrode in Ag+ solution using different cycles like 2, 3, 5, 10, and 15 cycles giving the optimum results for 2 cycles (Fig. S1 C). Optimum conditions for Ag+ electrodeposition were detected by applying CV technique to p-1,8-DAN/GC electrode (formed by 20 cycles) in a solution of 0.01 M KNO3 containing 0.01 M AgNO3 in the potential range from −0.9 to +0.6 V with scan rate of 0.05 V. s−1 for 2 cycles. (figure not shown) [16].
Figure 1 shows the electrochemical responses of p-1,8-DAN/GC and AgNPs@p-1,8-DAN/GC modified electrodes s in ABS using square wave voltammetry (SWV) technique in the potential range from - 0.9 V to +0.3 V. No electrochemical response was detected at p-1,8-DAN/GC electrode (Fig. 1a). The modified electrode has a well-defined anodic peak appeared at +0.45 V for Ag+ ions stripping with an anodic peak current of 49.0 μA (Fig. 1b). This indicates that the working potential window from - 0.9 V to 0.0 V is suitable for the studied heavy metal ions determination. The wide-range potential space offers a pronounced improvement of the electrode applications for ASV of heavy metals.
Surface and bulk characterization of AgNPs@p-1,8-DAN-modified glassy carbon electrode
Characterization of AgNPs@p-1,8-DAN-modified electrode was carried out using different techniques like FE-SEM, EDX, XPS and AFM (for surface analysis), while XRD and TEM (for bulk analysis). FE-SEM is employed to characterize the surface morphology of the electrode. Figure 2a shows a rough grayish black surface indicative for the presence of p-1,8-DAN/GC electrode. SEM image (Fig. 2b) indicates a homogenous dispersion of Ag NPs at p-1,8-DAN/GC surface. Furthermore, EDX experiments confirm the Ag deposition giving signals corresponding to Ag metal with a percentage of 97.4% (Fig. 2c).
TEM image represents the morphology of Ag NPs almost as a spherical in shape (Fig. 2 D) for which the particles size ranges from 10.0 nm to 60.0 nm with an average size around 35.5 nm as shown in Fig. 2c inset (diameter distribution histogram).
The crystallinity of Ag NPs was studied by XRD pattern for which diffractions peaks appeared at 38.3°, 44.25°, 64.72°, and 77.40° which are related to the (111), (200), (220), and (311) planes of Ag face-centered cubic, respectively (Fig. 3a) [17]. The strongest diffraction that arises from the (111) plane is a characteristic of such phase. The crystallite size was determined from the full-width at half maximum (FWHM) of the highest reflection of the (111) peak at 2θ = 38.3°. Determined crystallite size came out to be 39 nm.
Surface elemental analysis via XPS enabled further insight into the composition of the AgNPs@p-1,8-DAN/GC electrode surface. The modified electrode surface showed the presence of carbon, silver and nitrogen elements (not shown). The presence of three components in C1s high resolution region at 284.6, 285.8, and 288.2 eV (Fig. 3b) are ascribed to C–C, C–N, and amide bonding in the p-1,8-DAN film, respectively. For determination of the chemical environment and oxidation state of Ag NPs, XPS measurements were carried out at the Ag 3d core levels. Figure 3c displays the spectrum Ag 3d involves two peaks at 374.15 and 368.15 eV, which are attributed to binding energies for Ag 3d3/2 and Ag 3d5/2 of metallic Ag, respectively. Results are in good approval with other results which obtained from XRD which implies the presence of pure metallic Ag NPs.
Electrodes surfaces were visualized using tapping mode AFM. The root-mean-square (rms) roughness values were evaluated from 3D image topographies of p-1,8-DAN/GC and AgNPs@p-1,8-DAN/GC modified electrodes surfaces with 5.6 nm and 15.9 nm, respectively (Fig. 4a and b). Also, small protuberant peaks which were detected in 2D and 3D AFM images of the nanoprobe electrode surface confirming the successful deposition of Ag NPs on the electrode.
Electrochemical behavior and effective surface area of AgNPs@p-1,8-DAN-modified glassy carbon electrode
The electrochemical behaviors of GC, p-1,8-DAN/GC and AgNPs@p-1,8-DAN/GC electrodes were examined in 2.0 mM potassium ferrocyanide (K4[Fe (CN)6]) in 0.10 M KCl aqueous solution using CV technique as illustrated in Fig. S2 (a, b and c). Peak-to-peak separation (∆Ep) values were recorded at 0.93, 0.12 and 0.011 V for the three electrodes, respectively with increasing of peak current of AgNPs@p-1,8-DAN/GC electrode due to the presence of Ag NPs. The low ∆Ep for the previous electrode (nanoprobe) indicates a fast electron transfer higher than both GC and p-1,8-DAN/GC electrodes. This means that introducing Ag NPs at the surface of p-1,8-DAN/GC electrode develops its electrochemical performance.
The effective surface areas of p-1,8-DAN/GC and AgNPs@p-1,8-DAN/GC electrodes were calculated using Randles-Sevcik equation for a reversible process in [Fe (CN)6]3−/4− [18] as presented in Eq. 1:
where A (effective surface area of electrode), D (diffusion coefficient of [Fe (CN)6]3−/4− equal 7.6 × 10−5 cm2 s−1), C (bulk concentration of redox probe [1.0 mM [Fe (CN)6]3−/4−]), n (number of transferred electrons in [Fe (CN)6]3−/4− and equals equal one). The effective surface area of p-1,8-DAN/GC electrode was found to be 0.1311 cm2. The surface area increased by about 5 times when Ag NPs incorporated into p-1,8-DAN film to reach 0.5536 cm2.
Electrochemical determination of individual Pb2+, Cd2+ and Cu2+ heavy metal ions
The electrochemical determination of 10.0 μM Cd2+ ions in ABS at both GC and p-1,8-DAN/GC electrodes using ASV technique with accumulation time 120 s and scan rate of 0.005 V. s−1 were not detected (Fig. S3 A, a and b). At AgNPs@p-1,8-DAN/GC modified electrode, an anodic peak appeared at −0.98 V with an anodic peak current of 4.31 μA (Fig. S3 A, c). The electrochemical determination of 10.0 μM Pb2+ ions at the three electrodes were examined (Fig. S3 B). No peaks were detected at GC electrode (Fig. S3 B, a). Two anodic peaks appeared at −0.77 V and - 0.82 V with anodic peak currents of 3.6 μA and 7.2 μA were detected for both p-1,8-DAN/GC and AgNPs@P-1,8-DA/GC electrodes, respectively with a favorable higher value for the last electrode (Fig. S3 B, b and c). The anodic stripping voltammograms in Fig. S3 C illustrates the ability of GC electrode and p-1,8-DAN/GC electrode to detect 25.0 μM of Cu2+ ions at −0.27 V and - 0.23 V with anodic peaks currents of 0.4 μA and 8.4 μA, respectively (Fig. S3 C a and b). AgNPs@p-1,8-DAN/GC modified electrode showed more sensitivity as indicated by higher anodic peak current value to reach 11.9 μA at −0.29 V (Fig. S3 C c).
Previous results indicated that Pb2+ and Cu2+ ions can be detected individually at p-1,8-DAN/GC electrode while Cd2+ ions were not detected. This can be correlated to the presence of amine/imine function groups acting as chelating sites with for Pb2+ and Cu2+ ions [19]. On the other hand, the three ions were detected at AgNPs@P-1,8-DAN nanoprobe with peak potentials −0.82 V, −0.29 V and − 0.98 V, respectively with a remarkable raise in their current. The synergistic contributions from both P-1,8-DAN and Ag NPs improve the accumulation efficiency and the rate of charge transfer of the metal ions throughout ASV analysis. Additionally, Ag creates fused alloys with several heavy metals making it more readily to be reduced [19]. Such Ag alloys facilitated the nucleation process during heavy metal depositions in a similar manner to early described by Wang for Bi electrodes [3]. This can also explain why Cd2+ ions were detected only at AgNPs@p-1,8-DAN/GC electrode.
Effect of individual heavy metal Pb2+, Cd2+ and Cu2+ ions concentrations
Individual determination of low concentrations of different heavy metal ions were studied between −1.20 to 0.0 V (vs. Ag/AgCl) at ranges like: 0.90 nM - 9.0 μM for Cd2+, 2.0 nM - 24.0 μM for Pb2+, and 1.3 nM - 9.0 μM for Cu2+, while the presented data are in the concentration windows of 1.5–9.0 μM for Cd2+, 2.00–24.0 μM for Pb2+, and 2.00–9.0 μM for Cu2+. Figure 5a, b and c represents the anodic stripping voltammograms recorded at AgNPs@p-1,8-DAN/GC electrode with accumulation time 120 s and scan rate of 0.005 V. s−1, while Fig. 5 insets, represent the relation between different ions concentrations (in all ranges) and the corresponding anodic peaks currents. The anodic stripping peak currents were raised linearly upon increasing the concentrations of the studied metal ions. The calculated correlation coefficients (R2), LOD and limit of quantification (LOQ) are collected in Table S1 indicating the high sensitivity of the modified electrode towards the three heavy metal ions individual determination.
Anodic stripping voltammograms of 1.5–9.0 μM for Cd2+, 2.00–24.0 μM for Pb2+, and 2.00–9.0 μM for Cu2+ in ABS at AgNPs@p-1,8-DAN/GC electrode with accumulation time 120 s, scan rate of 0.005 V. s−1 and amplitude of 0.025 V between −1.20 to 0.0 V (vs. Ag/AgCl). Insets, relations between different ions concentrations and the corresponding anodic peaks currents
Simultaneous electrochemical determination of Pb2+, Cd2+, and Cu2+ heavy metal ions in a ternary mixture
The goal of this work is to detect Cd2+, Pb2+, and Cu2+ ions simultaneously. Anodic stripping voltammograms of a mixture of 0.5 μA Cd2+, 2.0 μA Pb2+ and 2.0 μA Cu2+ at p-1,8-DAN/GC and AgNPs@p-1,8-DAN/GC electrodes in ABS at a scan rate of 0.005 V. s−1 with accumulation time 120 s were recorded (Fig. S4 a and b). In Fig. S4 a, Cd2+, Pb2+ and Cu2+ ions were simultaneously detected at p-1,8-DAN/GC electrode at −1.06, −0,82 and 0.35 V with anodic stripping currents of 0.33 μA, 1.52 μA and 3.54 μA, respectively. At AgNPs@p-1,8-DAN/GC electrode (Fig. S4 b), peak potentials of Cd2+, Pb2+, and Cu2+ ions were detected at −1.02, −0.78 and − 0.32 V with anodic stripping currents of 1.22 μA, 5.43 μA and 9.76 μA, respectively. The remarkable differences in stripping peak potentials between Pb2+, Cd2+ and Cu2+ indicated the possibility of their simultaneous detection due to the strong activity of Ag NPs decorated p-1,8-DAN film. Peak currents at AgNPs@p-1,8-DAN/GC electrode compared with p-1,8-DAN/GC electrode demonstrates the role of Ag NPs towards the enhanced responses of Pb2+, Cd2+, and Cu2+ ions.
Simultaneous determination of different concentrations of studied heavy metal ions were studied in the voltage range from −1.20 to 0.0 V (vs. Ag/AgCl) at low concentrations ranges like: 0.15 nM - 3.0 μM for Cd2+, 0.13 nM - 12.0 μM for Pb2+, and 0.07 nM - 12.0 μM for Cu2+, while an ASVs of several Cd2+ ions concentrations (0.25–3.0 μM), Pb2+ and Cu2+ ions concentrations (1.0–12.0 μM) in ABS at AgNPs@p-1,8-DAN/GC are presented in Fig. 6. Stripping peaks were appeared at −0.98, −0.74 and − 0.28 V for Cd2+, Pb2+ and Cu2+, respectively. It is important to mention that at the higher concentration like1.0 μM, a second stripping peak appeared for Pb2+ ions. As the concentration was increased further, the magnitude of this second peak was also found to increase as presented in literature [20].
Anodic stripping voltammograms of Cd2+ ions concentrations (0.25–3.0 μM), Pb2+ and Cu2+ ions concentrations (1.0–12.0 μM) at AgNPs@p-1,8-DAN/GC electrode in ABS with accumulation time 120 s, scan rate of 0.005 V. s−1 and amplitude of 0.025 V between −1.20 to 0.0 V (vs. Ag/AgCl). Insets, relations between different ions concentrations and the corresponding anodic peaks currents
Figure 6 insets, represent the relation between different ions concentrations (in all ranges) and the corresponding anodic peaks currents. Results reveal that the anodic stripping peaks heights of all the studied heavy metal ions improved linearly with increasing their concentrations.
The resulting calibration plots exhibited an excellent linearity of current dependence for their corresponding concentrations with R2 values of 0.9938 for Cd2+, 0.9946 for Pb2+, and 0.9934 for Cu2+. LOD values were estimated to be 0.17 nM, 0.15 nM and 0.09 nM for Cd2+, Pb2+, and Cu2+ ions, respectively as collected in Table S2. As represented, the nanoprobe is highly sensitive for simultaneous determination of Cd2+, Pb2+, and Cu2+ ions in ternary mixture with lower LOD and LOQ values.
Repeatability
Repeatability was evaluated by six times successive determination of 0.5 μM Cd2+, 2.0 μM Pb2+ and 2.0 μM Cu2+ ions at the same nanoprobe by recording their peak responses (Fig. S5). The nanoprobe exhibited good repeatability and stability due to the high adsorption between Ag NPs and Cd2+, Pb2+ and Cu2+ ions [21].
Interference studies
Interferences of additional metal ions were examined for evaluating the ability of the nanoprobe for determination of Cd2+, Pb2+, and Cu2+ ions in presence of zinc (II) (Zn2+) and mercury (II) (Hg2+) ions. Interference studies performed using ASV technique with accumulation time 120 s and a scan rate of 0.005 V. s−1 by addition of 2.0 μM Zn2+ and 0.5 μM Hg2+ ions into the ABS containing 0.5 μM Cd2+, 2.0 μM Pb2+, and 2.0 μM Cu2+ ions. Well-defined sharp peaks of Cd2+, Pb2+, and Cu2+ were observed as illustrated in Fig. 7. The presence of both Zn2+ and Hg2+ ions did not affect peak responses for other ions with working voltages of −1.36 and + 0.9 V, respectively. The high surface area of Ag NPs supports high sensitivity for heavy metals ions detection and diminishes the interference for their simultaneously determination devoid of losing response intensities.
Real sample determination
The nanoprobe was tested in tap water collected and adjusted with ABS for estimating its performance. Neither Cd2+, Pb2+ nor Cu2+ traces were found (Fig. S6 a). Then, typical addition method had been used as an identified quantity were spiked into the water sample (of the previous ions) and ASV responses were examined with accumulation time 120 s and scan rate of 0.005 V. s−1 (Fig. S6 b). It was found that the electrode can successfully determine simultaneously Cd2+, Pb2+ and Cu2+ ions in tap water with high sensitivity. Table S3 optimizes the recoveries for results which obtained for two samples in absence and presence of spiked heavy metal ions. Results show high recoveries between 103.0% and 108.0%. Good results detected in recovery procedure indicated that the investigated AgNPs@p-1,8-DAN/GC electrode is suitable for the simultaneously determination of Cd2+, Pb2+ and Cu2+ ions in real water samples.
To further demonstrate the practicality of the present electrode, study also evaluated the simultaneous determination of Cd2+, Pb2+, and Cu2+ ions in contaminated three river water samples. The stripping signals of all studied ions were all observed with the presented nanoprobe with high good recoveries and confirmed by means of comparison with data obtained from ICP-OES method as presented in Table S4.
Electrochemical performance of the nanoprobe was compared with parallel systems in literatures used for the electrochemical determination of Cd2+, Pb2+ and Cu2+ ions (Table 1). Comparisons showed that the AgNPs@p-1,8-DAN/GC modified electrode can be a promising nanoprobe to be used for the determination of these heavy metal ions individually and simultaneously with high sensitivity and low LOD values.
Conclusion
The advantageous features of AgNPs@p-1,8-DAN/GC modified electrode have been revealed, this new nanoprobe was fabricated by electro polymerization method and Ag NPs were electrodeposited onto p-1,8-DAN film by CV technique and characterized via different surface and bulk analytical techniques. The AgNPs@p-1,8-DAN/GC modified electrode used for individual and simultaneous determination of Cd2+, Pb2+ and Cu2+ ions in ABS using ASV technique with high sensitivity and low values of LOD and LOQ. Additionally, it can detect other heavy metal ions like Zn2+ and Hg2+ in the same system. The presented nanoprobe exhibited highly electrocatalytic activity compared to the previous electrodes for electrochemical determination of these ions and the performance of the electrode shows high stability and sensitivity beside selectivity.
References
El Mhammedi MA, Achak M, Bakasse M (2013) Evaluation of a platinum electrode modified with hydroxyapatite in the lead (II) determination in a square wave voltammetric procedure. Arab J Chem 6(3):299–305. https://doi.org/10.1016/j.arabjc.2010.10.010
Pohl P (2009) Determination of metal content in honey by atomic absorption and emission spectrometries. Trends Anal Chem 28(1):117–128. https://doi.org/10.1016/j.trac.2008.09.015
Wang J (2005) Stripping analysis at bismuth electrodes: a review. Electroanal 17(15–16):1341–1346. https://doi.org/10.1002/elan.200403270
Su Z, Liu Y, Zhang Y, Xie Q, Chen L, Huang Y, Fu Y, Meng Y, Li X, Ma M, Yao S (2013) Thiol–ene chemistry guided preparation of thiolated polymeric nanocomposite for anodic stripping voltammetric analysis of Cd2+ and Pb2+. Analyst 138(4):1180–1186. https://doi.org/10.1039/C2AN36114K
Tang J, Li Z, Xia Q, Williams RS (2009) Fractal structure formation from Ag nanoparticle films on insulating substrates. Langmuir 25(13):7222–7225. https://doi.org/10.1021/la9010532
March G, Nguyen T, Piro B (2015) Modified electrodes used for electrochemical detection of metal ions in environmental analysis. Biosens 5(2):241–275. https://doi.org/10.3390/bios5020241
Wang Z, Ma L (2009) Gold nanoparticle probes. Coord Chem Rev 253(11–12):1607–1618. https://doi.org/10.1016/j.ccr.2009.01.005
Cheon J, Lee J-H (2008) Synergistically integrated nanoparticles as multimodal probes for Nanobiotechnology. Acc Chem Res 41(12):1630–1640. https://doi.org/10.1021/ar800045c
Berlina AN, Zherdev AV, Dzantiev BB (2019) Progress in rapid optical assays for heavy metal ions based on the use of nanoparticles and receptor molecules. Microchim Acta 186(3):172. https://doi.org/10.1007/s00604-018-3168-9
Hahm J-I (2013) Biomedical detection via macro- and nano-sensors fabricated with metallic and semiconducting oxides. J BIiomed Nanotechnol 9(1):1–25. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3766318/
Nishijo J, Oishi O, Judai K, Nishi N (2007) Facile and mass-producible fabrication of one-dimensional ag nanoparticle arrays. Chem Mater 19(19):4627–4629. https://doi.org/10.1021/cm071688i
Zheng J, Li X, Gu R, Lu T (2002) Comparison of the surface properties of the assembled silver nanoparticle electrode and roughened silver electrode. J Phys Chem B 106(5):1019–1023. https://doi.org/10.1021/jp012083r
Sandmann G, Dietz H, Plieth W (2000) Preparation of silver nanoparticles on ITO surfaces by a double-pulse method. J Electroanal Chem 491(1–2):78–86. https://doi.org/10.1016/S0022-0728(00)00301-6
Xing S, Xu H, Chen J, Shi G, Jin L (2011) Nafion stabilized silver nanoparticles modified electrode and its application to Cr (VI) detection. J Electroanal Chem 652(1–2):60–65. https://doi.org/10.1016/j.jelechem.2010.03.035
Hassan K, Abdel Azzem M (2015) Electrocatalytic oxidation of ascorbic acid, uric acid, and glucose at nickel nanoparticles/poly (1-amino-2-methyl-9,10-anthraquinone) modified electrode in basic medium. J Appl Electrochem 45(6):567–575. https://doi.org/10.1007/s10800-015-0805-4
Ng KH, Liu H, Penner RM (2000) Subnanometer silver clusters exhibiting unexpected electrochemical Metastability on graphite. Langmuir 16(8):4016–4023. https://doi.org/10.1021/la9914716
Shameli K, Ahmad MB, Jazayeri SD, Shabanzadeh P, Sangpour P, Jahangirian H, Gharayebi Y (2012) Investigation of antibacterial properties silver nanoparticles prepared via green method. Chem Cent J 6(1):73. https://doi.org/10.1186/1752-153x-6-73
Bard AJ, Faulkner LR (2001) Fundamentals and applications, vol 2. Electrochemical Methods
Salih FE, Ouarzane A, El Rhazi M (2017) Electrochemical detection of lead (II) at bismuth/poly(1,8-diaminonaphthalene) modified carbon paste electrode. Arab J Chem 10(5):596–603. https://doi.org/10.1016/j.arabjc.2015.08.021
Hutton LA, Newton ME, Unwin PR, Macpherson JV (2011) Factors controlling stripping voltammetry of Lead at polycrystalline boron doped diamond electrodes: new insights from high-resolution microscopy. Anal Chem 83(3):735–745. https://doi.org/10.1021/ac101626s
Hassan KM, Elsaid Gaber S, Fatehy M, Abdel Azzem M (2018) Novel sensor based on poly (1,2-Diaminoanthraquinone) for individual and simultaneous anodic stripping voltammetry of Cd2+, Pb2+, Cu2+ and Hg2+. J Electroanal 30(6):1155–1162. https://doi.org/10.1002/elan.201800097
Vu HD, Nguyen L-H, Nguyen TD, Nguyen HB, Nguyen TL, Tran DL (2015) Anodic stripping voltammetric determination of Cd2+ and Pb2+ using interpenetrated MWCNT/P1,5-DAN as an enhanced sensing interface. Ionics 21(2):571–578. https://doi.org/10.1007/s11581-014-1199-8
Guo Z, Li D-d, Luo X-k, Li Y-h, Zhao Q-N, Li M-m, Zhao Y-t, Sun T-s, Ma C (2017) Simultaneous determination of trace Cd (II), Pb (II) and Cu (II) by differential pulse anodic stripping voltammetry using a reduced graphene oxide-chitosan/poly-l-lysine nanocomposite modified glassy carbon electrode. J Colloid Interface Sci 490:11–22. https://doi.org/10.1016/j.jcis.2016.11.006
Hwang GH, Han WK, Park JS, Kang SG (2008) Determination of trace metals by anodic stripping voltammetry using a bismuth-modified carbon nanotube electrode. Talanta 76(2):301–308. https://doi.org/10.1016/j.talanta.2008.02.039
Wang Z, Wang H, Zhang Z, Liu G (2014) Electrochemical determination of lead and cadmium in rice by a disposable bismuth/electrochemically reduced graphene/ionic liquid composite modified screen-printed electrode. Sensors Actuators B Chem 199:7–14. https://doi.org/10.1016/j.snb.2014.03.092
Ouyang R, Zhu Z, Tatum CE, Chambers JQ, Xue Z-L (2011) Simultaneous stripping detection of Zn (II), Cd (II) and Pb (II) using a bimetallic Hg–Bi/single-walled carbon nanotubes composite electrode. J Electroanal Chem 656(1–2):78–84. https://doi.org/10.1016/j.jelechem.2011.01.006
Huang H, Chen T, Liu X, Ma H (2014) Ultrasensitive and simultaneous detection of heavy metal ions based on three-dimensional graphene-carbon nanotubes hybrid electrode materials. Anal Chim Acta 852:45–54. https://doi.org/10.1016/j.aca.2014.09.010
Li J, Guo S, Zhai Y, Wang E (2009) High-sensitivity determination of lead and cadmium based on the Nafion-graphene composite film. Anal Chim Acta 649(2):196–201. https://doi.org/10.1016/j.aca.2009.07.030
Pokpas K, Zbeda S, Jahed N, Mohamed N, Baker PG, Iwuoha EI (2014) Electrochemically reduced graphene oxide pencil-graphite in situ plated bismuth-film electrode for the determination of trace metals by anodic stripping voltammetry. Int J Electrochem Sci 9:736–759. http://hdl.handle.net/10566/3290
Chamjangali MA, Kouhestani H, Masdarolomoor F, Daneshinejad H (2015) A voltammetric sensor based on the glassy carbon electrode modified with multi-walled carbon nanotube/poly(pyrocatechol violet)/bismuth film for determination of cadmium and lead as environmental pollutants. Sensors Actuators B Chem 216:384–393. https://doi.org/10.1016/j.snb.2015.04.058
Serrano N, González-Calabuig A, del Valle M (2015) Crown ether-modified electrodes for the simultaneous stripping voltammetric determination of Cd (II), Pb (II) and Cu (II). Talanta 138:130–137. https://doi.org/10.1016/j.talanta.2015.01.044
Sahoo PK, Panigrahy B, Sahoo S, Satpati AK, Li D, Bahadur D (2013) In situ synthesis and properties of reduced graphene oxide/Bi nanocomposites: As an electroactive material for analysis of heavy metals. Biosens Bioelectron 43:293–296. https://doi.org/10.1016/j.bios.2012.12.031
Silva ACO, Oliveira LCF, Delfino AV, Meneghetti MR, Abreu FC (2016) Electrochemical study of carbon nanotubes/Nanohybrids for determination of metal species Cu2+ and Pb2+ in water samples. J AnalL Methods Chem 2016:1–12. https://doi.org/10.1155/2016/9802738
Chira A, Bucur B, Bucur MP, Radu GL (2014) Electrode-modified with nanoparticles composed of 4,4[prime or minute]-bipyridine-silver coordination polymer for sensitive determination of Hg (II), Cu (II) and Pb (II). New J Chem 38(11):5641–5646. https://doi.org/10.1039/C4NJ01245C
Sang S, Li D, Zhang H, Sun Y, Jian A, Zhang Q, Zhang W (2017) Facile synthesis of AgNPs on reduced graphene oxide for highly sensitive simultaneous detection of heavy metal ions. RSC Adv 7(35):21618–21624. https://doi.org/10.1039/C7RA02267K
Acknowledgements
The authors are appreciative to Alexander von Humboldt Foundation for providing some electrodes and accessories.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 147 kb)
Rights and permissions
About this article
Cite this article
Hassan, K.M., Elhaddad, G.M. & AbdelAzzem, M. Voltammetric determination of cadmium(II), lead(II) and copper(II) with a glassy carbon electrode modified with silver nanoparticles deposited on poly(1,8-diaminonaphthalene). Microchim Acta 186, 440 (2019). https://doi.org/10.1007/s00604-019-3552-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3552-0