Abstract
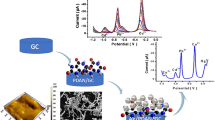
A glassy carbon electrode (GCE) modified with bismuth oxide nanoparticles (BiONPs) and the chitosan (CS) was fabricated, and used to simultaneously determine lead and cadmium by differential pulse anodic stripping voltammetry. This modified electrode can be self-designed and prepared at low-costs. Scanning electron microscopy image shows that the mean diameter of BiONPs is about 235 nm. The sensitivity of the BiONPs-CS-GCE is much better than that of the bare GCE and the CS-GCE. The effects of deposition potential, pH of the solution, and deposition time were optimized. Under the optimized conditions (pHABS = 5.0, Edep = −1.7 V, tdep = 180 s), the composite film electrode was then applied to the analysis of lead (II) and cadmium (II). The linear range of the electrode was 0.4–2.8 μM for Pb (II) and 0.8–5.6 μM for Cd (II), with a limit of detection of 0.15 μM for Pb (II) and 0.05 μM for Cd (II), respectively. The determination of Pb (II) and Cd (II) in spiked tap water was performed and gave recoveries ranging from 99 to 122 % for Pb (II), and from 96 to 120 % for Cd (II).

Bismuth oxide nanoparticles (BiONPs) and the chitosan (CS) were fabricated and used to simultaneously determine lead and cadmium by differential pulse anodic stripping voltammetry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The detection of metal ions, such as lead and cadmium is of importance [1, 2]. A variety of problems were caused by this pollution, such as ground water [3], tap water [4], and rice [5]. Lead is present in tap water primarily from household plumbing systems containing lead in pipes, solder, and so on [6]. At present, there are several methods for the determination of lead and cadmium in tap water. Modern testing analysis techniques, like atomic absorption spectroscopy [7] and X-ray fluorescence [8] are all widely adopted. As one of the cheapest ways to detect heavy metal ions, electrochemical methods have numerous advantages due to its easy operation and high sensitivity [9].
A sensitive DPASV method was used for the simultaneous determination of lead and cadmium on the self-designed bismuth film electrode. Compared with mercury, bismuth and bismuth salt are low toxicity and have satisfactory electrochemical performance (relatively wide potential window and easy to form alloys with lots of metal), and therefore bismuth and bismuth salt as alternatives to mercury have been employed for measurement of stripping voltammetry [10–11]. Furthermore, bismuth film exhibits numerous absorbing properties, including simple self-designing, high sensitivity, excellent peak resolution, insensitivity to dissolved oxygen and ability to employ in highly alkaline media [12–14]. The nanostructure materials play key roles in the detection of heavy metal ions because of the high ratios of surface area to volume, the high detection sensitivity for the target ions, and efficient acceleration of electron transfer for electrical current signal [15].
There are three main kinds of nanostructure electrode materials about bismuth: bismuth film, bismuth alloys, and bismuth oxide. Firstly, the bismuth film deposited on the bismuth film electrode, usually was fabricated by three ways: “in situ”, “ex situ” depositions, and bulk modification with a bismuth compound (Bi2O3, or a bismuth salt) [16] or a mixture of bismuth nanoparticles [17, 18]. Secondly, bismuth alloys like bismuth/antimony were simultaneously deposited on the surface of the GCE, which exhibited more excellent current response than the single metal film of electrode [19]. Thirdly, some bismuth precursor compounds [20] modified electrodes, like the carbon paste electrode was produced by mixing Bi2O3 [21] or bismuth salt [22] with graphite powder and ionic liquid.
Chitosan is regarded as a promising material for modification of the electrode because of its excellent stability, fine film-forming property and the good adhesion on surfaces [23, 24].
There are few reports on the electrochemical sensor based on the composite of nano Bi2O3 and CS. To the best of our knowledge, there is only one paper referred to the composite material applied to the determination of DNA [25]. We have fabricated a composite electrode by using chitosan and bismuth oxide nanoparticle which is synthesized by ourselves. Then, a bismuth film electrode (BiF-CS-GCE) was formed by electrochemical reduction of bismuth oxide. As far as we know, the method for preparing the BiF-CS-GCE by electrochemical reduction has, until now, not been found to be reported. Different parameters during the measurement procedure were investigated and optimized. Subsequently, under the optimized conditions, the bismuth film sensor prepared from Bi2O3 was applied to determine the trace Pb (II) and Cd (II) ions by DPASV.
Experimental
Chemicals
All unspecified chemicals were analytical grade without further treatment. Chitosan (biochemical reagent), sodium hydroxide and Bi(NO3)3·5H2O were obtained from Sinopharm Chemical Reagent Co., Ltd. Sodium lignin sulfonate (SLS) was obtained from Tianjin Fuchen Chemical Reagents Factory. Ethanol was obtained from Shanghai Chemical Reagent CO,.LTD. Deionized water (DW) (with a resistivity of 15.01 MΩ·cm) was used for all experiments.
Apparatus
Cyclic voltammetry (CV) and DPASV experiments were performed on the electrochemical workstation CHI660 (Shanghai Chenhua Instrument). Three-electrode system was conventional: a saturated calomel electrode (SCE) reference electrode, A Pt wire auxiliary electrode, and the modified disposable electrode. The inside diameter of GCE is 4 mm, and the out diameter is 8 mm. The whole experiments were carried out in one electrochemical cell (10 mL). All the experiments were performed at room temperature. No deaeration of the solutions was applied to remove the oxygen at any stage of this study.
The morphologies of the Bi2O3 nanoparticles were observed by using a field emission scanning electron microscope (SEM; JSM-7001F). The crystal structures analysis of the samples were conducted with a Bruker-AXS D8 Advance X-ray diffractometer (Cu Kα radiation, λ = 0.15406 nm).
Preparation of the bismuth oxide nanoparticles
14 g NaOH and 1.5 g SLS were weighted and dissolved into 175 mL of deionized water to form a homogeneous solution in the 500 mL beaker. The mixture solution was stirred with 20 r·s−1, heated to 80 °C in water bath. Then 25 mL 0.2 mol·L−1 Bi(NO3)3·5H2O solution was added to the beaker slowly with continuous stirring to generate a white precipitate which is quickly changed to yellow precipitate [26]. After 1 h, the solid precipitate was filtered and then dried in the oven at 60 °C for 1 h, the solid product was roasted in the box resistor-stove at 500 °C for 3 h.
Preparation of Bi2O3/CS emulsion
0.5 % CS solution was prepared by dissolving CS into 1 % acetic acid, and then sonicated for 6 h at room temperature until it was completely dissolved [25]. the Bi2O3 nanoparticle was dispersed into the chitosan solution by ultrasonic dispersion for 0.5 h. experiments showed that the low content of Bi2O3 is not conducive to the formation of Bi film, and the film-forming is poor when the content of CS is low. for these reasons, the appropriate mass ratio of Bi2O3 and CS was determined to be 4:1
Preparation of the modified electrodes
The surface of the GCE was polished with fine metallographic sandpaper, 0.1 μm alumina slurry over a wool cloth till mirror-like. For each process, the electrode would be rinsed thoroughly with deionized water (DW). Besides, the GCE is immersed in a DW, ethanol, DW for 1–2 min, respectively. Then the electrode was activated in 1 M H2SO4 solution by CV from −1.0 to 1.0 V. After the electrode surface drying in the air, 5 μL CS-Bi2O3 emulsion was dropped on the surface, and then the modified electrode was dried at room temperature.
Measurement procedure
The DPASV were performed in 0.2 M sodium acetate buffer (ABS, pH 5.0), if not stated otherwise. The reduction of bismuth oxide to bismuth on the electrode was employed at −1.2 V (vs. SCE) for 360 s, in 0.2 M NaOH solution with current time methods. DPASV was performed to simultaneously determine Pb2+ and Cd2+ in the ABS and the tap water. With the method, the pre-concentration step is at −1.7 V for 180 s. The parameters of DPASV are as follows: Edep = −1.7 V, tdep = 180 s; teq = 2 s, Ebegin = −0.9 V, Eend = −0.4 V, Estep = 4 mV, Eampl = 25 mV. Before each measurement, a clean step at potential of −0.4 V was employed for 30 s.
Results and discussion
Characterization
Figure 1 presents the XRD patterns of the sample. From this figure, almost all of the peaks can be indexed to the monoclinic crystal (JCPDS no. 41–1449). In addition, the diffraction peaks indicate that the Bi2O3 samples are well crystallized.
Fig. 2a is the SEM image of BiONPs-CS, which reveals that the morphology is largely consisted of irregular particles and membranes. Fig. 2b shows the size distribution graph for BiONPS obtained by manual counting of at least 200 times. From the statistical data, its mean particle size is 235.4 nm. Besides, most particles size is in the range of 173–274 nm. As can be seen in Fig. 2c, the morphology of Bi nanoparticles is different from that of the bismuth oxide nanoparticle due to the smaller size of the former. Fig. 2d shows the size distribution of bismuth nanoparticles, which indicates an average size of 109.8 nm. Moreover, in some areas in Fig. 2c, the size of the most of Bi nanoparticles is less than 100 nm, ranging from 47.1 to 87.2 nm.
After the determination of Pb(II) and Cd(II), the morphology of Bi is changed greatly, which is illustrated in Fig. 3a. During the determination of the two metal ions, the Bi nanoparticles changed from the irregular particle (Fig. 2c) to regular flake (Fig. 3a). EDS area (S 3) in the whole surface of regular flake Bi is shown in Fig. 3b and its EDS spectrum is shown in Fig. 3c. From the data analysis of the EDX spectrum, we can see that there is no residual lead and cadmium on the surface of GCE. Moreover, the result of analysis may illustrate that the clean potential of −0.4 V and the clean time of 30 s are enough to meet experimental requirements.
As shown in Fig. 4, the cyclic voltammogram from a BiONPs-CS-GCE was recorded in 0.2 M NaOH solution. During the anodic scan, the only one oxidation peak was found at −0.44 V, and two obvious reduction peaks found at −0.82 V and −0.98 V on the cathodic scan. The explanation of the three peaks had been given in the corresponding reference [27, 28].
To ensure the Bi film on the surface of GCE totally been produced by above reduction reaction, the potential of −1.2 V and the time of 360 s were chosen for reducing bismuth oxide at the electrode surface [28].
Fig. 5 shows that the DPASV of 1 μM Pb(II) and 2 μM Cd(II) at the bare GCE, CS modified GCE (CS-GCE) and Bismuth film modified GCE(BiF-CS-GCE). As is shown in Fig. 5a, the stripping peaks for Pb(II) and Cd(II) at the bare GCE(0.5 μA at −0.562 V for Pb(II) and 1.3 μA at −0.798 V for Cd(II)) were very poor. In contrast, the CS-GCE (Fig. 5b, 1 .9 μA at −0.574 V for Pb(II) and 2.9 μA at −0.821 V for Cd(II)) and the BiF-CS-GCE (Fig. 5c, 5.4 μA at −0.565 V for Pb(II) and 9.0 μA at −0.818 V for Cd(II)) obtained better significant in stripping process. The signals of Pb(II) and Cd(II) of the BiF-CS-GCE were nearly nine times and six times larger than the ones at bare GCE, respectively. The improved response of the CS-GCE electrode can be contributed to the absorption capacity of the chitosan for the metal ions. Furthermore, the current response of the BiF-CS-GCE is excellent, which can be ascribed to the ability of bismuth combining metal ions.
Optimization of method
The following parameters were optimized: (a) pH of supporting electrolyte; (b) deposition potential and (c) deposition time. Respective data and Figures are given in the Electronic Supporting Material. The following experimental conditions were found to give best results: (a) the pH 5.0 of ABS (Fig. S1a); (b) -1.7 V as the optimal deposition potential (Fig. S1b); (c) 180 s as the best condition of deposition time. (Fig. S1c).
Performance analysis
Calibrations for the simultaneous determination of Pb (II) and Cd (II) at the BiF-CS-GCE electrode were obtained by DPASV under the best conditions described above. The performance of the assemble electrode for the determination of two metal ions over the concentration range 0.4–2.8 μM for Pb2+ and 0.8–5.6 μM for Cd2+ were illustrated in Fig. 6b and c, respectively. The result shows that both metal ions have a good linear relationship with concentration. For lead, the linear regression equation is IPb (current/μA) = −2.489 + 9.288CPb (concentration/μM) (Cpb:0.4–2.8) with the R = 0.9943. For cadmium, the linear regression equation is ICd = −3.317+ 8.114CCd (concentration/μM) (CCd:0.8–5.6) with the R = 0.9951. The LOD was 0.15 μM for Pb2+ and 0.05 μM for Cd2+.
a Stripping voltammograms and calibration plots for b lead and c cadmium using BiF-CS-GCE electrode in 0.2 M ABS, pH 5.0. Deposition potential: −1.7 V; deposition time, 180 s. Other conditions were same as the Fig. 5. (down to up: 0.4, 0.8, 1.2, 1.6, 2.0, 2.4, 2.8 μM for lead, 0.8, 1.6, 2.4, 3.2, 4.0, 4.8, 5.6 μM for cadmium, respectively)
The response of the electrodes started to decrease gradually after used around eight successive stripping. Consequently, these electrodes can be used as semi-disposable dual sensors [29]. The electrode prepared in our work is comparable with or better than many works summarized in Table 1
Interference studies
The formation of metal alloy is a serious interference in the determination of lead and cadmium by DPASV on the electrode [9]. Therefore, in the major interference on the anodic striping voltammetry of Pb(II) and Cd(II) at bismuth electrode, the Cu(II) is usually been studied [37, 38]. As shown in Fig. 7, different concentrations of copper (0–16 μM) have a negative impact on the signal of the two metal ions at BiF-CS-GCE. Figure 7a clearly shows that Both Pb(II) and Cd(II) peaks decreased very quickly. And the peak of Cd(II) was more sensitive compare with the Pb(II) when the concentration of copper increased. Especially, the 16 μM copper seriously interferes with the current stripping of the lead and cadmium, at which the peak of lead is disappeared and cadmium is ignored.
The interference of Cu(II) widely distributed in the water sample is becoming one of the most important interference factors. Hence, a good recovery of Pb(II) and Cd(II) stripping signal in the solution which contained large amounts of Cu(II) is a meaningful matter when adding ferrocyanide. Figure 7b shows that the stripping responses current value of lead and cadmium were partially recovered by adding to potassium ferrocyanide in presence of 16 μM Cu(II) after the above experiment [37]. It is clearly seen that the peak value of cadmium increased faster than the lead peak by ferrocynide concentration less than 1.3 × 10−4 M. Then the peak was decreased slowly but the lead peak increased gradually in the range of ferrocynide concentration between 1.3 × 10−4 and 9.3 × 10−4 M possibly due to the competition process between the two metal ions on the stripping response. Additionally, as the copper iron still increased, the both current value inclined. The results show that 11.3 mM ferrocyanide was enough in alleviating the Cu (II) interference, with the two metal ions’ peak recovered at 83 % and 39 %, which is compared to the original stripping signal, respectively.
Tap water analysis
All values are the mean of three measurements.
The BiONPs-CS-GCE electrode was then applied to detect the Pb(II) and Cd(II) in tap water sample by using the standard addition method. The heavy metal content detected is reported in Table 2. The ability of the electrode for the tap water analysis was further investigated by the recovery experiment, with an average result of 107.3 % for Pb (II) and 106.7 % for Cd (II), respectively.
Conclusions
A composite film modified electrode was prepared from BiONPs, and chitosan for heavy metal ions sensing. Because of the good absorption capacity of chitosan, and the superior electrochemical property of bismuth oxide nanoparticles, the self-made electrode demonstrated excellent analytical performance. Under the optimized conditions, the bismuth film (BiF-CS-GCE) sensor obtained through electrochemical reduction of the BiONPs-CS-GCE was applied to determine the trace Pb (II) and Cd (II) ions by DPASV. The sensor displayed relatively low detection of limits. The Cu (II) interference can be partially eliminated to a certain degree when adding 11.3 mM ferrocyanide to the solution which contained large amounts of Cu (II). Furthermore, a satisfactory result is obtained from the detection of the trace Pb (II) and Cd (II) in tap water with the BiF-CS-GCE electrode. Our works put forward a semi-disposable sensor based on a composite of Bi2O3 nanomaterials and chitosan. The method of fabricating the Bi-CS-GCE sensor with the aid of an electroreduction is an attempt. Though it remains to be further improved, the properties of the self-designed bismuth film sensor are comparable with those of the reported in literature.
References
Zhang MK, Liu ZY, Wang H (2010) Use of single extraction methods to predict bioavailability of heavy metals in polluted soils to rice. Commu Soil Sci Plan 41(7):820–831
Dos Santos VB, Fava EL, De Miranda Curi NS, Fara RC, Fatibello-Filho O (2010) A thermostated electrochemical flow cell with a coupled bismuth film electrode for square-wave anodic stripping voltammetric determination of cadmium(II) and lead(II) in natural. Talanta 126:82–90
Wei BG, Yang LS (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94(2):99–107
Murai K, Okano M, Kuramitz H, Hata N, Kawakami T, Taguchi S (2008) Investigation of formaldehyde pollution of tap water and rain water using a novel visual colorimetry. Water Sci Technol 58(5):1055–1060
Wang ZQ, Wang H, Zhang ZH, Liu G (2014) Electrochemical determination of lead and cadmium in rice by a disposable bismuth/electrochemically reduced grapheme/ionic liquid composite modified screen-printed electrode. Sensor Actuat B-Chem 199:7–14
Getaneh Z, Mekonen S, Ambelu A (2014) Exposure and health risk assessment of lead in communities of jimma town. Bull Environ Contam Toxicol 93(2):245–250
Eiband MMSG, Trindade KC, De A, Gama K, De Melo JV, Martínez-Huitle CA, Ferro S (2014) Elimination of Pb2+ through electrocoagulation: applicability of adsorptive stripping voltammetry for monitoring the lead concentration during its elimination. J Electroanal Chem 717:213–218
Hillyer MM, Finch LE, Cerel AS, Dattelbaum JD, Leopold MC (2014) Multi-technique quantitative analysis and socioeconomic considerations of lead, cadmium, and arsenic in children’s toys and toy jewelry. Chemosphere 108:205–213
Liu BZ, Lu LY, Zi YQ (2008) A study of nafion-coated bismuth-film electrode for the determination of zinc, lead, and cadmium in blood samples. Electroanal 20(21):2363–2369
Baldrianova L, Svancara I, Sotiropoulos S (2007) Anodic stripping voltammetry at a new type of disposable bismuth-palted carbon paste mini-electrodes. Anal Chim Acta 599(2):249–255
Barton J, García MBG, Santos DH, Fanjul-Bolado P, Ribotti A, McCaul M, Diamond D, Magni P (2016) Screen-printed electrodes for environmental monitoring of heavy metal ions: a review. Microchimica Acta, on the Web. doi:10.1007/s00604-015-1651-0
Rehacek V, Hotovy I, Vojs M (2014) Bismuth film voltammetric sensor on pyrolyzed photoresist/alumina support for determination of heavy metals. Electroanal 26(5):898–903
Hutton EA, Hočevar SB, Ogorevc B (2005) Ex situ preparation of bismuth film microelectrode for use in electrochemical stripping microanalysis. Anal Chim Acta 527(1–2):285–292
Economou A (2005) Bismuth-film electrodes: recent developments and potentialities for electroanalysis. Trac-Trend Anal Chem 24(4):334–340
Li YS, Sun GQ, Zhang YH, Ge CW, Bao N, Wang YH (2014) A glassy carbon electron modified with bismuth nanotubes in a silsesquioxane framework for sensing of trace lead and cadmium by stripping voltammetry. Microchim Acta 181(7–8):751–757
Marinho JZ, Silva RAB, Barbosa TGG, Richter EM, Muňoz RAA, Lima RC (2015) Graphite-composite electrodes bulk-modified with (BiO)2CO3 and Bi2O3 plates-like nanotructures for trace metal determinaiton by anodic stripping voltammetry. Electroanal 25(3):765–760
Yang D, Wang L, Chen ZL, Megharaj M, Naidu R (2014) Anodic stripping voltammetric determination of traces of Pb (II) and Cd (II) using a glassy carbon electrode modified with bismuth nanoparticles. Microchim Acta 181:1199–1206
Nui PF, Fernández-Sánchez C, Gich M, Navarro-Hernández C, Fanjul-Bolado P, Roig A (2015) Screen-printed electrodes made of a bismuth nanoparticle porous carbon nanocomposite applied to the determination of heavy metal ions. Microchim Acta. doi:10.1007/s00604-015-1684-4
Sahoo S, Satpati AK, Reddy AVR (2015) Electrodeposited Bi-Au nanocomposite modified carbon paste electrode for the simultaneous determination of copper and mercury. RSC Adv 5(33):25794–25800
Lezi N, Economou A, Dimovasilis PA, Trikalitis PN, Prodromidis MI (2012) Disposable screen-printed sensors modified with bismuth precursor compounds for the rapid voltammetric screening of trace Pb (II) and Cd (II). Anal Chim Acta 728:1–8
Ping PJ, Wu J, Ying YB (2012) Determination of trace heavy metals in milk using an ionic liquid and bismuth oxide nanoparticles modified carbon paste electrode. Chinese Sci Bull 57(15):1781–1787
Sopha H, Baldrianová L, Tesařová E, Grincienė G, Weidlich T, Švancara I, Hočevar SB (2010) A new type of bismuth electrode for electrochemical stripping analysis based on the ammonium tetrafluorobismuthate bulk-modified carbon paste. Electroanal 22(13):1489–1493
Zeynali KA, Mollarasouli F (2014) Bismuth and bismuth-chistosan modified electrodes for determination of two synthetic food colorants by net analyte signal standard addition method. Cent Eur J Chem 12(6):711–718
Wu LQ, Gadre AP, Yi H, Kastantin MJ, Rubloff GW, Bentley WE, Payne GF, Ghodssi R (2002) Voltage-dependent assembly of the polysaccharide chitosan onto an electrode surface. Langmuir 18(22):8620–8625
Taufik S, Yusof NA, Tee TW, Ramli I (2011) Bismuth oxide nanoparticles/chitosan/modified electrodes as biosensor for DNA Hybrideization. J Electrochem Sc 6(6):1880–1891
Tseng TK, Choi J, Jung DW, Davidson M, Holloway PH (2010) Three-dimensional self-assembled hierarchical architectures of gamma-phase flowerlike bismuth oxide. Appl Mater Interfaces 2(4):943–946
Pauliukaite R, Metelka R, Švancara I, Krόlicka A, Bobrowski A, Vytřas K, Norkus E, Kalcher K (2002) Carbon paste electrodes modified with Bi2O3 as sensors for the determination of Cd and Pb. Anal Bioanal Chem 374(6):1155–1158
Tong ZZ, Han JS (1992) Potentiometric String Analysis. Nankai University Press, Tianjin, pp 63–69
Dimovasilis PA, Prodromidis MI (2013) Bismuth-dispersed xerogel-based composite films for trace Pb(II) and Cd(II) voltammetric determination. Anal Chim Acta 769(6):49–55
Rehacek V, Hotovy I, Vojs M, Kups T, Spiess L (2012) Nafion-coated bismuth film electrodes on pyrolyzed photoresist/alumina supports for analysis of trace heavy metals. Electrochim Acta 63:192–196
Zhu WW, Li NB, Luo HO (2006) Anodic stripping voltammetry determination of Pb(II) and Cd(II) at a bismuth/poly(aniline) film electrode. Anal Lett 39(11):2273–2284
Wang Z, Lee PM, Liu E (2013) Graphene thin film electrodes synthesized by thermally treating co-sputtered nickel-carbon mixed layers for detection of trace lead, cadmium and copper ions in acetate buffer solutions. Thin Solid Films 544(4):341–347
Injang U, Noyrod P, Siangproh W, Siangproh W, Dungchai W, Motomizu S, Chailapakul O (2010) Determination of trace heavy metals in herbs by sequential injection analysis-anodic stripping voltammetry using screen-printed carbon nanotubes electrodes. Anal Chim Acta 668(1):54–60
Xu H, Zeng LP, Xing SJ, Xian YZ, Shi GY (2008) Ultrasensitive voltammetric detection of trace lead(II) and cadmium(II) using MWCNTs-nafion/bismuth composite electrodes. Electroanal 20(32):2655–2662
Vicentini FC, Silva TA, Pellatieri A, Janegitz BC, Filho OF, Fara RC (2014) Pb(II) determination in natural water using a carbon nanotubes paste electrode modified with crosslinked chitosan. Microchem J 116:191–196
Kadara RO, Tothill IE (2008) Development of disposable bulk-modified screen printed electrode based on bismuth oxide for stripping chronopotentiometric analysis of lead(II) and cadmium(II) in soil and water samples. Anal Chim Acta 623(1):76–81
Yang D, Wang L, Chen Z, Megharaj M, Naidu R (2013) Investigation of copper(II) interference on the anodic stripping voltammetry of lead(II) and cadmium(II) at bismuth film electrode. Electroanal 25(12):2637–2644
Yang D, Wang L, Chen ZL, Megharaj M, Naidu R (2014) Voltammetric determination of lead(II) and cadmium(II) using a bismuth film electrode modified with mesoporous silica nanoparticles. Electrochim Acta 132(19):223–229
Acknowledgments
We gratefully acknowledge the National Natural Science Foundation of China (61571245), the Open Project Program of State Key Laboratory of Analytical Chemistry for Life Science (Nanjing University) (KLACLS1010), the Program of Department of Education of Jiangsu Province (12KJD610003), the Natural Science Foundation of Jiangsu Province (BK20131249), the Senior Personnel Scientific Research Foundation of Jiangsu University (15JDG084), and the Postgraduate Research and Innovation Project of Jiangsu University (KYXX_0026) for financial support of this research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests
Electronic supplementary material
ESM 1
(DOC 59 kb)
Rights and permissions
About this article
Cite this article
Hao, C., Shen, Y., Shen, J. et al. A glassy carbon electrode modified with bismuth oxide nanoparticles and chitosan as a sensor for Pb(II) and Cd(II). Microchim Acta 183, 1823–1830 (2016). https://doi.org/10.1007/s00604-016-1816-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1816-5