Abstract
It is shown that metallothionein-stabilized copper nanoclusters (MT-CuNCs) display catalase-like activity. In the presence of either lead(II) or mercury(II), the catalase-like activity is converted to a peroxidase-like activity. On addition of Pb(II) or Hg(II), the inhibitory effect of MT-CuNCs on the chromogenic reaction of 3,3′,5,5′-tetramethylbenzidine (TMB) with H2O2 is weakened. On the other hand, the catalytic effect of the nanoclusters on the chromogenic reaction is increased. The system MT-CuNCs-Pb(II)/Hg(II) exhibits high affinity for the substrates TMB and H2O2. Their catalytic behavior follows Michaelis-Menten kinetics. Based on these findings, a method was developed for visual detection (via the blue coloration formed) and spectrophotometric determination (at 450 nm) of Pb(II) and Hg(II). The linear range for Pb(II) extends from 0.7 to 96 μM, and the linear ranges for Hg(II) from 97 nM to 2.3 μM and from 3.1 μM to 15.6 μM. The detection limits are 142 nM for Pb(II) and 43.8 nM for Hg(II).

Metallothionein-stabilized copper nanoclusters (MT-CuNCs) display catalase-like activity. On addition of Pb(II) or Hg(II), the catalase-like activity is converted to a peroxidase-like activity. The latter catalyzes the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) by H2O2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Catalase is an antioxidant enzyme that catalyzes the decomposition of H2O2 into O2 and H2O. Catalase can effectively scavenge H2O2 in vivo so that H2O2 cannot react with O2 under the action of iron chelates to produce highly oxidative hydroxyl radicals (·OH) [1]. Natural peroxidase is an enzyme that catalyzes the oxidation of specific substrates by providing electrons for H2O2 (electron acceptor). Natural enzymes catalyze reactions under very mild biological conditions, which limits their applications [2]. Compared to natural enzymes, many artificial nanozymes, such as carbon nanotubes, magnetic nanomaterials [3, 4], noble metal-graphene complexes [5], and metal nanoclusters [6, 7], have the advantages of simple manufacturing, low cost, high catalytic activity, good stability, etc. [8]. In particular, metal nanoclusters have been widely applied [9].
Lead and mercury ions can pollute the environment and endanger health. In order to quantify the concentration of Pb(II) and Hg(II) in water samples, analytical methods such as inductively coupled plasma mass spectrometry (ICP-MS), atomic absorption spectroscopy (AAS) and electrochemical analysis have been developed. The disadvansatages of these methods are bulky instruments, high cost, the need for professionally operators [10, 11]. Therefore, it is still highly desirable to develop a rapid and convenient method for detecting Pb(II) and Hg(II).
Pb(II) and Hg(II) can inhibit or enhance the activity of artificial nanozymes under certain conditions thus establishing a new method for detecting Pb(II) or Hg(II)..Zhu [12] et al. found that Hg2+ inhibited the peroxidase-like activity of bovine serum albumin-capped AuNCs via Au+-Hg2+ interaction. Liao [13] et al. developed a Pb2+ assay due to the ability of Pb2+ to induce the aggregation of glutathione (GSH) stablized AuNCs and enhance their peroxidase-like activity. Tseng [14] et al. prepared bimetallic Pt/Au NPs and found that Hg2+ transformed this peroxidase-like activity into a catalase-like activity. Yuan [15] et al. researched that the peroxidase-like activity of molybdenum disulfide nanosheets (MoS2-NSs) was stimulated by Hg2+. Most of the platforms in related reports are based on the use of the Hg2+-Au+ reaction to change the enzyme activity of gold-containing nanozymes. By contrast, a study on the modfication of copper nanoclusters with enzyme-mimicking activity based on the heavy metal ions Pb2+ and Hg2+ has not been reported.
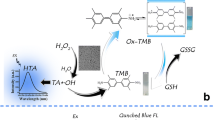
Herein, the effects of the heavy metal ions Pb2+ and Hg2+ on the enzyme-like activity of MT-CuNCs were explored, and Pb2+ and Hg2+ assay strategies were established. It is found that MT-CuNCs have catalase-like activity and that Pb2+/Hg2+ can induce the conversion of this catalase-like activity to peroxidase-like activity. Under certain conditions, H2O2 can self-oxidize to produce a small number of hydroxyl radicals (·OH), thus oxidizing TMB to 3,3′,5,5′-tetramethylbenzidine diimine (oxTMB). Correspondingly, the solution changes from colorless to light blue (Scheme 1a). The MT-CuNCs have catalase-like activity, which can decompose H2O2 and inhibit the oxidation of TMB. The solution color consequently become lighter (Scheme 1b). In the presence of Pb2+ or Hg2+, the catalase-like activity of the MT-CuNCs can be converted into peroxidase-like activity, thus catalyzing the oxidation and chromogenic reaction of TMB mediated by H2O2. Therefore, the solution changes from colorless to dark blue (Scheme 1c). Accordingly, a detection of Pb2+ or Hg2+ based on colorimetry and spectrophotometry was established. This detection method is selective,and feasible and has a wide linear range. The development of this method is informative for the progress of enzyme mimics.
Experimental section
Reagents and materials
Pb2+ and Hg2+ standard solutions were supplied by the Chinese Academy of Metrology (Beijing, China, www.nim.ac.cn). MT (induced by Zn2+ with a purity higher than 99%) was acquired from Yongkang Jiaxin Biological Technology Co., Ltd. (Beijing, China, www.jiaxintool.cn.globalimporter.net). CuCl2, catalase (CAT), TMB, phosphate buffered saline (PBS), 2′-diamino-bis-3-ethylbenzothiazolin-6-sulfonic acid (ABTS) and absolute ethanol were supplied by McLean Biochemical Technology Co., Ltd. (Shanghai, China, www.macklin.company.com). NaOAc, H2O2 and H2SO4 were obtained from Beijing Chemicals Reagent Company (Beijing, China, www.bicr.com). The electrical resistivity of the ultrapure water used was 18.25 MΩ·cm.
Apparatus
All ultraviolet-visible absorbance spectra obtained using a Shimadzu UV-2450 spectrophotometer (Kyoto, Japan, www.shimadzu.com). A THERMO-SHAKER thermostat mixer (Heros, China) was utilized for the synthesis of the MT-CuNCs. The fluorescence spectra were acquired on a Lumina spectrofluorometer (Thermo Fisher Scientific, USA, www.thermofisher.com). A Titan G2 60–300 transmission electron microscope (FEI, USA, www.fei.com), a K-Alpha 1063 X-ray photoelectron spectrometer (Thermo Fisher Scientific, USA, www.thermofisher.com) and a Shimadzu IR Prestige-21 Fourier transform infrared spectrometer (Kyoto, Japan, www.shimadzu.com) were used to characterize the MT-CuNCs mixed with Pb2+ or Hg2+.
Preparation of MT-CuNCs
Briefly, MT solution (600 μL, 2.25 mg·mL−1) and CuCl2 solution (100 μL, 3 mM) were mixed at 55 °C. NaOH solution (200 μL, 1.0 M) was added 15 min later. The reaction was proceeded with shaking at 800 rpm for 12 h at 55 °C. After filtering through a 0.22 μm filter membrane, the light purple solution was stored at 4 °C.
Determination of Pb2+ and Hg2+
In a 2 mL EP tube, NaOAc-HOAc buffer (pH 4.0, 80 μL, 200 mM), MT-CuNCs solution (30 μL), TMB solution (40 μL, 5 mM), H2O2 solution (60 μL, 10 mM), and a certain amount of Pb2+ standard solution were mixed. The reaction took place for 40 min at 40 °C in a thermostatic mixer. Then, H2SO4 solution (100 μL, 0.2 M) was introduced, and the measurement was performed by colorimetry. Moreover, the absorbance value of this system at 450 nm was measured.
Likewise, in a 2 mL EP tube, NaOAc-HOAc buffer (pH 3.25, 60 μL, 200 mM), MT-CuNCs solution (20 μL), TMB solution (50 μL, 5 mM), H2O2 solution (60 μL, 10 mM), and a certain amount of Hg2+ standard solution were mixed. The reaction took place for 40 min at 40 °C in a thermostatic mixer. Then, H2SO4 solution (100 μL, 0.2 M) was introduced, and the absorbance of the system at 450 nm was measured.
The absorbance change of the system was calculated as follows: ΔA = A - A0. A and A0 represent the absorbance values in the presence and absence of Pb2+/Hg2+, respectively.
Results and discussion
Transformation of the enzymatic activity of MT-CuNCs induced by Pb2+ or Hg2+
The catalase-like activity of MT-CuNCs was verified by monitoring the absorbance of H2O2 at 240 nm, because catalase can catalyze the decomposition of H2O2 [16]. As shown in Fig. S1, the MT-CuNCs have high catalase-like activity and that their relative activity is 2.8 times that of the positive control group (1.5 mg·mL−1 CAT).
The enzyme activity transition of MT-CuNCs after the addition of Pb2+/Hg2+ was verified by UV-Vis spectroscopy (Fig. 1a). Both TMB (a curve) and the MT-CuNCs-TMB system (b curve) have no absorption peak at 450 nm. The A450 of the TMB solution (c curve) increases significantly after reacting with H2O2, indicating that H2O2 may yield ·OH through self-oxidation under these conditions. The ·OH species oxidizes TMB into oxTMB. After the reaction was terminated with H2SO4, the solution appeared light yellow and exhibited an absorption peak at 450 nm. In the MT-CuNCs system (d curve), the TMB-H2O2 chromogenic reaction is suppressed, and the A450 value decreases. This effect may occur because MT-CuNCs with catalase-like activity catalyze the decomposition of H2O2 and inhibit the production of ·OH. When Pb2+ or Hg2+ was added, the A450 values of the MT-CuNCs-Pb2+/Hg2+-TMB-H2O2 system increased gradually with increasing Pb2+ or Hg2+ concentration, suggesting that Pb2+ or Hg2+ can convert the catalase-like activity of MT-CuNCs into peroxidase-like activity. At this time, H2O2 may be adsorbed on the surface of the MT-CuNCs and turned into highly active ·OH via O-O bond cleavage, thus generating the catalytic activity of the nanoclusters [17]. The kinetics of each system within 60 min were further investigated (Fig. 1b). The absorbance of the TMB-H2O2 system (a curve) at 652 nm increases over time. After the addition of MT-CuNCs (b curve), the absorbance value from 0 to 1700 s increases very slowly, and the slope of the kinetic curve after 1700 s is still lower than that of the TMB-H2O2 system. These results indicate that MT-CuNCs show catalase-like activity, which inhibites the chromogenic reaction of TMB. In the presence of Pb2+/Hg2+ and MT-CuNCs (c, d curve), the A652 value increases slowly from 0 to 1200 s. Later, the slope of the kinetic curve is larger than that of the TMB-H2O2 system. These results confirm our hypothesis that Pb2+ or Hg2+ can reduce and transform the catalase-like activity of MTs-CuNCs into peroxidase-like activity, The latter catalyzes the oxidation reaction of TMB.
Switching effects of Pb2+/Hg2+ on enzyme-like activity of MT-CuNCs a Absorption spectrum of the systems with acid termination. (a) TMB. (b) TMB-MT-CuNCs. (c) TMB-H2O2. (d) TMB-H2O2-MT-CuNCs. (e) TMB-H2O2-MT-CuNCs-Hg2+. (f) TMB-H2O2-MT-CuNCs-Pb2+. b Absorbance-time curves (at 652 nm) of the systems. (a) TMB-H2O2。 (b) TMB-H2O2-MT-CuNCs. (c) TMB-H2O2-MT-CuNCs-Pb2+. (d) TMB-H2O2-MT-CuNCs-Hg2+
Mechanistic studies
Because surface chemistry is significant in regulating electron transfer and substrate adsorption, it affects the catalytic activity of nanozymes. Liu et al. found that fluoride capping enhanced the oxidase activity of cerium oxide NPs by more than 100 times [18], while DNA increased the peroxidase activity of Fe3O4 NPs [19]. The mechanism of these effects was F− and DNA regulating the surface charges of nanozymes, thereby enhancing the electron transfer and substrate adsorption on the surface of the nanozymes. In this study, the positive charges on the surface of the MT-CuNCs increased after their interaction with Pb2+ or Hg2+, which might cause charge repulsion with positively charged TMB and weaken the catalytic activity of the MT-CuNCs in the oxidation of TMB. However, this hypothesis is not consistent with the enhancement of peroxidase activity in the experimental phenomenon. Hence, negatively charged ABTS was used as the chromatic substrate for validation (Fig. S2). The results are similar to that obtained when TMB is used as a substrate. Therefore, the transformation function of Pb2+/Hg2+ in the enzymatic activity of MT-CuNCs should not be attributed to the regulation of surface charges.
Changes in the morphology and size of nanomaterials may affect their intrinsic properties. Liao [13] et al. found that Pb2+ increased the peroxidase-like activity of GSH-AuNCs and confirmed that the reason was aggregation-induced enzyme activity enhancement, similar to that of aggregation-induced luminescence. TEM images (Fig. S3) show that both Pb2+ and Hg2+ can induce the aggregation of MT-CuNCs and an increase in the size of the MT-CuNCs. With the addition of Pb2+ or Hg2+, the photoluminescence property of the MT-CuNCs is decreased, and aggregation-induced luminescence enhancement is not observed. For further verification, the effect of Ag+ on the size and enzyme activity of MT-CuNCs was investigated. The size of MT-CuNCs is increased, but the absorption of oxTMB at 450 nm hardly enhance (Fig. S4). These results reveal that the enzymatic activity transformation of MT-CuNCs is not caused by the change of the morphology and size.
The catalytic activity of metal NCs and metal NPs is correlated with the valences of metal ions/complexes formed on their surfaces. Lien [20] investigated the influences of various metal ions on the oxidase activity, peroxidase-like activity and catalase-like activity, of AuNPs. They found that the formation of a metal-Au alloy over AuNPs greatly affected their catalytic performance. Therefore, in this study, the modulating function of Pb2+ or Hg2+ on the enzymatic activity of MT-CuNCs may be ascribed to the generation of Pb-Cu/Hg-Cu alloys, which was further verified by XPS (Fig. 2). The Cu 2p spectrum of MT-CuNCs show the characteristic peaks at 932.79 and 952.54 eV without the satellite peak of Cu(II) at approximately 942 eV, indicating the existence of Cu(0) and Cu(I) rather than Cu(II) (a) [21, 22]. After the interaction between MT-CuNCs and Pb2+, the Cu 2p spectrum did not change significantly. The characteristic Pb 4f peaks of Pb(II) and Pb(0) are observed at 138.34/143.29 eV and 136.29/141.35 eV, respectively (b) [23]. In a pH range of 3.5–4.5, the thiolate ligands (Zn2+-MT bonds) in MT were partially dissociated [24],and Pb2+ diffused into the α domains of MT and was reduced into Pb(0), which would be deposited on the surface of the MT-CuNCs to form a Pb-Cu alloy. In the Cu 2p spectrum of the MT-CuNCs-Hg2+ system (c), the characteristic satellite peak of Cu(II) is observed at 942.46 eV. Hg2+ might react with the Cu+ on the surface of the MT-CuNCs to generate Hg(0). The characteristic Hg4f peaks of Hg(II) and Hg(0) is observed at 100.86/104.91 eV and 99.54/103.59 eV, respectively (d) [25]. Hg2+ competed with Zn2+ to bind the sites in the α domains of MT, and a portion of Hg2+ was reduced to Hg(0) by MT. Consequently, the Pb-Cu/Hg-Cu alloy formed on the surface of the MT-CuNCs transformed their catalase-like activity into peroxidase-like activity.
XPS spectra of the elements (Cu, Pb, Hg) in the absence and presence of Pb2+/Hg2+. a Cu 2p curve of MT-CuNCs. b Pb 4f curve in MT-CuNCs-Pb2+ system. c Cu 2p curve in MT-CuNCs-Hg2+ system. d Hg4f curve in MTs-CuNCs-Hg system. The solutions of MT-CuNcs and MT-CuNCs-Pb2+/Hg2+ were prepared respectively. XPS measurement were performed after vacuum drying at low temperature. cPb(II) = 10 μM, cHg(II) = 2.0 μM
The structural changes in MT-CuNCs after the addition of Pb2+/Hg2+ (Fig. 3) were analyzed with FTIR. After the reaction with Pb2+, the number of hydrogen bonds (at 3000–3600 cm−1) in the MT-CuNCs system is increased, and the asymmetric stretching vibration of +N-H···O− (at 2110 cm−1) is intensified [26], along with the production of the N-H stretching peak (amide II bonds, at 1552 cm−1). Likewise, after the interaction between Hg2+ and MT-CuNCs, the transmittance values of the bands corresponding to C-N/C-C (amide III bonds, at 1398 cm−1), C=O/N-H (amide I bonds, at 1641 cm−1) and N-H/O-H decrease significantly, and bands corresponding to amide II bonds appear [27]. All these indicate that the interactions among Pb2+/Hg2+, Cu/Cu+ and MT change the coordination bonds in the MT-CuNCs.
Fourier transform infrared spectrum of MT-CuNCs in (a) the absence and presence of (b) Pb2+ or (c) Hg2+. The solutions of the systems (MT-CuNCs, MT-CuNCs-Pb2+ and MT-CuNCs-Hg2+) were prepared respectively. The solutions were dropped onto KBr flakes for determination. cPb(II) = 10 μM, cHg(II) = 2.0 μM
Steady-state kinetic analysis
The affinity of MT-CuNCs-Pb2+/Hg2+ for H2O2 and TMB was investigated via steady-state kinetic analysis [28]. The results (Figs. S5 and S6) show that after the reaction between the MT-CuNCs and Pb2+/Hg2+, the process of catalyzing the TMB-H2O2 reaction follow the typical Michaelis-Menten model (a, b). Using the Lineweaver-Burk double-reciprocal method, it is found that there is a positive correlation between the reciprocal of the initial rate (1/v) and the reciprocal of the TMB concentration (1/[S]) (c, d) [29]. After linear fitting, the values of maximum reaction rate (vmax) and the Michaelis constant (Km) were received. The affinities of MT-CuNCs-Pb2+/Hg2+ (Km = 1.08/3.45) for H2O2 is higher than that of HRP for H2O2 (Km = 3.70), showing that only a small amount of H2O2 is enough to commence the catalytic oxidation reaction in the MT-CuNC-Pb2+/Hg2+ systems (Table S1).
Detection of Pb2+ and Hg2+
In order to improve analysis performance, the reaction conditions were optimized (Figs. S7 - S12). Under the selected reaction conditions, the selectivity of this method for Pb2+ was investigated with a 10 μM Pb2+ solution as a reference. According to the experimental results (Fig. S12a), within a relative deviation of ±5%, species at 500 times (Zn2+ or Na+), 250 times (Mg2+, K+, or Cd2+), 100 times (Ca2+ or Mn2+), 50 times (Al3+), 25 times (Ag+ or Cu2+), and 10 times (Hg2+ or Fe3+) greater concentrations do not interfere with the determination of Pb2+. Moreover, in the system for Hg2+ determination, the influence of several likely interfering components on the determination of Hg2+ was tested with a 2 μM Hg2+ solution as a reference. Within a relative deviation of ±5%, species at 500 times (K+ or Ca2+), 250 times (Na+ or Cd2+), 100 times (Mg2+, Zn2+, Mn2+ or Pb2+), 50 times (Al3+), 25 times (Ag+ or Cu2+), and 10 times (Fe3+) greater concentrations do not interfere with the determination of Hg2+ (Fig. S12b). In other words, this approach has great selectivity performance.
The calibration plot for the determination of Pb2+ and Hg2+ were plotted under the optimized experimental conditions. Within the concentration range cPb(II) of 707 nM-96 μM, a great linear relationship is observed (Fig. 4). The equation of linear regression was ΔA = 0.0113cPb(II) (μM)-0.034 with a correlation coefficient (r) of 0.9987. The detection limit (LOD) was calculated to be 142 nM based on the equation LOD = 3Sb/k. Precision experiments (n = 11) were carried out for three standard solutions with Pb2+ concentrations of 1.5, 60, and 96 μM. The relative standard deviations were 1.65%, 0.5% and 3.11%, respectively. These results show that this method for detecting Pb2+ has high accuracy. In the cHg(II) ranges of 97 nM-2.325 μM and 3.10 μM–15.59 μM, the ΔA value and Hg2+ concentration are in a good linear relationship (Fig. 5). The regression equations were ΔA = 0.0413cHg(II) (μM) + 0.0231 (r = 0.9950) and ΔA = 0.0157cHg(II) (μM) + 0.0159 (r = 0.9961), respectively. LOD of this method was 43.8 nM. Three standard Hg2+ solutions with concentrations of 0.775, 2.325, and 12.46 μM were each measured 11 times, and the relative standard deviations were 3.02%, 1.62%, and 2.32%, respectively. These demonstrate that the precision of this method for detecting Hg2+ is good. In comparison with other analytical methods for Pb2+/Hg2+ determination, the data in the Table 1 show that the method for detecting Pb2+/Hg2+ has a wide linear range and relatively good sensitivity.
a Absorption spectra (at 450 nm) of the TMB-H2O2-MT-CuNCs system in the absance and presence of different Pb2+ concentrations (from a to q: 0, 0.75, 1.5, 3, 6, 9, 12, 15, 18, 24, 36, 42, 48, 54, 60, 72, and 96 μM). Insert: the chromogenic photograph of different Pb2+ concentrations (from left to right: 0, 1.5, 6, 12, 18, 24, 36, 42, 48, 54, 60, 72, 96 μM). The photograph was obtained without performing acid termination, because blue is easier to distinguish than yellow. b Calibration plot of Pb2+ detection (concentration is consistent with that in the absorption spectra)
Absorption spectra (at 450 nm) of the TMB-H2O2-MT-CuNCs system in the absance and presence of different Hg2+ concentrations. Insert: the chromogenic photograph of different Hg2+ concentrations. The photograph was obtained without performing acid termination. a (from a to h: 0, 0.097, 0.195, 0.39, 0.775, 1.55, and 2.325 μM). Insert: (from left to right: 0, 0.097, 0.39, 0.775, 1.55, and 2.325 μM). c (from a to i: 3.1, 3.875, 4.672, 6.23, 7.788, 9.345, 10.903, 12.46, and 15.56 μM). Insert: (from left to right: 3.1, 4.672, 7.788, 9.345, 10.903, 12.46, and 15.56 μM). b, d Calibration plot of Hg2+ detection (concentration is consistent with that in the absorption spectra)
Also, the method was employed to determine the ion concentrations in environmental water samples. After filtering twice with quantitative filter paper, all environmental water samples (collected from tap water, the pond water, Xiangjiang River,) were heated and boiled for 10 min. After cooling to room temperature, the solution was filtered through a 0.22 μm filter membrane for subsequent analysis. The recovery test of spiked samples were performed. The results (Tables S2,S3) demonstrate obviously that this method can be supplied for environmental water samples.
Conclusions
The relative catalase-like activity of MT-CuNCs is 2.8 times that of 1.5 mg·mL−1 CAT. Due to the generation of the Pb-Cu/Hg-Cu alloy, Pb2+/Hg2+ induced the conversion of the catalase-like enzymatic activity of MT-CuNCs to peroxidase-like enzymatic activity. The latter catalyzed TMB-H2O2 oxidation according to a typical Michaelis-Menten model, and exhibited a higher affinity for H2O2 than HRP. Furthermore, a reliable colorimetry/spectrophotometry method for detecting Pb2+/Hg2+ was established.. which had great selectivity, wide linear ranges and high sensitivity. The assay displays potential application for monitoring Pb2+/Hg2+ concentration in environmental water sample.
References
Zhang HL, Ma KX, Yang YJ, Shi CY, Chen GW, Liu DZ (2018) Molecular cloning, characterization, expression and enzyme activity of catalase from planarian Dugesia japonica in response to environmental pollutants. Ecotoxicol Environ Saf 165:88–95
Shi Y, Yang M, Liu L, Pang Y, Long Y, Zheng H (2018) GTP as a peroxidase-mimic to mediate enzymatic cascade reaction for alkaline phosphatase detection and alkaline phosphatase-linked immunoassay. Sensors Actuators B Chem 275:43–49
Zhang T, Cao C, Tang X, Cai Y, Yang C, Pan Y (2017) Enhanced peroxidase activity and tumour tissue visualization by cobalt-doped magnetoferritin nanoparticles. Nanotechnology 28:045704
Wei H, Wang E (2008) Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal Chem 80:2250–2254
Tao Y, Lin YH, Huang ZZ, Ren JS, Qu X (2013) Incorporating graphene oxide and gold nanoclusters: a synergistic catalyst with surprisingly high peroxidase-like activity over a broad pH range and its application for cancer cell detection. Adv Mater 25:2510–2510
Tao Y, Lin YH, Ren J, Qu X (2013) A dual fluorometric and colorimetric sensor for dopamine based on BSA-stabilized Au nanoclusters. Biosens Bioelectron 42:41–46
Liu Y, Ding D, Zhen YL, Guo R (2017) Amino acid-mediated ‘turn-off/turn-on’ nanozyme activity of gold nanoclusters for sensitive and selective detection of copper ions and histidine. Biosens Bioelectron 92:140–146
Santhosh M, Chinnadayyala SR, Kakoti A, Goswami P (2014) Selective and sensitive detection of free bilirubin in blood serum using human serum albumin stabilized gold nanoclusters as fluorometric and colorimetric probe. Biosens Bioelectron 59:370–376
Tao Y, Lin Y, Huang Z, Ren J, Qu X (2012) Poly(acrylic acid)-templated silver nanoclusters as a platform for dual fluorometric turn-on and colorimetric detection of mercury (II) ions. Talanta 88:290–294
Li CL, Liu KT, Lin YW, Chang HT (2011) Fluorescence detection of lead(II) ions through their induced catalytic activity of DNAzymes. Anal Chem 83:225–230
Li T, Dong SJ, Erkang W (2009) Label-free colorimetric detection of aqueous mercury ion (Hg2+) using Hg2+-modulated G-quadruplex-based DNAzymes. Anal Chem 81:2144–2149
Zhu R, Zhou Y, Wang XL, Zhang HJ, Huang XX, Zheng HZ (2013) Detection of Hg2+ based on the selective inhibition of peroxidase mimetic activity of BSA-Au clusters. Talanta 117:127–132
Liao H, Liu G, Liu Y, Li R, Fu W, Hu L (2017) Aggregation-induced accelerating peroxidase-like activity of gold nanoclusters and their applications for colorimetric Pb(2+) detection. Chem Commun (Camb) 53:10160–10163
Tseng CW, Chang HY, Chang JY, Huang CC (2012) Detection of mercury ions based on mercury-induced switching of enzyme-like activity of platinum/gold nanoparticles. Nanoscale 4:6823–6830
Lu Y, Yu J, Ye WC, Xin Y, Zhou PP, Zhang HX (2016) Spectrophotometric determination of mercury(II) ions based on their stimulation effect on the peroxidase-like activity of molybdenum disulfide nanosheets. Mikrochim Acta 183:2481–2489
Zhang HC, Ma KX, Yang YJ, Shi CY, Chen GW, Liu DZ (2018) Molecular cloning, characterization, expression and enzyme activity of catalase from planarian Dugesia japonica in response to environmental pollutants. Ecotoxicol Environ Saf 165:88–95
Liao H, Hu L, Zhang Y, Yu X, Liu Y, Li R (2018) A highly selective colorimetric sulfide assay based on the inhibition of the peroxidase-like activity of copper nanoclusters. Mikrochim Acta 185:143
Liu B, Huang Z, Liu J (2016) Boosting the oxidase mimicking activity of nanoceria by fluoride capping: rivaling protein enzymes and ultrasensitive F(−) detection. Nanoscale 8:13562–13567
Liu B, Liu J (2015) Accelerating peroxidase mimicking nanozymes using DNA. Nanoscale 7:13831–13835
Lien CW, Chen YC, Chang HT, Huang CC (2013) Logical regulation of the enzyme-like activity of gold nanoparticles by using heavy metal ions. Nanoscale 5:8227–8234
Bhamore JR, Jha S, Mungara AK, Singhal RK, Sonkeshariya D, Kailasa SK (2016) One-step green synthetic approach for the preparation of multicolor emitting copper nanoclusters and their applications in chemical species sensing and bioimaging. Biosens Bioelectron 80:243–248
Han A, Xiong L, Hao S, Yang Y, Li X, Fang G, Liu J, Pei Y, Wang S (2018) Highly bright self-assembled copper nanoclusters: a novel Photoluminescent probe for sensitive detection of histamine. Anal Chem 90:9060–9067
Huang YQ, Yang LN, Wang YS, Xue JH, Chen SH (2018) Protamine-stabilized gold nanoclusters as a fluorescent nanoprobe for lead(II) via Pb(II)-Au(I) interaction. Mikrochim Acta 185:483
Li JR, Xuan W, Li XP, Xiong CH, Wang XC (2010) Research progress in metallothionein. Food Sci 31:392–396
Huang YQ, Fu S, Wang YS, Xue JH, Xiao XL, Chen SH, Zhou B (2018) Protamine-gold nanoclusters as peroxidase mimics and the selective enhancement of their activity by mercury ions for highly sensitive colorimetric assay of Hg(II). Anal Bioanal Chem 410:7385–7394
Vener MV, Odinokov AV, Wehmeyer C, Sebastiani D (2015) The structure and IR signatures of the arginine-glutamate salt bridge. Insights from the classical MD simulations. J Chem Phys 142:215106
Yan Z, Niu Q, Mou M, Wu Y, Liu X, Liao S (2017) A novel colorimetric method based on copper nanoclusters with intrinsic peroxidase-like for detecting xanthine in serum samples. J Nanopart Res 19:235
Wang GL, Dong YM, Jin LY, Wu XM, Li ZY (2015) Intrinsic enzyme mimicking activity of gold nanoclusters upon visible light triggering and its application for colorimetric trypsin detection. Biosens Bioelectron 64:523–529
Hu L, Yuan Y, Zhang L, Zhao S, Majeed J, Xu G (2013) Copper nanoclusters as peroxidase mimetics and their applications to H2O2 and glucose detection. Anal Chim Acta 762:83–86
Wu HM, Liang JG, Han HY (2008) A novel method for the determination of Pb2+ based on the quenching of the fluorescence of CdTe quantum dots. Microchim Acta 161:81–86
Guo YM, Wang Z, Qu WS, Shao HW, Jiang XY (2011) Colorimetric detection of mercury, lead and copper ions simultaneously using protein-functionalized gold nanoparticles. Biosens Bioelectron 26:4064–4069
Chai F, Wang C, Wang TT, Li L, Su ZM (2010) Colorimetric detection of Pb2+ using glutathione functionalized gold nanoparticles. ACS Appl Mater Interfaces 2:1466–1470
Rameshkumar P, Manivannan S, Ramaraj R (2013) Silver nanoparticles deposited on amine-functionalized silica spheres and their amalgamation-based spectral and colorimetric detection of Hg(II) ions. J Nanopart Res 15:1639–1647
Zhang YF, Yuan Q, Chen T, Zhang XB, Chen Y, Tan WH (2012) DNA-capped mesoporous silica nanoparticles as an ion-responsive release system to determine the presence of mercury in aqueous solutions. Anal Chem 84:1956–1962
Zhao Y, Qiang H, Chen ZB (2017) Colorimetric determination of Hg(II) based on a visually detectable signal amplification induced by a Cu@Au-Hg trimetallic amalgam with peroxidase-like activity. Microchim Acta 184:107–115
Acknowledgments
The authors gratefully acknowledge the support of the National Natural Science Foundation of China (No. 81473021).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 2141 kb)
Rights and permissions
About this article
Cite this article
Liu, R., Zuo, L., Huang, X. et al. Colorimetric determination of lead(II) or mercury(II) based on target induced switching of the enzyme-like activity of metallothionein-stabilized copper nanoclusters. Microchim Acta 186, 250 (2019). https://doi.org/10.1007/s00604-019-3360-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3360-6