Abstract
A facile synthetic method to decorate amine-functionalized silica spheres (SiO2) by silver nanoparticles (Ag NPs) is reported. The transmission electron microscopic (TEM) images showed that spherical Ag NPs with an average particle size of 14 nm were deposited on 250 nm-sized SiO2 spheres (SiO2/Ag NPs). The spectral and colorimetric detection of Hg(II) ions were carried out using the synthesized SiO2/Ag NPs with an experimental detection limit of 5 μM. It was found that the addition of Hg(II) ions (150 μM) into the solution of SiO2/Ag NPs completely quenched the SPR band of the Ag NPs due to the formation of anisotropic Ag amalgam crystals (AgHg). The selective detection of Hg(II) ions by SiO2/Ag NPs in the presence of other environmentally relevant metal ions was also demonstrated using spectral and colorimetric methods.
Graphical abstract
Amine-functionalized silica spheres are decorated by in situ formation of silver nanoparticles and their spectral and colorimetric detection of Hg(II) ions is reported.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The metal nanoparticles (NPs) embedded in silicate sol–gel (SSG) find various applications in the field of catalysis and sensors and hence, the designing of various synthetic methods to prepare metal NPs embedded in silica sol–gel has attracted much attention toward the preparation of catalytic and sensor materials (Maduraiveeran and Ramaraj 2009; Bharathi et al. 1999; Manivannan and Ramaraj 2011; Lev et al. 1997; Pandikumar et al. 2010). Silica (SiO2) spheres are widely used as a supporting material because of their inert character and moreover, they provide higher active surface area by accommodating more number of metal NPs on their surface for catalytic and sensing applications (Zanella et al. 2006). The SiO2 spheres qualify as hard substrates for many reasons (Pol et al. 2002). The SiO2/metal NPs (Ag or Au) structures have received much attention over the years because of their potential applications (Jean et al. 2010; Serra et al. 2009; Lee and El-Sayed 2006; Xu et al. 2009). The metal NPs, especially silver (Ag) NPs, are very sensitive to analytes and the position of surface plasmon resonance (SPR) band of the Ag NPs is highly influenced by the local environment (Manivannan and Ramaraj 2012; Maduraiveeran and Ramaraj 2011; Prasad et al. 2008).
Heavy metal contamination arising from various polluting sources causes serious health and environmental hazards (Clarkson et al. 2003; Campbell et al. 2003; Harris et al. 2003). Among the heavy metals, mercury (Hg(II) ions) is one of the most toxic metals which has drawn much attention due to its toxic impact on environment and also on human health. As the Hg(II) ion possesses a high enough electrochemical potential to oxidize Ag to Ag+, it can alter the absorption band of the Ag NPs and, therefore, Ag NPs could be used for the detection of Hg(II) ions (Li et al. 2010). The nanomaterials used for the detection of Hg(II) ions usually carry functional ligands as major binding sites for the Hg(II) ions (Kim et al. 2010; Huang and Chang 2007; Lee et al. 2007; Xue et al. 2008; Wang et al. 2010). The synthesis of effective adsorbents for metal ions introducing functional ligands on mesoporous SiO2 is one of the most effective strategies for the removal of metal ions (Yantasee et al. 2007, 2008, 2010). Therefore, while developing metal NPs-based sensor for the sensing of metal ions, it should be an ideal design to anchor suitable ligands on the surface of metal NPs by suitable surface functionalization method, which provides an easy access to the incoming analyte molecules without interparticles coupling which could reduce the resolution of sensing (Jean et al. 2010). Hence, we have chosen amine-functionalized SiO2 spheres as a solid support for the in situ formation of Ag NPs embedded in amine-functionalized SSG matrix. Recently, our group has reported the sensing of Hg(II) ions using Ag quantum dots dispersed in functionalized SSG matrix (Maduraiveeran et al. 2011). The optical sensing of melamine using Ag NPs deposited on SiO2 spheres was reported (Jean et al. 2010). It is understood that the SiO2 sphere acts as a well-defined colloidal spacer for the Ag NPs ensuring the isolation of Ag NPs on a support material. In the present work, SiO2 spheres were functionalized using a triamine-silicate matrix to enhance the interaction between Hg(II) ions and Ag NPs on the surface of the SiO2 spheres. Ascorbic acid (AA) is a weak reducing agent that ensures the nucleation and growth of Ag NPs on the amine-functionalized SiO2 spheres (Wilson et al. 2005).
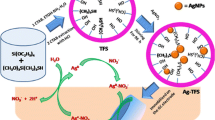
Here, we report Ag–Hg amalgam-based spectral and colorimetric methods for the detection of Hg(II) ions using SiO2/Ag NPs. Colorimetric method, in particular, is extremely attractive because the detection can easily be read out with naked eyes. It was found that the addition of optimum level of Hg(II) ions into the solution of SiO2/Ag NPs completely quenched the SPR band of Ag NPs due to the formation of anisotropic Ag amalgam crystals (AgHg). We have also demonstrated the selective detection of Hg(II) ions using the SiO2/Ag NPs in the presence of various metal ions using spectral and colorimetric methods.
Experimental
Materials and methods
Tetraethylorthosilicate (TEOS), N-[3-(trimethoxysilyl)propyl] diethylenetriamine (TPDT), silver nitrate (AgNO3), β-cyclodextrin (CD), benzyldimethylhexadecylammonium chloride (BDAC), and HgCl2 were received from Sigma-Aldrich. AA, Hg(NO3)2, PbCl2, CuCl2·2H2O, CoCl2·6H2O, CdCl2, CaCl2·2H2O, NiCl2·6H2O, ZnCl2, MnCl2·4H2O, FeCl2·6H2O, FeCl3·6H2O, NaCl, NaOCl, and H2O2 were obtained from Merck. All glassware was thoroughly cleaned with aqua regia (1:3 HNO3/HCl (v/v)). Absorption spectra were recorded with an Agilent Technologies 8453 spectrophotometer using 1 cm quartz cell. Surface morphology was studied using the scanning electron microscopy (SEM) on a HITACHI (Model S-3400) instrument. The aqueous solutions of corresponding Ag NPs were drop-casted and air-dried over the microscopic slide (1 cm × 1 cm) and gold sputtering was carried out over the sample surface prior to the SEM analysis. The high-resolution transmission electron microscopy images were recorded with a FEI TECNAI 30 G2 S-TWIN instrument. Samples for TEM studies were prepared by placing a drop of fresh SiO2/Ag NPs on carbon-coated copper grid and then evaporating the solvent under vacuum.
Synthesis of SiO2/Ag NPs
The amine-functionalized SSG (TPDT)-covered SiO2 spheres were prepared by modified Stober method (Guo et al. 2008). Briefly, ammonium hydroxide (1.7 mL; 25–28 %) was added to ethanol (50 mL) along with TEOS (1.5 mL) and water (1 mL) under vigorous stirring and after 3 h, an additional 1 mL of TEOS was added. The SiO2 spheres were obtained after 12 h of stirring and then TPDT (0.4 mL) was added to the solution and the stirring was continued for further 6 h. The TPDT-functionalized SiO2 particles were purified twice by centrifugation and redispersed in water (40 mL). The Ag NPs deposition on SiO2 spheres was carried out by the following procedure (Manivannan and Ramaraj 2013). First, 25 μL of 1 M TPDT silane was added to 5 mL pre-stirred aqueous mixture containing each 7 mM β-CD and BDAC and then further stirred for 1 h. To this β-CD–TPDT–BDAC composite, 5 mg of pre-formed amine-functionalized SiO2 was added and sonicated for 5 min. To this resulting solution, 50 μL of 0.1 M AgNO3 and 50 μL of 0.1 M AA were added. The formation of yellowish orange color solution indicated the formation of Ag NPs.
Spectral and colorimetric detection of Hg(II) ions
The spectral detection of Hg(II) ions using the SiO2/Ag NPs was performed using Agilent 8453 absorption spectrophotometer. The absorption spectra of SiO2/Ag NPs were recorded upon the addition of different concentrations of Hg(II) ions. For the absorption spectral measurements, freshly prepared solution of Hg(II) ions in microliters was added into a 2 mL of SiO2/Ag NPs, shaken well, and subjected to constant resting time (5 min). Unless otherwise mentioned, the HgCl2 was used for the Hg(II) ions sensing. For selective and colorimetric detection, an optimum level of analyte was added into the SiO2/Ag NPs solution, shaken well, and subjected to constant resting time and the changes were monitored by recording absorption spectra or observing with naked eye. The chloride salts of all other metal ions were also used for sensing studies.
Results and discussion
Spectral studies of SiO2/Ag NPs
The Ag NPs were deposited on the TPDT-functionalized SiO2 spheres by in situ wet chemical synthesis at three different AgNO3 concentrations. Decoration of Ag NPs on SiO2 spheres was feasible up to 3 mM of AgNO3 and further increase in the concentration of AgNO3 led to the aggregation of SiO2/Ag NPs. Figure 1 shows the comparison of absorption spectra of colloidal solutions of SiO2 (a), SiO2/Ag (1 mM) NPs (b), SiO2/Ag (2 mM) NPs (c), and SiO2/Ag (3 mM) NPs (d). It shows the characteristic SPR bands at 412, 409, and 404 nm due to the presence of 1, 2, and 3 mM Ag NPs, respectively, on SiO2. As shown in Fig. 1 (inset), the SiO2/Ag (3 mM) NPs showed an intense yellowish orange color due to the SPR band of Ag NPs. Excitation of collective oscillations of electron density, called SPR, determines the optical properties of Ag NPs. The frequency of the resonance can be tuned across the broad spectral range by selecting the particle size, shape as well as the surrounding dielectric medium of the Ag NPs. The observed narrow and sharp SPR band suggests the formation of higher amount of Ag NPs with uniform size and oscillating at a particular wavelength. It is known that the SPR absorption band of Ag NPs is very sensitive to their size and local environment (Manivannan and Ramaraj 2012; Maduraiveeran and Ramaraj 2011; Prasad et al. 2008; El-Sayed 2001; Kelly et al. 2003; Daniel and Astruc 2004; Catherine et al. 2005). The TPDT SSG was used as a protecting agent and as a consequence, the Ag NPs showed superior stability, which was monitored by recording the SPR spectra over a period of 3 months. This suggests that the amine-functionalized SiO2 spheres provided comfortable accommodation to the Ag NPs and this can be ascribed by the interaction of –NH2 groups of TPDT SSG with the Ag NPs.
SEM and TEM studies of SiO2 and SiO2/Ag NPs
Figure 2 shows the SEM images of SiO2 spheres at different magnifications (a and b) and the TEM images of SiO2/Ag NPs at Ag concentrations of 1 and 3 mM (c and d), respectively. The SEM images of the amine-functionalized SiO2 spheres showed that the SiO2 particles were spherical in shape and the average particle size was ~250 nm. Figure 2c, d displays the effect of AgNO3 concentrations on the decoration of pre-formed amine-functionalized SiO2 spheres by Ag NPs, i.e., the number of Ag NPs deposited on SiO2 spheres is higher in the case of SiO2/Ag (3 mM) NPs (Fig. 2d) than that of the SiO2/Ag (1 mM) NPs (Fig. 2c). Furthermore, Fig. 2d shows that the decorated Ag NPs are spherical in shape, distributed without aggregation, and the average particle size was calculated as 14 nm. When the concentration of AgNO3 was increased above 3 mM, aggregation and sedimentation of particles were observed. Further close analysis of Fig. 2d suggests that the thickness of the TPDT SSG layer on the SiO2 spheres was around 10–20 nm (marked in Fig. 2d). The lower magnified SEM image of the bulk SiO2/Ag (3 mM) NPs is shown in Fig. S2. The decoration/deposition of metal NPs on the pre-formed SiO2 spheres was already reported (Pol et al. 2002; Guo et al. 2008; Kim et al. 2006). For the first time we have introduced the in situ approach for the Ag NPs decoration/deposition using triamine-functionalized SSG (TPDT SSG). Our group has recently reported newer methodologies for the in situ synthesis of SSG matrix-embedded Au and Ag NPs (Maduraiveeran and Ramaraj 2009; Manivannan and Ramaraj 2011; Pandikumar et al. 2010) and observed remarkable stability of the metal NPs for several months due to the interaction between the amine-functionalized SSG matrix and the metal NPs. The amine-functionalized (TPDT SSG) SiO2 spheres could adsorb the Ag+ ions due to their interaction with the amine groups of the TPDT leading to the in situ formation of Ag NPs upon the addition of AA. The prepared SiO2/Ag NPs were found to be stable for more than 3 months.
Spectral and colorimetric sensing of Hg(II) ions by SiO2/Ag NPs
Since Hg(II) ion possesses high enough electrochemical potential (0.85 V) than the Ag(I)/Ag couple (0.8 V), it could alter the absorption band of the Ag NPs (Li et al. 2010) by quenching their SPR band. To evaluate the sensing ability of the Ag NPs at three different concentrations on the amine-functionalized (TPDT SSG) SiO2 spheres (SiO2/Ag NPs), different concentrations of Hg(II) ions were added to the solution containing known amount of SiO2/Ag NPs. The SPR absorbance changes were monitored by recording the absorption spectra of SiO2/Ag NPs after every 5 min. The absorbance of SiO2/Ag NPs was sensitive to the concentration of Hg(II) ions and the intensity of the SPR band was decreased linearly with an observable blue shift in the SPR band while increasing the concentration of Hg(II) ions for all the three SiO2/Ag NPs (Figs. 3a, S3-A, B). This SPR band shift toward lower wavelength is attributed to the deposition of mercury layer on the surface of the Ag NPs by forming Ag amalgam (Morris et al. 2002) and it can also be attributed to the oxidation of Ag(0) to Ag(I) (Li et al. 2010). An improved sensing of Hg(II) ions was observed at the SiO2/Ag (3 mM) NPs (Figs. 3a, S3C-c). It is obvious that the higher amount of Ag NPs which were deposited on the amine-functionalized (TPDT SSG) SiO2 spheres showed an improved sensing when compared to the lower concentrations of Ag NPs. Hence, the SiO2/Ag (3 mM) NPs were chosen for the sensing of Hg(II) ions at lower concentration and Fig. S4 shows the sensing at each addition of 5 μM Hg(II) ions. The change in absorbance was higher up to the addition of 20 μM Hg(II) ions and above this concentration the change in absorbance became lower with good linearity. Previous reports (Katsikas et al. 1996; Henglein and Brancewicz 1997; Ramesh and Radhakrishnan 2011; Fan et al. 2009) revealed that the adsorption of Hg onto Ag NPs causes a blue shift in the SPR band of the Ag NPs. According to Raj and co-workers (Bera et al. 2010), two possible reactions can be considered for the formation of Hg(0) by redox reaction: (i) surface coating of zero-valent Hg and (ii) formation of amalgam between Hg and Ag NPs. A suitable explanation can be given for the two linear ranges obtained for the Hg(II) ions sensing at the SiO2/Ag (3 mM) NPs (Fig. S4-B). Up to the addition of 20 μM Hg(II) ions, the quenching of SPR band intensity of the SiO2/Ag NPs was linear and above 20 μM concentration a gradual formation of AgHg crystals occured, i.e., the Hg(0) layer formed around the Ag NPs (Morris et al. 2002) which shows the second linear range. The citrate-stabilized plain Ag NPs (Lee and Meisel 1982; Henglein and Giersig 1999) were tested for the sensing of Hg(II) ions at different concentrations; however, a noticeable quenching of the SPR band of the plain Ag NPs was not observed. To find out the optimum concentration of Hg(II) ions required for the complete quenching of the SPR band of Ag NPs and for the formation of AgHg crystals, addition of 150 μM Hg(II) ions (by each addition of 25 μM) into the SiO2/Ag NPs solution was carried out (Fig. S5). It is clearly shown that the SPR band of the SiO2/Ag (3 mM) NPs gradually decreased and completely disappeared over the addition of 150 μM Hg(II) ions. This observation indicates that the blue shift in the SPR band brings about a morphological change in the Ag NPs (Bera et al. 2010). Upon the addition of 150 μM Hg(II) ions, the yellowish orange color of the SiO2/Ag (3 mM) NPs completely turned into colorless (Fig. S5-inset). Furthermore, the optical sensing of Hg(II) ions by using Hg(NO3)2 was carried out to confirm the Hg(II) ions detection and the chloride ion interference (Fig. S6). The result obtained from this experiment is very similar to that of the HgCl2.
Further experiments were carried out to evolve the selective sensing of Hg(II) ions by SiO2/Ag NPs in the presence of other commonly encountered metal ions (Pb(II), Cu(II), Co(II), Cd(II), Ca(II), Ni(II), Zn(II), Mn(II), Fe(II), Fe(III), and Na(I)) by adding 500 μM of different metal ions together into the SiO2/Ag (3 mM) NPs solution under similar experimental conditions as in the case of Hg(II) ions (Fig. 4). Remarkably, no observable SPR band quenching was noticed with these metal ions (Fig. S7). The selective sensing of Hg(II) ions in the presence of other commonly encountered metal ions is clearly demonstrated using the SiO2/Ag NPs (Fig. 4) and the addition of Hg(II) ions into the SiO2/Ag NPs solution resulted in the decolorization of the Ag NPs with the formation of AgHg crystals. Due to the lack of high electrochemical potential to oxidize Ag(0) to Ag(I) (Bera et al. 2010; Bard and Faulkner 2004), the quenching of the SPR band or decolorization of the SiO2/Ag NPs was not observed in the case of other metal ions when compared to the Hg(II) ions. Only Hg(II) ions could react with the Ag NPs and significantly alter the SPR band of the Ag NPs. In a previous report (Jean et al. 2010), SiO2/Ag NPs were prepared and used for the optical sensing of melamine. From this report, it is understood that the SiO2 spheres act as a well-defined colloidal stabilizer and as a spacer for the Ag NPs leading to the well-defined separation between the Ag NPs either in the colloidal or film form. In this work, the surface of the SiO2 spheres was functionalized with the amine-functionalized SSG which brought about the interaction between the amine silicate and the Hg(II) ions in the vicinity of the Ag NPs at SiO2 spheres. In our previous report (Maduraiveeran and Ramaraj 2011), the biomolecules-mediated synthesis of assembly of Ag NPs embedded in diamine-functionalized SSG network and sensing of cysteine, adenosine, and NADH are reported. In the present study, we have used new synthetic route to prepare highly stable (more than 3 months) Ag NPs supported on silica spheres using triamine-functionalized SSG matrix (SiO2/Ag NPs) and used for the optical and colorimetric sensing of Hg(II) ions.
Mechanistic study of Hg(0) deposition and AgHg crystal formation on/around SiO2/Ag NPs
Selective sensing of Hg(II) ions was evaluated by following the SPR band quenching along with an appreciable blue shift in the SPR band of the SiO2/Ag NPs. Previous studies (Bera et al. 2010) revealed that the redox reaction between Ag NPs and Hg(II) ions would alter the morphology of the Ag NPs by oxidative etching process. Hence, the TEM and EDX measurements were carried out to investigate the morphological change in the SiO2/Ag NPs after the addition of Hg(II) ions. Figure 5a and b displays the TEM images obtained for the SiO2/AgHg (1 mM) NPs (a) and SiO2/AgHg (3 mM) NPs after the addition of Hg(II) ions. It can be seen from the TEM images that the spherical Ag NPs were transformed into anisotropic Ag nanostructures after the reaction with the Hg(II) ions. As shown in Fig. 5a and b, the formed AgHg crystals were collectively leached out from the SiO2 spheres over the addition of higher concentration of Hg(II) ions to the SiO2/Ag NPs and the resultant AgHg crystals aggregated on the SiO2 spheres. To our knowledge, this is the first time the formation of aggregated AgHg crystals on the SiO2 spheres was observed. These results are supported by the observed blue shift in the SPR band of the SiO2/Ag NPs coupled with a decrease in the SPR band intensity due to the redox interaction between the Hg(II) ions and the Ag NPs. Due to the difference in the redox potentials of Hg(II)/Hg couple (0.85 V) and Ag(I)/Ag couple (0.8 V), the Ag is oxidized to Ag(I) by Hg(II) ions and thus the Hg layer forms on the Ag NPs through the formation of amalgam (Henglein and Brancewicz 1997; Bera et al. 2010) and wipes out the Ag NPs from the SiO2 spheres as AgHg crystals. Figure 6 displays the SAED patterns of the SiO2/Ag (3 mM) NPs (a) and SiO2/AgHg NPs (b), and the EDX spectrum of the SiO2/AgHg crystals (c). The diffused rings observed from the SAED pattern for the SiO2/Ag NPs (3 mM) (Fig. 6a) clearly suggest that the Ag NPs were encapsulated by the amorphous TPDT SSG matrix and the brighter rings with dark spots observed for the SiO2/AgHg NPs (Fig. 6b). The SAED pattern showing diffused (Fig. 6a) and brighter spots (Fig. 6b) for amorphous and crystalline materials, respectively, confirms the enhancement of crystallinity of the Ag NPs in the presence of Hg(II) ions (Clszkowska et al. 1994; Liu et al. 2011). The SAED patterns of SiO2/Ag (3 mM) NPs showed the Ag crystal planes of (1 1 1), (2 2 0), and (4 2 0) with the d values of 2.3448, 1.4166, and 0.8947 Å, respectively (Fig. 6a) which are consistent with the standard database values of Ag. The crystal planes observed for Ag from the SAED patterns of SiO2/AgHg NPs showed the Ag crystal planes of (1 1 1), (2 0 0), (2 2 0), (3 1 1), and (4 2 0) with the d values of 2.2666, 1.9428, 1.4468, 1.2142, and 0.9066 Å, respectively (Fig. 6b). The EDX spectrum of the SiO2/AgHg NPs (Fig. 6c) clearly shows the presence of Hg element in the SiO2/AgHg NPs, indicating the formation of AgHg crystals on the surface of the SiO2 spheres. The amalgamation-based Hg(II) ions sensing by SiO2/Ag NPs would also find application in the removal of Hg(II) ions from solution as silver amalgam in addition to its sensing.
Conclusions
A facile synthetic method to decorate amine-functionalized SiO2 spheres with Ag NPs was reported. Both the spectral and colorimetric detection of Hg(II) ions were demonstrated with a detection limit of 5 μM Hg(II) ions using SiO2/Ag (3 mM) NPs. A significant blue shift accompanied with a decrease in the SPR band intensity for the Ag NPs was noticed due to the formation of the amalgam-like structures between Ag and Hg. The addition of an optimum concentration of the Hg(II) ions (150 μM) to a solution of SiO2/Ag NPs led to a complete quenching of SPR band of the SiO2/Ag NPs due to the formation of AgHg amalgam. The selective detection of Hg(II) ions in the presence of several other commonly encountered metal ions present in the environment was also established.
References
Bard AJ, Faulkner LR (2004) Electrochemical methods, fundamentals and applications. Wiley, New York, p 808
Bera RK, Das AK, Raj CR (2010) Enzyme-cofactor-assisted photochemical synthesis of Ag nanostructures and shape-dependent optical sensing of Hg(II) ions. Chem Mater 22:4505
Bharathi S, Fishelson N, Lev O (1999) Direct synthesis and characterization of gold and other noble metal nanodispersions in sol–gel-derived organically modified silicates. Langmuir 15:1929
Campbell LM, Dixon DG, Hecky RE (2003) A review of mercury in lake Victoria, East Africa: implications for human and ecosystem health. J Toxicol Environ Health B 6:325
Catherine JM, Tapan KS, Anand MG, Christopher JO, Gao J, Gou L, Simona EH, Li T (2005) Anisotropic metal nanoparticles: synthesis, assembly, and optical applications. J Phys Chem B 109:13857
Clarkson TW, Magos L, Myers GJ (2003) The toxicology of mercury-current exposures and clinical manifestations. N Engl J Med 349:1731
Clszkowska M, Donten M, Stojek Z (1994) Preparation of a mercury disk microelectrode based on solid silver amalgam. Anal Chem 66:4112
Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104:293
El-Sayed MA (2001) Some interesting properties of metals confined in time and nanometer space of different shapes. Acc Chem Res 34:257
Fan Y, Liu Z, Wang L, Zhan J (2009) Synthesis of starch-stabilized Ag nanoparticles and Hg2+ recognition in aqueous media. Nanoscale Res Lett 4:1230
Guo S, Zhai J, Fang Y, Dong S, Wang E (2008) Nanoelectrocatalyst based on high-density Au/Pt hybrid nanoparticles supported on a silica nanosphere. Chem Asian J 3:1156
Harris HH, Pickering IJ, George GN (2003) The chemical form of mercury in fish. Science 301:1203
Henglein A, Brancewicz C (1997) Absorption spectra and reactions of colloidal bimetallic nanoparticles containing mercury. Chem Mater 9:2164
Henglein A, Giersig M (1999) Formation of colloidal silver nanoparticles: capping action of citrate. J Phys Chem B 103:9533
Huang C–C, Chang H-T (2007) Parameters for selective colorimetric sensing of mercury(II) in aqueous solutions using mercaptopropionic acid-modified gold nanoparticles. Chem Comm 25:1215
Jean R-D, Chiu K-C, Chen T-H, Chen C-H, Liu D-M (2010) Functionalized silica nanoparticles by nanometallic Ag decoration for optical sensing of organic molecule. J Phys Chem C 114:15633
Katsikas L, Gutie′rrez M, Henglein A (1996) Bimetallic colloids: silver and mercury. J Phys Chem 100:11203
Kelly KL, Coronado E, Zhao LL, Schatz GC (2003) The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J Phys Chem B 107:668
Kim J, Lee JE, Lee J, Jang Y, Kim S-W, An K, Yu JH, Hyeon T (2006) Generalized fabrication of multifunctional nanoparticle assemblies on silica spheres. Angew Chem Int Ed 45:4789
Kim Y-R, Mahajan RK, Kim JS, Kim H (2010) Highly sensitive gold nanoparticle-based colorimetric sensing of mercury(II) through simple ligand exchange reaction in aqueous media. ACS Appl Mater Interfaces 2:292
Lee KS, El-Sayed MA (2006) Gold and silver nanoparticles in sensing and imaging: sensitivity of plasmon response to size, shape, and metal composition. J Phys Chem B 110:19220
Lee PC, Meisel D (1982) Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J Phys Chem 86:3391
Lee J-S, Han MS, Mirkin CA (2007) Colorimetric detection of mercuric ion (Hg2+) in aqueous media using DNA-functionalized gold nanoparticles. Angew Chem Int Ed 46:4093
Lev O, Wu Z, Bharathi S, Glezer V, Modestov A, Gun J, Rabinovich L, Sampath S (1997) Sol-gel materials in electrochemistry. Chem Mater 9:2354
Li W, Guo Y, McGill K, Zhang P (2010) A facile synthesis of Ag nanoparticles for mercury ion detection with high sensitivity and selectivity. New J Chem 34:1148
Liu Y, Zhu Z, Liu G, Xu Z, Kuznicki SM, Zhang H (2011) A novel method to improve crystallinity of supported nanoparticles using low melting point metals. J Phys Chem C 115:14591
Maduraiveeran G, Ramaraj R (2009) Potential sensing platform of silver nanoparticles embedded in functionalized silicate shell for nitroaromatic compounds. Anal Chem 81:7552
Maduraiveeran G, Ramaraj R (2011) Silver nanoparticles embedded in amine-functionalized silicate sol–gel network assembly for sensing cysteine, adenosine and NADH. J Nanopart Res 13:4267
Maduraiveeran G, Tamilmani V, Ramaraj R (2011) Silver quantum dots for selective detection of mercuric ions. Curr Sci 100:199
Manivannan S, Ramaraj R (2011) Polymer-embedded gold and gold/silver nanoparticle modified electrodes and their applications in catalysis and sensors. Pure Appl Chem 83:2041
Manivannan S, Ramaraj R (2012) Assemblies of silicate sol–gel matrix encapsulated core/shell Au/Ag nanoparticles: interparticles surface plasmon coupling. J Nanopart Res 14:961
Manivannan S, Ramaraj R (2013) Silver nanoparticles embedded in cyclodextrin–silicate composite and their applications in Hg(II) ion and nitrobenzene sensing. Analyst 138:1733
Morris T, Copeland H, McLinden E, Wilson S, Szulczewski G (2002) The effects of mercury adsorption on the optical response of size-selected gold and silver nanoparticles. Langmuir 18:7261
Pandikumar A, Murugesan S, Ramaraj R (2010) Functionalized silicate sol–gel-supported TiO2–Au core–shell nanomaterials and their photoelectrocatalytic activity. ACS Appl Mater Interfaces 2:1912
Pol VG, Srivastava DN, Palchik O, Palchik V, Slifkin MA, Weiss AM, Gedanken A (2002) Sonochemical deposition of silver nanoparticles on silica spheres. Langmuir 18:3352
Prasad BL, Sorensen CM, Klabunde KJ (2008) Gold nanoparticle superlattices. Chem Soc Rev 37:1871
Ramesh GV, Radhakrishnan TP (2011) A universal sensor for mercury (Hg, HgI, HgII) based on silver nanoparticle-embedded polymer thin film. ACS Appl Mater Interfaces 3:988
Serra A, Fillippo E, Re M, Palmisano M, Vittori-Antisari M, Buccolieri A, Manno D (2009) Non-functionalized silver nanoparticles for a localized surface plasmon resonance-based glucose sensor. Nanotechnology 20:165501
Wang Y, Yang F, Yang X (2010) Colorimetric detection of mercury(II) ion using unmodified silver nanoparticles and mercury-specific oligonucleotides. ACS Appl Mater Interfaces 2:339
Wilson OM, Scott RWJ, Garcia-Martinez JC, Crooks RM (2005) Synthesis, characterization, and structure-selective extraction of 1–3-nm diameter AuAg dendrimer-encapsulated bimetallic nanoparticles. J Am Chem Soc 127:1015
Xu K, Wang JX, Kang XL, Chen JF (2009) Fabrication of antibacterial monodispersed Ag–SiO2 core–shell nanoparticles with high concentration. Mater Lett 63:31
Xue X, Wang F, Liu X (2008) One-step, room temperature, colorimetric detection of mercury (Hg2+) using DNA/nanoparticle conjugates. J Am Chem Soc 130:3244
Yantasee W, Lin Y, Hongskirikam K, Fryxell GE, Addleman R, Timchalk C (2007) Electrochemical sensors for the detection of lead and other toxic heavy metals: the next generation of personal exposure biomonitors. Environ Health Perspect 115:1683
Yantasee W, Charnhattakorn B, Fryxell GE, Timchalk C, Addleman RS (2008) Detection of Cd, Pb, and Cu in non-pretreated natural waters and urine with thiol functionalized mesoporous silica and nafion composite electrodes. Anal Chim Acta 620:55
Yantasee W, Rutledge RD, Chouyyok W, Sukwarotwat V, Orr G, Warner CL, Warner MG, Fryxell GE, Wiacek RJ, Timchalk C, Addleman RS (2010) Functionalized nanoporous silica for the removal of heavy metals from biological systems: adsorption and application. ACS Appl Mater Interfaces 2:2749
Zanella R, Sandoval A, Santiago P, Basiuk VA, Saniger JM (2006) New preparation method of gold nanoparticles on SiO2. J Phys Chem B 110:8559
Acknowledgments
The financial support from the Department of Science and Technology (DST), New Delhi is gratefully acknowledged. SMV is the recipient of CSIR-Senior research fellowship. The TEM images were recorded at CECRI, Karaikudi and PSG Institute of Advanced Studies, Coimbatore.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rameshkumar, P., Manivannan, S. & Ramaraj, R. Silver nanoparticles deposited on amine-functionalized silica spheres and their amalgamation-based spectral and colorimetric detection of Hg(II) ions. J Nanopart Res 15, 1639 (2013). https://doi.org/10.1007/s11051-013-1639-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-013-1639-9