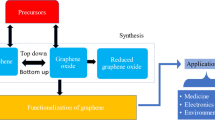
Abstract
This review (with 155 refs.) summarizes the progress made in the past few years in the field of electrochemical sensors based on graphene-derived materials for the determination of heavy metal ions. Following an introduction of this field and a discussion of the various kinds of modified graphenes including graphene oxide and reduced graphene oxide, the review covers graphene based electrodes modified (or doped) with (a) heteroatoms, (b) metal nanoparticles, (c) metal oxides, (d) small organic molecules, (e) polymers, and (f) ternary nanocomposites. Tables are provided that afford an overview of representative methods and materials for fabricating electrochemical sensors. Furthermore, sensing mechanisms are discussed. A concluding section presents new perspectives, opportunities and current challenges.

Schematic illustration of electrochemical sensor for heavy metal ion sensing based on heteroatom-doped graphene, metal-modified graphene, metal-oxide-modified graphene, organically modified graphene, polymer-modified graphene, and ternary graphene based nanocomposites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal ions (e.g. those of Cd, Pb, and Hg), and semimetals (e.g. As) are highly toxic and may cause serious damage to health. Owing to growing concerns in this area, many international and national organizations have defined maximum contaminant levels for drinking water. For example, the maximum permissible levels set by the World Health Organization (WHO) for Cd and Pb are 0.003 and 0.010 mg L-1, respectively [1]. Electrochemical sensors are very promising for heavy metal monitoring, as they offer desirable characteristics such as sensitivity, selectivity, inexpensiveness, robustness, and field-deployability [2].
Graphene and its derivatives, including graphene oxide (GO), reduced graphene oxide (rGO), and three-dimensional (3D) graphene, are widely used in electrochemistry [3]. Graphene consists of a one-atom-thick planar sheet comprising a closely packed honeycomb carbon lattice. By definition, graphene contains only sp2 carbons without oxygen (or nitrogen). Pristine graphene has a high electron transfer rate, a large surface area (2630 m2 g-1) [4], and a high conductivity (64 mS cm-1) [5, 6]. Although some studies [7, 8] claim that pristine graphene was used, in fact they used graphite, GO or rGO. GO, a monolayer of graphite oxide, contains many defects and numerous oxygen functional groups, mostly in the form of hydroxyl and epoxy groups on the basal plane, with smaller amounts of carboxyl, carbonyl, phenol, lactone, and quinone groups at the sheet edges [9]. Compared with pristine graphene, the polar oxygen functional groups provide GO with good dispersibility in many polar solvents, particularly in water (concentration < 1 mg mL-1). In addition, the oxygen functional groups serve as sites for immobilizing various electroactive species via covalent or noncovalent bonds. However, the large amount of oxygen functional groups in GO causes some loss of electrical conductivity, which may limit the direct application of GO in electrically active materials and devices [9].
Reduced graphene oxide (rGO) is obtained by the chemical/electrochemical reduction of GO. The charge transportation ability of rGO is enhanced compared with that of GO owing to the removal of some oxygen-containing functional groups and partial remediation of the sp2 conducting structure. Moreover, the chemical and electrical properties of rGO are tunable and the content of oxygen functional groups and defects is sufficient for facilitating analyte adsorption [10]. Although rGO and its composites have been widely employed as sensing materials [11], the dispersion ability remains challenging.
In addition to these two-dimensional (2D) nano-carbon materials, 3D graphene has received considerable interest owing to its outstanding properties, such as an interconnected porous structure, an enormous specific surface area, good mechanical stability, and flexibility to tailorable surface chemistry [12]. Based on these advantages, 3D-graphene-based sensors have been exploited for the electrochemical sensing of heavy metal ions. However, 3D graphene shows an inferior sensing ability for heavy metal ions [13] owing to the restricted diffusion of aqueous analytes in the hydrophobic 3D connected framework [12]. To improve the sensing ability, various active species have been introduced on the 3D framework of graphene [12]. An excellent review related to 3D-graphene-based electrochemical sensors has been published by Baig et al. [14]. A summary of the merits and disadvantages of graphene, GO, rGO, and 3D graphene are listed in Table 1.
In general, the direct use of pristine graphene or graphene derivatives for electrochemical sensing suffers from low sensitivity, interference from other substrates, and easy agglomeration. Therefore, sensors based on graphene-derived nanomaterials have been widely investigated [15, 16]. Although Gan et al. has addressed the preparation of sensors based on various 2D nanomaterials and their sensing properties for heavy metal ions [17], a systematic investigation of the mechanisms by which graphene-derived nanomaterials achieve improved detection of heavy metal ions is still needed. Our review is organized according to the type of sensing elements, which are classified as heteroatom-doped graphene, metal-modified graphene, metal oxide-modified graphene, organically modified graphene, polymer-modified graphene, and ternary graphene-based nanocomposites. Corresponding hybrid materials are also introduced.
Sensors using heteroatom-doped graphene and GO
Heteroatom (N, S, F, etc.) doped graphene can exhibit various new or improved electromagnetic, physicochemical, optical, and structural properties [18]. For example, Xing et al. synthesized N-doped graphene via a one-step electrochemical strategy. The incorporation of pyridine-like N and pyrrole-like N in graphene was found to greatly enhance the performance for electrochemical determination of Cd2+, Pb2+, Cu2+, and Hg2+ compared with rGO. The detection limit were estimated to be 0.05 μM for Cd2+ and Hg2+, and 0.005 μM for Pb2+ and Cu2+. [19]. Liu et al. reported a nanocarbon paste electrode modified with N-doped graphene for trace Pb and Cd determination using square wave anodic stripping voltammetry. The presence of N atoms in graphene increased the number of catalytically active sites and enhanced the electron transfer ability of the modified electrode [20]. In the presence of dibenzyl disulfide and a silica template, Manna et al. synthesized S-doped porous rGO by thermal annealing, and the material was used for efficient removal and electrochemical determination of Hg2+. As shown in Fig. 1, the presence of a large amount of thiophenic S and the porous structure provided a detection limit as low as 0.5 nM [21]. Antony et al. reported fluorinated GO for the simultaneous detection of Cd2+, Pb2+, Cu2+ and Hg2+ using square wave anodic stripping voltammetry. The incorporation of F into GO improved the sensitivity owing to the interactions between electron-donating F and electron-deficient heavy metal ions [22].
The morphology of S-doped porous rGO and application in Hg2+ electrochemical sensing and removing. Reproduced from [21] with permission of ACS
Sensors using metal-modified graphene
Graphene modified with noble metal nanoparticles (NPs) has been used for heavy metal ions sensing because noble metal NPs exhibit high catalytic activity via the size effect. Moreover, graphene can transfer electrons acquired from catalytic process of the metal NPs to electrodes, which may accelerate the catalytic process [23]. Among NPs that form nanocomposite with graphene, Au NPs are the most widely applied for the detection of metal ions because they offer the advantages of high chemical stability and easy preparation processes [15]. For example, Au NPs decorated graphene synthesized via electrodeposition method was used for sensitive determination of Hg2+. Compared with their bulk electrode counterpart, Au NP modified electrodes are promising because they can eliminate the memory effect and increase the sensitivity for heavy metal ion detection [15]. The detection limit for Hg2+ was estimated to be 0.03 pM, which is well below the guideline value set by the WHO [24]. In addition to Hg2+ electroanalysis, Au NPs/rGO nanocomposites have also been widely used for the analysis of As3+. For instance, Liu et al. utilized an Au NPs-electroreduced graphene oxide (ERGO) composite film for the determination of As3+. The obtained good sensitivity (limit of detection = 2.7 nM) was attributed to the formation of Au–As intermetallic compounds that enhance the efficiency for cathodic preconcentration of As(0) [25]. Moreover, the effect of the supporting electrolyte (0.20 M aqueous HClO4, 0.20 M aqueous HCl, or 0.10 M aqueous H2SO4) on the magnitude of the detected signal was also evaluated. The detection performance in 0.20 M aqueous HCl was better than that in the other two supporting electrolytes, which was attributed to improved electron kinetics resulting from the complexation of Cl- ions with As3+. However, the detection of inorganic As in highly acidic media could cause problems such as hydrogen evolution or undesirable corrosion reactions [26].
In addition to Au NPs, graphene decorated with Ag NPs has also been used in heavy metal ion sensing. For example, Sang et al. synthesized Ag NPs/rGO via an in situ method. A nanocomposite-modified glassy carbon electrode was used for simultaneous electrochemical sensing of Cd2+, Pb2+, Cu2+ and Hg2+, and this modified electrode showed excellent selectivity [27]. However, expensive materials like Au and Ag are of limited practicality when fabricating macroelectrodes, which require large amount of material, owing to cost considerations [28].
Alternatives like Bi NPs or Sn NPs have also been used in heavy metal ion sensing. For example, Sahoo et al. prepared Bi NPs modified rGO sheets via an in situ method [29]. Lee et al. decorated rGO with Sn NPs via an electrodeposition method and realized electrochemical sensing of Cd2+, Pb2+ and Cu2+ using square wave anodic stripping voltammetry [30]. Sn has similar electroanalytical properties to Bi, but it is less toxic and cheaper [31].
Apart from metal NPs, metal films have also been hybridized with graphene to construct sensors for heavy metal determination. For example, Ping et al. fabricated an electrochemical sensing platform based on a screen-printed electrode modified with an electrochemically rGO. After in situ plating with a Bi film, the electrode was used for the simultaneous determination of Cd2+ and Pb2+ [32]. The mechanism of Cd2+ and Pb2+ detection at the surface of Bi based electrode involves the capacity of Bi to form a “fused alloy” with heavy metal ions [33]. Compared with a previously widely used Hg film, Bi is less toxic to the environment and has excellent mechanical stability [33]. Unfortunately, compared with the Hg-modified electrode, the Bi-modified electrode has various limitations, such as a narrow potential window (below the oxidation potential of Bi) and easy oxidation upon contact with air [34].
Sb film electrodes exhibiting similar electroanalytical performance to the Bi film electrodes have also been applied to heavy metal sensing. For example, Ruengpirasiri et al. used GO-Sb film-modified electrode for the simultaneous determination of Cd2+, Pb2+, Cu2+, and Hg2+ [35]. In situ preparation of Sb films can be conducted in a wider pH range than Bi films because bismuth hydroxide will form at pH 4, which results in irreproducible measurements [36, 37]. Thus, Sb film electrodes are a valuable and complementary alternative to Bi film electrodes for measurement under an oxidative potential or in acidic media (e.g. determination of Cu2+ and Hg2+). However, the toxicity of Sb metal ions, especially Sb3+, cannot be ignored completely [36]. The overview on metal-modified graphene as sensing material for electrochemical sensing of heavy metal ions was displayed in Table 2.
Sensors using metal-oxide-modified graphene
As an alternative to metal NPs and metal films, metal oxides have frequently been used in heavy metal ion sensing owing to their large surface areas and high electrocatalytic activities. The sensing mechanism of metal oxide for heavy metal ion is strong adsorption ability, or electrocatalytic activity, or both simultaneously [28, 55,56,57]. However, most metal oxides have inferior conductivities and stabilities, which are unfavorable for electron transfer during the detection process and decrease the long-term stability of the electrode. However, when a metal oxide is combined with graphene, the nanocomposite is expected to provide a new electrochemical platform for heavy metal ion sensing [58]. Up to now, Fe3O4, ZnO, MnO2, Cu2O, Fe2O3, SnO2, TiO2, and Co3O4-based graphene nanocomposites have been successfully applied to the detection of heavy metal ions in aqueous solution. The morphology and average size of the metal oxide can greatly influence the performance of the modified electrode. As shown in Fig. 2, Sun et al. synthesized rGO decorated with three different shapes of Fe3O4 via a one-step in-situ co-precipitation method. The sensitivity for analysis of Pb2+ decreased in the following order: band Fe3O4/rGO > spherical Fe3O4/rGO > rod Fe3O4/rGO [59]. Karthik et al. synthesized Co-doped ZnO/rGO as a heavy metal ion sensor for Cd2+ and Pb2+. Compared with ZnO/rGO, Co-doped ZnO/rGO exhibited better catalytic activity toward Cd2+ and Pb2+ sensing with detection limits of 8.36 nM for Cd2+ and 4 nM for Pb2+ [60].
Preparation processes of three shapes of Fe3O4/rGO by adjusting the mole ratio of Fe2+/Fe3+ via in-situ co-precipitation method. Reproduced from [59] with permission of Elsevier
Compared with monometallic oxides, bimetallic oxides exhibit better electrochemical activity owing to electron hopping between different valence states of metals in oxygen sites [61]. Huang et al. compared the detection performance of NiCo2O4 with those of Co3O4 and NiO. NiCo2O4 exhibited better performance for electrochemical determination of Pb2+ and Cu2+ than the other two materials [62]. Based on this concept, Xiong et al. used a 1,6-hexanediamine (HDA)- functionalized MgFe2O4/rGO composite for the electrochemical determination Cu2+. The amino group in HDA has high activity for coordination with heavy metal ions. The detection limit was estimated to be 0.2 nM with a sensitivity of 0.0172 μA nM-1 [63]. The same group investigated polyethyleneimine (PEI) (or ethanediamine (EDA)) functionalized CoFe2O4/rGO composite for electrochemical detection of ultra-trace Cu2+, and explored the interaction mechanism. Cyclic voltammetry and X-ray photoelectron spectroscopy results indicated that the interaction between the composite and Cu2+ involved an adsorption control process [64]. Zhou et al. synthesized GO incorporating mesoporous MnFe2O4 for the electrochemical determination of Pb2+. The mesoporous structure of MnFe2O4 increased the specific surface area of GO and enhanced the electrochemical activity toward Pb2+ analysis [65]. The overview on metal oxide-modified graphene as sensing material for electrochemical sensing of heavy metal ions was shown in Table 3.
Sensors using organically modified graphene
The modification of graphene with organic molecules is believed to increase the sensitivity and selectivity of graphene-based electrochemical sensors for heavy metal ion through two different recognition mechanisms, namely, chemical affinity and cavity entrapment (or both simultaneously). Various kinds of organic molecule including small organic molecules (containing electron-rich groups such as –OH, –SH, and –NH2) and caged molecules (calixarenes and cyclodextrins) have been investigated [1]. For instance, Muralikrishna et al. synthesized L-cysteine functioned GO by reacting the carboxyl groups in graphene with the amino group in L-cysteine. This material was used for the simultaneous electrochemical determination of Cd2+, Pb2+, Cu2+, and Hg2+. The oxygen–containing groups of GO and the electron donor group in L-cysteine facilitated the adsorption process of heavy metal ions [81]. Yuan et al. reported high–density 2-amino-5-mercapto-1,3,4-thiodiazole (AMT)–grafted GO prepared via an amidation reaction between GO and AMT under strong basic conditions. The high grafting density was attributed to the high density carboxyl groups on GO. The detection signal during electroanalysis of Cu2+ was amplified by the abundant N, O, and S donor atoms of AMT.
To exploit the coordination between heavy metal ions and N atoms in piperazine, our group synthesized piperazine–grafted GO through nucleophilic ring–opening of epoxy groups on GO with the amino groups of piperazine. After chemical reduction by ascorbic acid, the modified glassy carbon electrode was used for the detection of Hg2+ with a detection limit of 0.2 nM [82]. Based on the same synthetic mechanism, Zhou et al. reported cysteamine-functionalized GO for the selective determination of Hg2+. In addition to interacting with the Au electrode surface through the formation of Au–S bonds, the residual mercapto groups in cysteamine can selectively interact with Hg2+ [83]. Göde et al. functionalized rGO with calixarenes using 1-ethyl-3-(3-dimethylaminoprophy) carbondiimide hydrochloride (EDC) and N-hydroxy succinimide (NHS) to activate the carboxylic acid (–COOH) groups on rGO. The nanocomposite was used for simultaneous determination of Fe3+, Cd2+, and Pb2+. As shown in Fig. 3, the 3D basket, cup, or bucket shapes of calixarenes can effectively entrap metal ions and the oxygen–containing groups can form complexes with the metal ions, thus increasing the sensitivity and selectivity of the sensor for these metal ions [84].
Preparation of calixarene/rGO/GCE and nano-sensing of the guest metal ions. Reproduced from [84] with permission of Elsevier
Yu et al. fabricated N-[(1-pyrenyl-sulfonamido)-heptyl]-gluconamide (PG) modified graphene for ultrasensitive and selective sensing of heavy metal ions. Owing to the large π system of pyrene, a stable interaction can occur between the pyrene residue and graphene. Whereas functional groups such as hydroxyls and imines in glucose can act as coordination sites for Hg2+ during the detection process [85]. Magerusan et al. used an N–doped graphene/chitosan nanocomposite for selective Pb2+ detection. The electron-donating functional groups such as hydroxyls and amines in chitosan and the N doping groups in rGO can easily coordinate electron-deficient heavy metal ions. Moreover, positively charged chitosan can interact with negatively charged rGO to increase the stability of the nanocomposite [86]. Yang et al. constructed an As3+ sensor with excellent selectivity using an Au microelectrode decorated with amino-functionalized GO. Benefited from the synergetic effect of the strong adsorption capability of NH2-GO and the excellent electrocatalytic ability of Au microwire, resulting in a low detection limit of 2.16 nM [87]. The overview on organically modified graphene as sensing material for electrochemical sensing of heavy metal ions were listed in Table 4.
Sensors using polymer modified graphene
Polymers with a high number of reactive sites allows for analyte preconcentration on the electrode surface and are thus expected to increase the sensitivity when used for heavy metal ion sensing. Among the various types of polymers, conducting polymers have received much attention owing to their superior electrical conductivities and anti-fouling capabilities [115]. Moreover, the morphology of the conducting polymers (fiber, wire, film, or particle) and the dopants are related to the detection performance (sensing range, limit of detection, and response/recovery time) of the modified electrode [116, 117]. Conducting polymers including polyaniline (PANI), polypyrrole (PPy), and poly(3,4-ethylenedioxythiophene) (PEDOT) have been widely used in heavy metal determination. For example, in our previous work, we synthesized PEDOT nanorods/GO nanocomposite via interfacial polymerization as a new electrode material for electrochemical detection of Hg2+. The specific doping and de-doping properties of PEDOT could be controlled by varying the deposition potential, providing a selective sensing platform for Hg2+ determination. Moreover, in the nanocomposite, the PEDOT nanorods can function as electro-active sites to facilitate electron transfer during the determination process [118]. Dai synthesized PPy/GO nanocomposites via in situ chemical oxidation polymerization, and phytic acid molecules were functionalized with nanocomposites through electrostatic attraction. Owing to the presence of phosphoric acid groups in phytic acid and N-containing groups in PPy, the sensor was utilized for the simultaneous determination of Cd2+ and Pb2+ with detection limits of 19 and 1.98 nM, respectively [119]. Muralikrishna et al. described PANI/GO hydrogels for highly sensitive electrochemical determination of Pb2+. The hydrogels were synthesized through in situ polymerization of aniline in the presence of GO nanosheets followed by hydrogel formation at an elevated temperature [120].
Besides conducting polymers, other electroactive polymers including Nafion, poly(dimethylsiloxane) (PDMS), polydopamine, poly-L-lysine (PLL), polyallylamine, and polyethyleneimine have also been used in heavy metal ion sensing. For example, Li et al. reported a Nafion–graphene nanocomposite for ultrasensitive determination of Cd2+, with a detection limit of 0.044 nM [121]. The addition of Nafion can increase the mechanical robustness of the electrode and avoid interference from anionic in the sample (NO3-, SO42-, or CO32-). Chałupniak et al. prepared a microfluidic lab-on-a-chip platform for heavy metals preconcentration and electrochemical detection based on a GO-PDMS nanocomposite. The use of GO–PDMS significantly improve the sensitivity for the electrochemical detection of heavy metals with a low detection limit of 0.34 pM [122]. Guo et al. prepared an electrode modified with an GO and chitosan hybrid matrix through drop casting, and a PLL film was coated on the electrode through electropolymerization via a cyclic voltammetry method. The amino and hydroxyl groups in this system effectively coordinated metal ions. Moreover, the PLL films which had excellent permselectivity, good stability, strong adherence to the electrode surface, and an increased amount of active sites enhanced the electrocatalytic activity of the modified electrode. When used for the simultaneous electrochemical detection of Cd2+, Pb2+, and Cu2+, detection limits of 0.089, 0.097, and 0.31 nM, respectively, were obtained [123]. Liu et al. constructed polyallylamine-hydrochloride-functionalized via a non-covalent method. The –NH2 functional groups of polyallylamine hydrochloride improved the performance or trace detection of Cu2+, with a relatively low detection limit of approximately 0.35 nM [124]. Through nucleophilic substitution reactions between the surface-exposed epoxy groups in GO and the active amine groups in PEI, Hu et al. synthesized PEI-rGO nanocomposites. When combined with Nafion, the hybrid modified electrode showed selectivity for Cu2+ electrochemical determination, with a detection limit of 0.3 μM [125]. In conclusion, polymer-modified interfaces have many outstanding merits, but the application of these systems is still limited by potential swelling or denaturation of the polymers during prolonged accumulation times and slow diffusion across the films [126]. In addition, the overview on polymer modified graphene as sensing material for electrochemical sensing of heavy metal ions was showed in Table 5.
Sensors using ternary graphene-based nanocomposite
Compared with binary graphene-based nanocomposites, ternary or quaternary graphene nanocomposites such as metal-conducting polymers, metal-carbon nanotube (CNT) or conducting polymer-CNT hybrid with graphene show better performance [23]. For example, Dong et al. constructed an Au NPs/PANI/graphene modified electrode for sensitive detection of Pb2+. Compared with Au NPs, PANI, or graphene modified glassy carbon electrodes, the ternary hybrid showed improved detection performance, which was attributed to the synergetic effects of these three materials [138]. Moreover, PANI can function as a protective layer for Au NPs, avoiding interparticle aggregation via van der Waals attraction [1].
Wang et al. reported that the passivation of modified electrodes is problematic real sample analysis because various surface-active species may be adsorbed on the electrode. However, fouling of the electrode can be effectively alleviated by modification of the electrode with a dialysis membrane layer, such as Nafion, PLL, or cellulose acetate. Commonly used membrane modification methods usually involve a solvent evaporation procedure, which results in unsatisfactory homogeneity and reproducibility of the membrane. Therefore, they adopted an electrodeposition method to modify rGO/glassy carbon electrode with p-aminobenzene sulfonic acid. Compared with the afore-mentioned modification method, this electropolymerization method had the advantages of strong adhesion, controllable film thickness, uniform structure, and good stability. After in-situ plating a stannum film, the sensor was used for the sensitive determination of trace Cd2+ [139].
Cui et al. prepared thiazole-derivative functionalized graphene decorated with SnO2 NPs and compared the influence of different halogen anions (F, Cl, or I) on the detection performance of the composite. The F@SnO2/thiazole derivative-functionalized graphene exhibited superior performance for the detection of Cu2+ than the other two materials [140]. Recently, sensors based on a flexible substrate have gained attention, owing to their potential application as wearable sensors to monitor heavy metal ion in sweat, saliva, tears, or other body fluids. For example, Xuan et al. fabricated a fully integrated, miniaturized, and flexible electrochemical sensor based on a micro-patterned rGO and CNT composite on a flexible Au substrate as a working electrode (Fig. 4). After plating with a Bi film, the sensor exhibited separated and well-defined stripping peaks for Cd2+ and Pb2+ [141].
Photographs of the fabricated miniaturized, integrated, and flexible heavy metal ion sensor with micro-patterned rGO and a CNT composite working electrode. Photo images (a, d) of a fabricated flexible heavy metal ion sensor, (b) microscope image of 3 electrodes, and (c) working electrode. (Gap size: 50 μm, total effective working electrode area: 1.5 mm2, total working electrode thickness: ∼1 μm). Reproduced from [141] with permission of Elsevier
Sensors based on films have also received attention owing to their potential as disposable electrodes for heavy metal ion sensing. For example, Dong et al. synthesized a sandwich structured ionic liquid-CNT-graphene film via an effective inkjet printing method for electrochemical determination of Cd2+ and Pb2+. The sensor exhibited high sensitivity, a wide linear range, and a low detection limit owing to the synergetic effects of these materials including fast charge transferability, sufficient surface active sites, and a large surface area [142]. Table 6 shows the overview on ternary or quaternary graphene-based nanocomposite as sensing material for electrochemical sensing of heavy metal ions.
Conclusions and perspectives
Graphene-based nanocomposites have been widely investigated as chemical sensors with high sensitivity and selectivity. We reviewed the sensing principles of graphene-based hybrids, including heteroatom-doped graphene, metal-modified graphene, metal-oxide-modified graphene, organically modified graphene, polymer-modified graphene, and ternary graphene based nanocomposite, which provide sensitive, selective and stable platforms for heavy metal ions determination. On one hand, searching for new materials and new methodologies to control the morphology and structure of sensing materials to fabricate new sensors is an important direction for graphene-based sensors. On the other hand, optimizing the performance of current sensor systems, including sensitivity, selectivity, and stability, is of equal importance. Furthermore, the development of flexible or wearable sensors for detecting heavy metal ions in real samples or human body fluids is an important endeavor.
References
Aragay G, Pons J, Merkoci A (2011) Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem Rev 111(5):3433–3458. https://doi.org/10.1021/cr100383r
Rassaei L, Marken F, Sillanpää M, Amiri M, Cirtiu CM, Sillanpää M (2011) Nanoparticles in electrochemical sensors for environmental monitoring. TrAC-Trend Anal Chem 30(11):1704–1715. https://doi.org/10.1016/j.trac.2011.05.009
Xu JH, Wang YZ, Hu SS (2017) Nanocomposites of graphene and graphene oxides: synthesis, molecular functionalization and application in electrochemical sensors and biosensors. A review. Microchim Acta 184(1):1–44. https://doi.org/10.1007/s00604-016-2007-0
Wu S, He Q, Tan C, Wang Y, Zhang H (2013) Graphene-based electrochemical sensors. Small 9(8):1160–1172. https://doi.org/10.1002/smll.201202896
Pumera M (2009) Electrochemistry of graphene: new horizons for sensing and energy storage. Chem Rec 9(4):211–223. https://doi.org/10.1002/tcr.200900008
Chen X, Wu G, Jiang Y, Wang Y, Chen X (2011) Graphene and graphene-based nanomaterials: the promising materials for bright future of electroanalytical chemistry. Analyst 136(22):4631–4640. https://doi.org/10.1039/c1an15661f
Li F, Liu S, Wu T, Zhang Q, Dong X, Niu L (2018) Disposable graphene sensor with an internal reference electrode for heavy metals stripping analysis. Anal Methods 10(17):1986–1992. https://doi.org/10.1039/c8ay00221e
Chao H, Fu L, Li Y, Li X, Du H, Ye J (2013) Sensitive stripping determination of cadmium(ii) and lead(ii) on disposable graphene modified screen-printed electrode. Electroanal 25(9):2238–2243. https://doi.org/10.1002/elan.201300239
Chen D, Feng H, Li J (2012) Graphene oxide: preparation, functionalization, and electrochemical applications. Chem Rev 112(11):6027–6053. https://doi.org/10.1021/cr300115g
Naveen MH, Gurudatt NG, Shim YB (2017) Applications of conducting polymer composites to electrochemical sensors: a review. Applied Mater Today 9:419–433. https://doi.org/10.1016/j.apmt.2017.09.001
Fang Y, Wang E (2013) Electrochemical biosensors on platforms of graphene. Chem Commun 49(83):9526–9539. https://doi.org/10.1039/c3cc44735a
Zhao D, Zhang L, Siebold D, DeArmond D, Alvarez NT, Shanov VN, Heineman WR (2017) Electrochemical studies of three dimensional graphene foam as an electrode material. Electroanal. 29(6):1506–1512. https://doi.org/10.1002/elan.201700057
Shi L, Li Y, Rong X, Wang Y, Ding S (2017) Facile fabrication of a novel 3D graphene framework/Bi nanoparticle film for ultrasensitive electrochemical assays of heavy metal ions. Anal Chim Acta 968:21–29. https://doi.org/10.1016/j.aca.2017.03.013
Baig N, Saleh TA (2018) Electrodes modified with 3D graphene composites: a review on methods for preparation, properties and sensing applications. Microchim Acta 185(6):283. https://doi.org/10.1007/s00604-018-2809-3
Waheed A, Mansha M, Ullah N (2018) Nanomaterials-based electrochemical detection of heavy metals in water: current status, challenges and future direction. TrAC-Trend Anal Chem 105:37–51. https://doi.org/10.1016/j.trac.2018.04.012
Shao Y, Wang J, Wu H, Liu J, Aksay IA, Lin Y (2010) Graphene based electrochemical sensors and biosensors: a review. Electroanal 22(10):1027–1036. https://doi.org/10.1002/elan.200900571
Gan X, Zhao H, Schirhagl R, Quan X (2018) Two-dimensional nanomaterial based sensors for heavy metal ions. Microchim Acta 185(10):478. https://doi.org/10.1007/s00604-018-3005-1
Wang X, Sun G, Routh P, Kim DH, Huang W, Chen P (2014) Heteroatom-doped graphene materials: syntheses, properties and applications. Chem Soc Rev 43(20):7067–7098. https://doi.org/10.1039/c4cs00141a
Xing H, Xu J, Zhu X, Duan X, Lu L, Wang W, Zhang Y, Yang T (2016) Highly sensitive simultaneous determination of cadmium (II), lead (II), copper (II), and mercury (II) ions on N-doped graphene modified electrode. J Electroanal Chem 760:52–58. https://doi.org/10.1016/j.jelechem.2015.11.043
Liu XS, Li ZJ, Ding RM, Ren BB, Li YH (2016) A nanocarbon paste electrode modified with nitrogen-doped graphene for square wave anodic stripping voltammetric determination of trace lead and cadmium. Microchim Acta 183(2):709–714. https://doi.org/10.1007/s00604-015-1713-3
Manna B, Raj CR (2018) Nanostructured sulfur-doped porous reduced graphene oxide for the ultrasensitive electrochemical detection and efficient removal of Hg(II). ACS Sustain Chem Eng 6(5):6175–6182. https://doi.org/10.1021/acssuschemeng.7b04884
Thiruppathi AR, Sidhureddy B, Keeler W, Chen A (2017) Facile one-pot synthesis of fluorinated graphene oxide for electrochemical sensing of heavy metal ions. Electrochem Commun 76:42–46. https://doi.org/10.1016/j.elecom.2017.01.015
Meng FL, Guo Z, Huang XJ (2015) Graphene-based hybrids for chemiresistive gas sensors. TrAC-Trend Anal Chem 68:37–47. https://doi.org/10.1016/j.trac.2015.02.008
Gong J, Zhou T, Song D, Zhang L (2010) Monodispersed Au nanoparticles decorated graphene as an enhanced sensing platform for ultrasensitive stripping voltammetric detection of mercury(II). Sensor Actuat B-Chem 150(2):491–497. https://doi.org/10.1016/j.snb.2010.09.014
Liu Y, Huang Z, Xie Q, Sun L, Gu T, Li Z, Bu L, Yao S, Tu X, Luo X, Luo S (2013) Electrodeposition of electroreduced graphene oxide-Au nanoparticles composite film at glassy carbon electrode for anodic stripping voltammetric analysis of trace arsenic(III). Sensor Actuat B-Chem 188:894–901. https://doi.org/10.1016/j.snb.2013.07.113
Gao C, Huang XJ (2013) Voltammetric determination of mercury(II). TrAC-Trend Anal Chem 51:1–12. https://doi.org/10.1016/j.trac.2013.05.010
Sang S, Li D, Zhang H, Sun Y, Jian A, Zhang Q, Zhang W (2017) Facile synthesis of AgNPs on reduced graphene oxide for highly sensitive simultaneous detection of heavy metal ions. RSC Adv 7(35):21618–21624. https://doi.org/10.1039/c7ra02267k
Welch CM, Compton RG (2006) The use of nanoparticles in electroanal: a review. Anal Bioanal Chem 384(3):601–619. https://doi.org/10.1007/s00216-005-0230-3
Sahoo PK, Panigrahy B, Sahoo S, Satpati AK, Li D, Bahadur D (2013) In situ synthesis and properties of reduced graphene oxide/Bi nanocomposites: as an electroactive material for analysis of heavy metals. Biosens Bioelectron 43:293–296. https://doi.org/10.1016/j.bios.2012.12.031
Lee PM, Chen Z, Li L, Liu E (2015) Reduced graphene oxide decorated with tin nanoparticles through electrodeposition for simultaneous determination of trace heavy metals. Electrochim Acta 174:207–214. https://doi.org/10.1016/j.electacta.2015.05.092
Molina J, Cases F, Moretto LM (2016) Graphene-based materials for the electrochemical determination of hazardous ions. Anal Chim Acta 946:9–39. https://doi.org/10.1016/j.aca.2016.10.019
Ping J, Wang Y, Wu J, Ying Y (2014) Development of an electrochemically reduced graphene oxide modified disposable bismuth film electrode and its application for stripping analysis of heavy metals in milk. Food Chem 151:65–71. https://doi.org/10.1016/j.foodchem.2013.11.026
Arduini F, Calvo JQ, Palleschi G, Moscone D, Amine A (2010) Bismuth-modified electrodes for lead detection. TrAC-Trend Anal Chem 29(11):1295–1304. https://doi.org/10.1016/j.trac.2010.08.003
Pan D, Wang Y, Chen Z, Lou T, Qin W (2009) Nanomaterial/ionophore-based electrode for anodic stripping voltammetric determination of lead: an electrochemical sensing platform toward heavy metals. Anal Chem 81(12):5088–5094. https://doi.org/10.1021/ac900417e
Ruengpirasiri P, Punrat E, Chailapakul O, Chuanuwatanakul S (2017) Graphene oxide-modified electrode coated with in-situ antimony film for the simultaneous determination of heavy metals by sequential injection-anodic stripping voltammetry. Electroanal 29(4):1022–1030. https://doi.org/10.1002/elan.201600568
Zhang XZ, Qu KM, Li DP (2015) Advance in the stripping voltammetry using alloy electrodes for the determination of heavy metal ions. Int J Electrochem Sci 10(10):8497–8512
Wang T, Yue W (2017) Carbon nanotubes heavy metal detection with stripping voltammetry: a review paper. Electroanal 29(10):2178–2189. https://doi.org/10.1002/elan.201700276
Ding L, Liu Y, Zhai J, Bond AM, Zhang J (2014) Direct electrodeposition of graphene-gold nanocomposite films for ultrasensitive voltammetric determination of mercury(II). Electroanal 26(1):121–128. https://doi.org/10.1002/elan.201300226
Sahoo PK, Sahoo S, Satpati AK, Bahadur D (2015) Solvothermal synthesis of reduced graphene oxide/Au nanocomposite-modified electrode for the determination of inorganic mercury and electrochemical oxidation of toxic phenolic compounds. Electrochim Acta 180:1023–1032. https://doi.org/10.1016/j.electacta.2015.09.018
Sahoo S, Sahoo PK, Satpati AK (2017) Gold nano particle and reduced graphene oxide composite modified carbon paste electrode for the ultra trace detection of arsenic (III). Electroanal 29(5):1400–1409. https://doi.org/10.1002/elan.201600676
Li WW, Kong FY, Wang JY, Chen ZD, Fang HL, Wang W (2015) Facile one-pot and rapid synthesis of surfactant-free Au-reduced graphene oxide nanocomposite for trace arsenic (III) detection. Electrochim Acta 157:183–190. https://doi.org/10.1016/j.electacta.2014.12.150
Lee PM, Wang Z, Liu X, Chen Z, Liu E (2015) Glassy carbon electrode modified by graphene–gold nanocomposite coating for detection of trace lead ions in acetate buffer solution. Thin Solid Films 584:85–89. https://doi.org/10.1016/j.tsf.2015.03.017
Wang S, Wang Y, Zhou L, Li J, Wang S, Liu H (2014) Fabrication of an effective electrochemical platform based on graphene and AuNPs for high sensitive detection of trace Cu2+. Electrochim Acta 132:7–14. https://doi.org/10.1016/j.electacta.2014.03.114
Liu M, Pan D, Pan W, Zhu Y, Hu X, Han H, Wang C, Shen D (2017) In-situ synthesis of reduced graphene oxide/gold nanoparticles modified electrode for speciation analysis of copper in seawater. Talanta 174:500–506. https://doi.org/10.1016/j.talanta.2017.06.054
Gnanaprakasam P, Jeena SE, Premnath D, Selvaraju T (2016) Simple and robust green synthesis of Au NPs on reduced graphene oxide for the simultaneous detection of toxic heavy metal ions and bioremediation using bacterium as the scavenger. Electroanal 28(8):1885–1893. https://doi.org/10.1002/elan.201600002
Han H, Pan D, Wang C, Zhu R (2017) Controlled synthesis of dendritic gold nanostructures by graphene oxide and their morphology-dependent performance for iron detection in coastal waters. RSC Adv 7(26):15833–15841. https://doi.org/10.1039/c6ra27075a
Zhu Y, Pan D, Hu X, Han H, Lin M, Wang C (2017) An electrochemical sensor based on reduced graphene oxide/gold nanoparticles modified electrode for determination of iron in coastal waters. Sensor Actuat B-Chem 243:1–7. https://doi.org/10.1016/j.snb.2016.11.108
Xu Y, Zhang W, Shi J, Zou X, Li Y, Haroon Elrasheid T, Huang X, Li Z, Zhai X, Hu X (2017) Electrodeposition of gold nanoparticles and reduced graphene oxide on an electrode for fast and sensitive determination of methylmercury in fish. Food Chem 237:423–430. https://doi.org/10.1016/j.foodchem.2017.05.096
Dar RA, Khare NG, Cole DP, Karna SP, Srivastava AK (2014) Green synthesis of a silver nanoparticle-graphene oxide composite and its application for As(III) detection. RSC Adv 4(28):14432–14440. https://doi.org/10.1039/c4ra00934g
Kempegowda R, Antony D, Malingappa P (2014) Graphene platinum nanocomposite as a sensitive and selective voltammetric sensor for trace level arsenic quantification. Inter J Smart Nano Mater 5(1):17–32. https://doi.org/10.1080/19475411.2014.898710
Hu X, Pan D, Lin M, Han H, Li F (2016) Graphene oxide-assisted synthesis of bismuth nanosheets for catalytic stripping voltammetric determination of iron in coastal waters. Microchim Acta 183(2):855–861. https://doi.org/10.1007/s00604-015-1733-z
Lee S, Park SK, Choi E, Piao Y (2016) Voltammetric determination of trace heavy metals using an electrochemically deposited graphene/bismuth nanocomposite film-modified glassy carbon electrode. J Electroanal Chem 766:120–127. https://doi.org/10.1016/j.jelechem.2016.02.003
Lin X, Lu Z, Zhang Y, Liu B, Mo G, Li J, Ye J (2018) A glassy carbon electrode modified with a bismuth film and laser etched graphene for simultaneous voltammetric sensing of Cd(II) and Pb(II). Microchim Acta 185(9):438. https://doi.org/10.1007/s00604-018-2966-4
Wang J, Chen X, Wu K, Zhang M, Huang W (2016) Highly-sensitive electrochemical sensor for Cd2+ and Pb2+ based on the synergistic enhancement of exfoliated graphene nanosheets and bismuth. Electroanal 28(1):63–68. https://doi.org/10.1002/elan.201500447
Cui L, Wu J, Ju H (2015) Electrochemical sensing of heavy metal ions with inorganic, organic and bio-materials. Biosens Bioelectron 63:276–286. https://doi.org/10.1016/j.bios.2014.07.052
Campbell FW, Compton RG (2010) The use of nanoparticles in electroanal: an updated review. Anal Bioanal Chem 396(1):241–259. https://doi.org/10.1007/s00216-009-3063-7
Arino C, Serrano N, Diaz-Cruz JM, Esteban M (2017) Voltammetric determination of metal ions beyond mercury electrodes. A review. Anal Chim Acta 990:11–53. https://doi.org/10.1016/j.aca.2017.07.069
Khan M, Tahir MN, Adil SF, Khan HU, Siddiqui MRH, Al-warthan AA, Tremel W (2015) Graphene based metal and metal oxide nanocomposites: synthesis, properties and their applications. J Mater Chem A 3(37):18753–18808. https://doi.org/10.1039/c5ta02240a
Sun Y, Zhang W, Yu H, Hou C, Li D, Zhang Y, Liu Y (2015) Controlled synthesis various shapes Fe3O4 decorated reduced graphene oxide applied in the electrochemical detection. J Alloy Compd 638:182–187. https://doi.org/10.1016/j.jallcom.2015.03.061
Karthik R, Thambidurai S (2017) Synthesis of cobalt doped ZnO/reduced graphene oxide nanorods as active material for heavy metal ions sensor and antibacterial activity. J Alloy Compd 715:254–265. https://doi.org/10.1016/j.jallcom.2017.04.298
Lu XF, Gu LF, Wang JW, Wu JX, Liao PQ, Li GR (2017) Bimetal-organic framework derived CoFe2O4/C porous hybrid nanorod arrays as high-performance electrocatalysts for oxygen evolution reaction. Adv Mater 29(3):1604437. https://doi.org/10.1002/adma.201604437
Yu XY, Yao XZ, Luo T, Jia Y, Liu JH, Huang XJ (2014) Facile synthesis of urchin-like NiCo2O4 hollow microspheres with enhanced electrochemical properties in energy and environmentally related applications. ACS Appl Mater Interfaces 6(5):3689–3695. https://doi.org/10.1021/am4060707
Xu J, Li Z, Yue X, Xie F, Xiong S (2018) Electrochemical detection of Cu(ii) using amino-functionalized MgFe2O4/reduced graphene oxide composite. Anal Methods 10(17):2026–2033. https://doi.org/10.1039/c8ay00452h
Xiong S, Ye S, Hu X, Xie F (2016) Electrochemical detection of ultra-trace Cu(ii) and interaction mechanism analysis between amine-groups functionalized CoFe2O4/reduced graphene oxide composites and metal ion. Electrochim Acta 217:24–33. https://doi.org/10.1016/j.electacta.2016.09.060
Zhou SF, Han XJ, Fan HL, Huang J, Liu YQ (2018) Enhanced electrochemical performance for sensing Pb(II) based on graphene oxide incorporated mesoporous MnFe2O4 nanocomposites. J Alloy Compd 747:447–454. https://doi.org/10.1016/j.jallcom.2018.03.037
Mahmoudian MR, Alias Y, Basirun WJ, Woi PM, Sookhakian M, Jamali-Sheini F (2015) Synthesis and characterization of Fe3O4 rose like and spherical/reduced graphene oxide nanosheet composites for lead (II) sensor. Electrochim Acta 169:126–133. https://doi.org/10.1016/j.electacta.2015.04.050
Sun YF, Chen WK, Li WJ, Jiang TJ, Liu JH, Liu ZG (2014) Selective detection toward Cd2+ using Fe3O4/RGO nanoparticle modified glassy carbon electrode. J Electroanal Chem 714-715:97–102. https://doi.org/10.1016/j.jelechem.2013.12.030
Chimezie AB, Hajian R, Yusof NA, Woi PM, Shams N (2017) Fabrication of reduced graphene oxide-magnetic nanocomposite (rGO-Fe3O4) as an electrochemical sensor for trace determination of As(III) in water resources. J Electroanal Chem 796:33–42. https://doi.org/10.1016/j.jelechem.2017.04.061
Devi P, Sharma C, Kumar P, Kumar M, Bansod BKS, Nayak MK, Singla ML (2017) Selective electrochemical sensing for arsenite using rGO/Fe3O4 nanocomposites. J Hazard Mater 322:85–94. https://doi.org/10.1016/j.jhazmat.2016.02.066
Xiong S, Yang B, Cai D, Qiu G, Wu Z (2015) Individual and simultaneous stripping voltammetric and mutual interference analysis of Cd2+, Pb2+ and Hg2+ with reduced graphene oxide-Fe3O4 nanocomposites. Electrochim Acta 185:52–61. https://doi.org/10.1016/j.electacta.2015.10.114
Prakash A, Chandra S, Bahadur D (2012) Structural, magnetic, and textural properties of iron oxide-reduced graphene oxide hybrids and their use for the electrochemical detection of chromium. Carbon 50(11):4209–4219. https://doi.org/10.1016/j.carbon.2012.05.002
Lee S, Oh J, Kim D, Piao Y (2016) A sensitive electrochemical sensor using an iron oxide/graphene composite for the simultaneous detection of heavy metal ions. Talanta 160:528–536. https://doi.org/10.1016/j.talanta.2016.07.034
Wei Y, Gao C, Meng FL, Li HH, Wang L, Liu JH, Huang XJ (2012) SnO2/reduced graphene oxide nanocomposite for the simultaneous electrochemical detection of cadmium(ii), lead(ii), copper(ii), and mercury(ii): an interesting favorable mutual interference. J Phy Chem C 116(1):1034–1041. https://doi.org/10.1021/jp209805c
Zhang H, Shuang S, Wang G, Guo Y, Tong X, Yang P, Chen A, Dong C, Qin Y (2015) TiO2–graphene hybrid nanostructures by atomic layer deposition with enhanced electrochemical performance for Pb(ii) and Cd(ii) detection. RSC Adv 5(6):4343–4349. https://doi.org/10.1039/c4ra09779c
Xie YL, Zhao SQ, Ye HL, Yuan J, Song P, Hu SQ (2015) Graphene/CeO2 hybrid materials for the simultaneous electrochemical detection of cadmium(II), lead(II), copper(II), and mercury(II). J Electroanal Chem 757:235–242. https://doi.org/10.1016/j.jelechem.2015.09.043
Zuo Y, Xu J, Jiang F, Duan X, Lu L, Xing H, Yang T, Zhang Y, Ye G, Yu Y (2017) Voltammetric sensing of Pb(II) using a glassy carbon electrode modified with composites consisting of Co3O4 nanoparticles, reduced graphene oxide and chitosan. J Electroanal Chem 801:146–152. https://doi.org/10.1016/j.jelechem.2017.07.046
Devi P, Bansod B, Kaur M, Bagchi S, Nayak MK (2016) Co-electrodeposited rGO/MnO2 nanohybrid for arsenite detection in water by stripping voltammetry. Sensor Actuat B-Chem 237:652–659. https://doi.org/10.1016/j.snb.2016.06.124
Zhang W, Xu Y, Zou X (2017) A ZnO–RGO-modified electrode coupled to microwave digestion for the determination of trace cadmium and lead in six species fish. Anal Methods 9(30):4418–4424. https://doi.org/10.1039/c7ay01574g
Lu YY, Chen MN, Gao YL, Yang JM, Ma XY, Liu JY (2015) Preparation of zinc oxide-graphene composite modified electrodes for detection of trace Pb(II). Chin J Anal Chem 43(9):1395–1401. https://doi.org/10.1016/s1872-2040(15)60862-3
Ramesha GK, Sampath S (2011) In-situ formation of graphene-lead oxide composite and its use in trace arsenic detection. Sensors Actuat B-Chem 160(1):306–311. https://doi.org/10.1016/j.snb.2011.07.053
Muralikrishna S, Sureshkumar K, Varley TS, Nagaraju DH, Ramakrishnappa T (2014) In situ reduction and functionalization of graphene oxide withl-cysteine for simultaneous electrochemical determination of cadmium(ii), lead(ii), copper(ii), and mercury(ii) ions. Anal Methods 6(21):8698–8705. https://doi.org/10.1039/c4ay01945h
Zuo Y, Xu J, Xing H, Duan X, Lu L, Ye G, Jia H, Yu Y (2018) Simple and green synthesis of piperazine-grafted reduced graphene oxide and its application for the detection of Hg(II). Nanotechnology 29(16):165502. https://doi.org/10.1088/1361-6528/aaaf4a
Zhou H, Wang X, Yu P, Chen X, Mao L (2012) Sensitive and selective voltammetric measurement of Hg2+ by rational covalent functionalization of graphene oxide with cysteamine. Analyst 137(2):305–308. https://doi.org/10.1039/c1an15793k
Gode C, Yola ML, Yilmaz A, Atar N, Wang S (2017) A novel electrochemical sensor based on calixarene functionalized reduced graphene oxide: application to simultaneous determination of Fe(III), Cd(II) and Pb(II) ions. J Colloid Interf Sci 508:525–531. https://doi.org/10.1016/j.jcis.2017.08.086
Yu C, Guo Y, Liu H, Yan N, Xu Z, Yu G, Fang Y, Liu Y (2013) Ultrasensitive and selective sensing of heavy metal ions with modified graphene. Chem Commun 49(58):6492–6494. https://doi.org/10.1039/c3cc42377h
Magerusan L, Socaci C, Coros M, Pogacean F, Rosu MC, Gergely S, Pruneanu S, Leostean C, Pana IO (2017) Electrochemical platform based on nitrogendoped graphene/chitosan nanocomposite for selective Pb2+ detection. Nanotechnology 28(11):114001. https://doi.org/10.1088/1361-6528/aa56cb
Yang M, Jiang TJ, Wang Y, Liu JH, Li LN, Chen X, Huang XJ (2017) Enhanced electrochemical sensing arsenic(III) with excellent anti-interference using amino-functionalized graphene oxide decorated gold microelectrode: XPS and XANES evidence. Sensor Actuat B-Chem 245:230–237. https://doi.org/10.1016/j.snb.2017.01.139
Zhan F, Gao F, Wang X, Xie L, Gao F, Wang Q (2016) Determination of lead(II) by adsorptive stripping voltammetry using a glassy carbon electrode modified with β-cyclodextrin and chemically reduced graphene oxide composite. Microchim Acta 183(3):1169–1176. https://doi.org/10.1007/s00604-016-1754-2
Lv M, Wang X, Li J, Yang X, Ca Z, Yang J, Hu H (2013) Cyclodextrin-reduced graphene oxide hybrid nanosheets for the simultaneous determination of lead(II) and cadmium(II) using square wave anodic stripping voltammetry. Electrochim Acta 108:412–420. https://doi.org/10.1016/j.electacta.2013.06.099
Li Y, Sun G, Zhang Y, Ge C, Bao N, Wang Y (2013) Sensitive and selective stripping voltammetric determination of copper(II) using a glassy carbon electrode modified with amino-reduced graphene oxide and β-cyclodextrin. Microchim Acta 181(7-8):751–757. https://doi.org/10.1007/s00604-013-1082-8
Mo Z, Liu H, Hu R, Gou H, Li Z, Guo R (2017) Amino-functionalized graphene/chitosan composite as an enhanced sensing platform for highly selective detection of Cu2+. Ionics 24(5):1505–1513. https://doi.org/10.1007/s11581-017-2309-1
Li Z, Chen L, He F, Bu L, Qin X, Xie Q, Yao S, Tu X, Luo X, Luo S (2014) Square wave anodic stripping voltammetric determination of Cd2+ and Pb2+ at bismuth-film electrode modified with electroreduced graphene oxide-supported thiolated thionine. Talanta 122:285–292. https://doi.org/10.1016/j.talanta.2014.01.062
Piek M, Fendrych K, Smajdor J, Piech R, Paczosa-Bator B (2017) High selective potentiometric sensor for determination of nanomolar con-centration of Cu(II) using a polymeric electrode modified by a graphene/7,7,8,8-tetracyanoquinodimethane nanoparticles. Talanta 170:41–48. https://doi.org/10.1016/j.talanta.2017.03.068
Abraham AA, Rezayi M, Manan NSA, Narimani L, Rosli ANB, Alias Y (2015) A novel potentiometric sensor based on 1,2-bis(n’-benzoylthioureido)benzene and reduced graphene oxide for determination of lead (ii) cation in raw milk. Electrochim Acta 165:221–231. https://doi.org/10.1016/j.electacta.2015.03.003
Priya T, Dhanalakshmi N, Thennarasu S, Thinakaran N (2018) A novel voltammetric sensor for the simultaneous detection of Cd2+ and Pb2+ using graphene oxide/kappa-carrageenan/L-cysteine nanocomposite. Carbohyd Polym 182:199–206. https://doi.org/10.1016/j.carbpol.2017.11.017
Priya T, Dhanalakshmi N, Thennarasu S, Thinakaran N (2018) Ultra sensitive detection of Cd (II) using reduced graphene oxide/carboxymethyl cellulose/glutathione modified electrode. Carbohyd Polym 197:366–374. https://doi.org/10.1016/j.carbpol.2018.06.024
Liu R, Lei C, Zhong T, Long L, Wu Z, Huan S, Zhang Q (2016) A graphene/ionic liquid modified selenium-doped carbon paste electrode for determination of copper and antimony. Anal Methods 8(5):1120–1126. https://doi.org/10.1039/c5ay02945g
Li F, Pan D, Lin M, Han H, Hu X, Kang Q (2015) Electrochemical determination of iron in coastal waters based on ionic liquid-reduced graphene oxide supported gold nanodendrites. Electrochim Acta 176:548–554. https://doi.org/10.1016/j.electacta.2015.07.011
Bagheri H, Afkhami A, Khoshsafar H, Rezaei M, Sabounchei SJ, Sarlakifar M (2015) Simultaneous electrochemical sensing of thallium, lead and mercury using a novel ionic liquid/graphene modified electrode. Anal Chim Acta 870:56–66. https://doi.org/10.1016/j.aca.2015.03.004
Wang Z, Wang H, Zhang Z, Liu G (2014) Electrochemical determination of lead and cadmium in rice by a disposable bismuth/electrochemically reduced graphene/ionic liquid composite modified screen-printed electrode. Sensor Actuat B-Chem 199:7–14. https://doi.org/10.1016/j.snb.2014.03.092
Zhou W, Li C, Sun C, Yang X (2016) Simultaneously determination of trace Cd2+ and Pb2+ based on L-cysteine/graphene modified glassy carbon electrode. Food Chem 192:351–357. https://doi.org/10.1016/j.foodchem.2015.07.042
Liu L, Wang CY, Wang GX (2013) Novel cysteic acid/reduced graphene oxide composite film modified electrode for the selective detection of trace silver ions in natural waters. Anal Methods 5(20):5812–5822. https://doi.org/10.1039/c3ay40888d
Qiu N, Liu Y, Guo R (2016) A novel sensitive electrochemical sensor for lead ion based on three-dimensional graphene/sodium dodecyl benzene sulfonate hemimicelle nanocomposites. Electrochim Acta 212:147–154. https://doi.org/10.1016/j.electacta.2016.06.136
Wang B, Luo B, Liang M, Wang A, Wang J, Fang Y, Chang Y, Zhi L (2011) Chemical amination of graphene oxides and their extraordinary properties in the detection of lead ions. Nanoscale 3(12):5059–5066. https://doi.org/10.1039/c1nr10901d
Yuan YH, Zhu XH, Wen SH, Liang RP, Zhang L, Qiu JD (2018) Electrochemical assay for As (III) by combination of highly thiol-rich trithiocyanuric acid and conductive reduced graphene oxide nanocomposites. J Electroanal Chem 814:97–103. https://doi.org/10.1016/j.jelechem.2018.02.039
Çelik GK, Üzdürmez AF, Erkal A, Kılıç E, Solak AO, Üstündağ Z (2016) 3,8-diaminobenzo[c]cinnoline derivatived graphene oxide modified graphene oxide sensor for the voltammetric determination of Cd2+ and Pb2+. Electrocatal 7(3):207–214. https://doi.org/10.1007/s12678-015-0297-3
Choi SM, Kim DM, Jung OS, Shim YB (2015) A disposable chronocoulometric sensor for heavy metal ions using a diaminoterthiophene-modified electrode doped with graphene oxide. Anal Chim Acta 892:77–84. https://doi.org/10.1016/j.aca.2015.08.037
Xing H, Xu J, Zhu X, Duan X, Lu L, Zuo Y, Zhang Y, Wang W (2016) A new electrochemical sensor based on carboimidazole grafted reduced graphene oxide for simultaneous detection of Hg2+ and Pb2+. J Electroanal Chem 782:250–255. https://doi.org/10.1016/j.jelechem.2016.10.043
Gupta VK, Yola ML, Atar N, Ustundağ Z, Solak AO (2013) A novel sensitive Cu(II) and Cd(II) nanosensor platform: graphene oxide terminated p-aminophenyl modified glassy carbon surface. Electrochim Acta 112:541–548. https://doi.org/10.1016/j.electacta.2013.09.011
Yuan X, Chai Y, Yuan R, Zhao Q, Yang C (2012) Functionalized graphene oxide-based carbon paste electrode for potentiometric detection of copper ion(ii). Anal Methods 4(10):3332–3337. https://doi.org/10.1039/c2ay25674f
Kang M, Peng D, Zhang Y, Yang Y, He L, Yan F, Sun S, Fang S, Wang P, Zhang Z (2015) An electrochemical sensor based on rhodamine B hydrazide-immobilized graphene oxide for highly sensitive and selective detection of Cu(ii). New J Chem 39(4):3137–3144. https://doi.org/10.1039/c5nj00157a
Ziółkowski R, Górski Ł, Malinowska E (2017) Carboxylated graphene as a sensing material for electrochemical uranyl ion detection. Sensor Actuat B-Chem 238:540–547. https://doi.org/10.1016/j.snb.2016.07.119
Zhang Y, Qi M, Liu G (2015) C-C bonding of graphene oxide on 4-aminophenyl modified gold electrodes towards simultaneous detection of heavy metal ions. Electroanal 27(5):1110–1118. https://doi.org/10.1002/elan.201400591
Zhang W, Wei J, Zhu H, Zhang K, Ma F, Mei Q, Zhang Z, Wang S (2012) Self-assembled multilayer of alkyl graphene oxide for highly selective detection of copper(ii) based on anodic stripping voltammetry. J Mater Chem 22(42):22631. https://doi.org/10.1039/c2jm34795d
March G, Nguyen T, Piro B (2015) Modified electrodes used for electrochemical detection of metal ions in environmental analysis. Biosensors 5(2):241–275. https://doi.org/10.3390/bios5020241
Kuilla T, Bhadra S, Yao D, Kim NH, Bose S, Lee JH (2010) Recent advances in graphene based polymer composites. Prog Polym Sci 35(11):1350–1375. https://doi.org/10.1016/j.progpolymsci.2010.07.005
Hui Y, Bian C, Xia S, Tong J, Wang J (2018) Synthesis and electrochemical sensing application of poly(3,4-ethylenedioxythiophene)-based materials: a review. Anal Chim Acta 1022:1–19. https://doi.org/10.1016/j.aca.2018.02.080
Zuo Y, Xu J, Zhu X, Duan X, Lu L, Gao Y, Xing H, Yang T, Ye G, Yu Y (2016) Poly(3,4-ethylenedioxythiophene) nanorods/graphene oxide nanocomposite as a new electrode material for the selective electrochemical detection of mercury (II). Synthetic Met 220:14–19. https://doi.org/10.1016/j.synthmet.2016.05.022
Dai H, Wang N, Wang D, Ma H, Lin M (2016) An electrochemical sensor based on phytic acid functionalized polypyrrole/graphene oxide nanocomposites for simultaneous determination of Cd(II) and Pb(II). Chem Eng J 299:150–155. https://doi.org/10.1016/j.cej.2016.04.083
Muralikrishna S, Nagaraju DH, Balakrishna RG, Surareungchai W, Ramakrishnappa T, Shivanandareddy AB (2017) Hydrogels of polyaniline with graphene oxide for highly sensitive electrochemical determination of lead ions. Anal Chim Acta 990:67–77. https://doi.org/10.1016/j.aca.2017.09.008
Li J, Guo S, Zhai Y, Wang E (2009) Nafion–graphene nanocomposite film as enhanced sensing platform for ultrasensitive determination of cadmium. Electrochem Commun 11(5):1085–1088. https://doi.org/10.1016/j.elecom.2009.03.025
Chalupniak A, Merkoci A (2017) Graphene oxide-poly(dimethylsiloxane)-based lab-on-a-chip platform for heavy-metals preconcentration and electrochemical detection. ACS Appl Mater Interfaces 9(51):44766–44775. https://doi.org/10.1021/acsami.7b12368
Guo Z, Li D, Luo X, Li Y, Zhao QN, Li M, Zhao Y, Sun T, Ma C (2017) Simultaneous determination of trace Cd(II), Pb(II) and Cu(II) by differential pulse anodic stripping voltammetry using a reduced graphene oxide-chitosan/poly-L-lysine nanocomposite modified glassy carbon electrode. J Colloid Interf Sci 490:11–22. https://doi.org/10.1016/j.jcis.2016.11.006
Liu H, Li S, Sun D, Chen Y, Zhou Y, Lu T (2014) Layered graphene nanostructures functionalized with NH2-rich polyelectrolytes through self-assembly: construction and their application in trace Cu(ii) detection. J Mater Chem B 2(16):2212–2219. https://doi.org/10.1039/c4tb00104d
Hu R, Gou H, Mo Z, Wei X, Wang Y (2015) Highly selective detection of trace Cu2+ based on polyethyleneimine-reduced graphene oxide nanocomposite modified glassy carbon electrode. Ionics 21(11):3125–3133. https://doi.org/10.1007/s11581-015-1499-7
Suherman AL, Tanner EEL, Compton RG (2017) Recent developments in inorganic Hg2+ detection by voltammetry. TrAC-Trend Anal Chem 94:161–172. https://doi.org/10.1016/j.trac.2017.07.020
Zhao ZQ, Chen X, Yang Q, Liu JH, Huang XJ (2012) Beyond the selective adsorption of polypyrrole-reduced graphene oxide nanocomposite toward Hg2+: ultra sensitive and selective sensing Pb2+ by stripping voltammetry. Electrochem Commun 23:21–24. https://doi.org/10.1016/j.elecom.2012.06.034
Palanisamy S, Thangavelu K, Chen SM, Velusamy V, Chang MH, Chen TW, Al-Hemaid FMA, Ali MA, Ramaraj SK (2017) Synthesis and characterization of polypyrrole decorated graphene/β-cyclodextrin composite for low level electrochemical detection of mercury (II) in water. Sensor Actuat B-Chem 243:888–894. https://doi.org/10.1016/j.snb.2016.12.068
Seenivasan R, Chang WJ, Gunasekaran S (2015) Highly sensitive detection and removal of lead ions in water using cysteine-functionalized graphene oxide/polypyrrole nanocomposite film electrode. ACS Appl Mater Interfaces 7(29):15935–15943. https://doi.org/10.1021/acsami.5b03904
Ruecha N, Rodthongkum N, Cate DM, Volckens J, Chailapakul O, Henry CS (2015) Sensitive electrochemical sensor using a graphene-polyaniline nanocomposite for simultaneous detection of Zn(II), Cd(II), and Pb(II). Anal Chim Acta 874:40–48. https://doi.org/10.1016/j.aca.2015.02.064
Promphet N, Rattanarat P, Rangkupan R, Chailapakul O, Rodthongkum N (2015) An electrochemical sensor based on graphene/polyaniline/polystyrene nanoporous fibers modified electrode for simultaneous determination of lead and cadmium. Sensor Actuat B-Chem 207:526–534. https://doi.org/10.1016/j.snb.2014.10.126
Nguyen TD, Dang TTH, Hoang T, Nguyen LH, Tran DL, Piro B, Pham MC (2016) One-step electrosynthesis of poly(1,5-diaminonaphthalene)/graphene nanocomposite as platform for lead detection in water. Electroanal 28(8):1907–1913. https://doi.org/10.1002/elan.201501075
Li J, Guo S, Zhai Y, Wang E (2009) High-sensitivity determination of lead and cadmium based on the Nafion-graphene composite film. Anal Chim Acta 649(2):196–201. https://doi.org/10.1016/j.aca.2009.07.030
Lee PM, Ng HW, Lim JD, Khun NW, Chen Z, Liu E (2016) Nanostructure restoration of thermally reduced graphene oxide electrode upon incorporation of Nafion for detection of trace heavy metals in aqueous solution. Electroanal 28(9):2037–2043. https://doi.org/10.1002/elan.201501099
Chaiyo S, Mehmeti E, Zagar K, Siangproh W, Chailapakul O, Kalcher K (2016) Electrochemical sensors for the simultaneous determination of zinc, cadmium and lead using a Nafion/ionic liquid/graphene composite modified screen-printed carbon electrode. Anal Chim Acta 918:26–34. https://doi.org/10.1016/j.aca.2016.03.026
Huang N, Zhang S, Yang L, Liu M, Li H, Zhang Y, Yao S (2015) Multifunctional electrochemical platforms based on the michael addition/schiff base reaction of polydopamine modified reduced graphene oxide: construction and application. ACS Appl Mater Interfaces 7(32):17935–17946. https://doi.org/10.1021/acsami.5b04597
Wang Y, Zhou L, Wang S, Li J, Tang J, Wang S, Wang Y (2016) Sensitive and selective detection of Hg2+ based on an electrochemical platform of PDDA functionalized rGO and glutaraldehyde cross-linked chitosan composite film. RSC Adv 6(74):69815–69821. https://doi.org/10.1039/c6ra10075a
Dong Y, Zhou Y, Ding Y, Chu X, Wang C (2014) Sensitive detection of Pb(ii) at gold nanoparticle/polyaniline/graphene modified electrode using differential pulse anodic stripping voltammetry. Anal Methods 6(23):9367–9374. https://doi.org/10.1039/c4ay01908c
Wang Z, Wang H, Zhang Z, Yang X, Liu G (2014) Sensitive electrochemical determination of trace cadmium on a stannum film/poly(p-aminobenzene sulfonic acid)/electrochemically reduced graphene composite modified electrode. Electrochim Acta 120:140–146. https://doi.org/10.1016/j.electacta.2013.12.068
Cui X, Fang X, Zhao H, Li Z, Ren H (2018) Fabrication of thiazole derivatives functionalized graphene decorated with fluorine, chlorine and iodine@SnO2 nanoparticles for highly sensitive detection of heavy metal ions. Colloid Surfaces A 546:153–162. https://doi.org/10.1016/j.colsurfa.2018.03.004
Xuan X, Park JY (2018) A miniaturized and flexible cadmium and lead ion detection sensor based on micro-patterned reduced graphene oxide/carbon nanotube/bismuth composite electrodes. Sensors Actuat B-Chem 255:1220–1227. https://doi.org/10.1016/j.snb.2017.08.046
Dong S, Wang Z, Asif M, Wang H, Yu Y, Hu Y, Liu H, Xiao F (2017) Inkjet printing synthesis of sandwiched structured ionic liquid-carbon nanotube-graphene film: toward disposable electrode for sensitive heavy metal detection in environmental water samples. Ind Eng Chem Res 56(7):1696–1703. https://doi.org/10.1021/acs.iecr.6b04251
Wang Z, Li L, Liu E (2013) Graphene ultrathin film electrodes modified with bismuth nanoparticles and polyaniline porous layers for detection of lead and cadmium ions in acetate buffer solutions. Thin Solid Films 544:362–367. https://doi.org/10.1016/j.tsf.2013.02.098
Lu ZZ, Yang SL, Yang Q, Luo SL, Liu CB, Tang YH (2013) A glassy carbon electrode modified with graphene, gold nanoparticles and chitosan for ultrasensitive determination of lead(II). Microchim Acta 180(7-8):555–562. https://doi.org/10.1007/s00604-013-0959-x
Zhou N, Li J, Chen H, Liao C, Chen L (2013) A functional graphene oxide-ionic liquid composites-gold nanoparticle sensing platform for ultrasensitive electrochemical detection of Hg2+. Analyst 138(4):1091–1097. https://doi.org/10.1039/c2an36405k
Al-Hossainy AF, Abd-Elmageed AAI, Ibrahim ATA (2015) Synthesis, structural and optical properties of gold nanoparticle-graphene-selenocysteine composite bismuth ultrathin film electrode and its application to Pb(II) and Cd(II) determination. Arab J Chem. https://doi.org/10.1016/j.arabjc.2015.06.020
Ghanei-Motlagh M, Karami C, Taher MA, Hosseini-Nasab SJ (2016) Stripping voltammetric detection of copper ions using carbon paste electrode modified with aza-crown ether capped gold nanoparticles and reduced graphene oxide. RSC Adv 6(92):89167–89175. https://doi.org/10.1039/c6ra10267k
Dahaghin Z, Kilmartin PA, Mousavi HZ (2018) Simultaneous determination of lead(II) and cadmium(II) at a glassy carbon electrode modified with GO@Fe3O4 @benzothiazole-2-carboxaldehyde using square wave anodic stripping voltammetry. J Mol Liq 249:1125–1132. https://doi.org/10.1016/j.molliq.2017.11.114
Mejri A, Mars A, Elfil H, Hamzaoui AH (2018) Graphene nanosheets modified with curcumin-decorated manganese dioxide for ultrasensitive potentiometric sensing of mercury(II). fluoride and cyanide. Microchim Acta 185(12):529. https://doi.org/10.1007/s00604-018-3064-3
Martín-Yerga D, González-García MB, Costa-García A (2012) Use of nanohybrid materials as electrochemical transducers for mercury sensors. Sensor Actuat B-Chem 165(1):143–150. https://doi.org/10.1016/j.snb.2012.02.031
Cui L, Wu J, Ju H (2015) Synthesis of bismuth-nanoparticle-enriched nanoporous carbon on graphene for efficient electrochemical analysis of heavy-metal ions. Chem 21(32):11525–11530. https://doi.org/10.1002/chem.201500512
Yang F, He D, Zheng B, Xiao D, Wu L, Guo Y (2016) Self-assembled hybrids with xanthate functionalized carbon nanotubes and electro-exfoliating graphene sheets for electrochemical sensing of copper ions. J Electroanal Chem 767:100–107. https://doi.org/10.1016/j.jelechem.2016.01.005
Khan AAP, Khan A, Rahman MM, Asiri AM, Oves M (2016) Lead sensors development and antimicrobial activities based on graphene oxide/carbon nanotube/poly(O-toluidine) nanocomposite. Int J Biol Macromol 89:198–205. https://doi.org/10.1016/j.ijbiomac.2016.04.064
Yu L, Zhang Q, Yang B, Xu Q, Xu Q, Hu X (2018) Electrochemical sensor construction based on Nafion/calcium lignosulphonate functionalized porous graphene nanocomposite and its application for simultaneous detection of trace Pb2+ and Cd2+. Sensor Actuat B-Chem 259:540–551. https://doi.org/10.1016/j.snb.2017.12.103
Gumpu MB, Veerapandian M, Krishnan UM, Rayappan JBB (2018) Amperometric determination of As(III) and Cd(II) using a platinum electrode modified with acetylcholinesterase, ruthenium(II)-tris(bipyridine) and graphene oxide. Microchim Acta 185(6):297. https://doi.org/10.1007/s00604-018-2822-6
Acknowledgments
We are grateful to the National Natural Science Foundation of China (21665010, 51762020 and 31741103), the Outstanding Youth Fund of Jiangxi Province (20162BCB23027), the Natural Science Foundation of Jiangxi Province (20171BAB203015, 20171ACB20026 and 20181BAB206015), the Jiangxi Provincial Department of Education (GJJ170662), and the One Hundred Person Yuan Hang Project (2017) for their financial support of this work. Yinxiu Zuo is greatly acknowledged Yunyong Hu for his support and encouragement during this writing process.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zuo, Y., Xu, J., Zhu, X. et al. Graphene-derived nanomaterials as recognition elements for electrochemical determination of heavy metal ions: a review. Microchim Acta 186, 171 (2019). https://doi.org/10.1007/s00604-019-3248-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3248-5