Abstract
An aptamer based colorimetric assay is described for the determination of zearalenone (ZEN). It is based on the inhibition of the peroxidase-mimicking activity of gold nanoparticles (AuNPs) by the ZEN aptamer. However, in the presence of ZEN, the aptamer is bound by ZEN and can no longer inhibit the peroxidase-mimicking activity of AuNPs. The color change of solution is related to ZEN concentration and observed with bare eyes. Under optimal conditions, the absorbance (at 630 nm) increases linearly in the ZEN concentration range of 10–250 ng·mL−1, and the limit of detection is 10 ng·mL−1. The specificity of the assay was verified by studying the effect of potential interferents. The recoveries from ZEN spiked corn and corn oil range from 92 to 110%, and the relative standard deviations are between 2.4 and 6.4%. The results are in good agreement with those obtained by an ELISA.

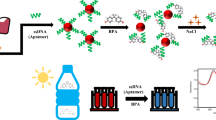
Schematic presentation of colorimetric assay for rapid and sensitive determination of zearalenone (ZEN) based on the inhibition of ZEN aptamer on the the peroxidase-like activity of gold nanoparticle (AuNPs).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zearalenone (ZEN) is an estrogenic mycotoxin produced by several fusarium species [1]. ZEN and its derivatives can widely contaminate agricultural products especially wheat, corn, barley, soybeans, as well as oats. ZEN in contaminated agricultural and animal products can accumulate in the human body through food chains and poses a great threat to human health. Previous studies have indicated zearalenone would bring strong mutagenicity, teratogenicity, neurotoxicity, reproductive toxicity and induced-cancer to animals and human [2]. ZEN has the characteristics of wide distribution and fast production. It is highly resistive in nature with long residual time and very difficult to clear away. To protect human from exposure to ZEN and reduce probable economic losses, the limits of the concentration of ZEN in different foodstuffs are set from 20 μg·kg−1 to 1000 μg·kg−1 by governments around the world [3]. Therefore, efficient, sensitive and specific methods were required in detecting and monitoring the level of ZEN in food products.
Various instrumental methods including high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS) and gas chromatography-mass spectrometry (GC-MS) are commonly used for ZEN detection [4]. Though these methods have high sensitivity and accuracy, majority of them need laborious sample pretreatments, sophisticated instruments and skilled professional. As alternative strategies, enzyme-linked immunosorbent assay (ELISA) that characterized with simpleness and quickness has been increasingly used for detection of ZEN in agricultural and food samples [5]. Other immunosensors such as immunochromatographic strip [6], electrochemical immunoassay [7] and fluorescence immunoassay [8] were also used for the rapid detection of ZEN. However, these immunoassays are seriously reliant on the utilization of the antibodies. The prepartion of the antibodies via animal immunization are often time-consuming, costly and susceptible. In addition, the ELISA kits are easy to be inactivated during storage and transportation and can’t be reused.
Aptamers possess the characteristic of easy synthesis in vitro without animal need, broad range of targets, high affinity and specificity, easy preservation and modification, low cost, high stability as well as reusability [9]. Based on the above outstanding advantages, aptamers have been considered as better alternative recognition elements to antibody and attract increasing interest in the construction of biosensors. For detection of mycotoxin, a series of aptamer-based biosensors were developed for the detection of ochratoxin A and aflatoxin B1 [10,11,12,13]. Few studies have been done in the rapid detection of ZEN based on the aptamer biosensors [3, 14, 15]. As a potential analysis tool for rapid test, optical aptasensors with easy operation, visible detection and high sensitivity have attracted great interest [16]. On the basis of unique optical property and easyness of synthesis, the gold nanoparticle (AuNPs) has become one of the most widely applied materials in these biosensors [17]. However, these AuNP-based assays often rely on the aggregation of disperse AuNPs under high salinity. Due to the uncontrolled aggregation caused by complex environmental factors, it might produce unsatisfied results by using above AuNP-based assay [18]. The recent discovery of peroxidase-like activity of AuNPs has aroused researchers’ interest [19, 20]. On the account of the higher peroxidase-like activity stability, lower production cost, higher flexibility and controlled size [21], AuNPs have been used in the biosensing assay by ways of substituting natural enzymes such as horse radish peroxidase (HRP). This assay strategy was independent of the AuNP aggregation and has been successfully used for the rapid detection of H2O2 [22], glucose [23], thrombin [24], heparin [25] and food contaminants such as pesticide [26], antibiotics [27] and so on. Few researches have been reported for the mycotoxin detection based on the utilization of AuNP peroxidase-like activity individually or combining with aptamers.
In this paper, a sensitive and specific colorimetric array for the rapid detection of ZEN was built based on the inhibition of ZEN aptamer on the peroxidase-like activity of AuNPs. The key parameters and the reliability of assay were investigated by a series of control experiments. In addition, the selectivity and specificity of the assay for ZEN detection were verified against various interferents. ZEN in real corn and oil samples were successfully detected using this colorimetric assay.
Experimental
Materials and instruments
ZEN aptamers with the sequences of 5′-GAT GGG GAA AGG GTC CCC CTG GGT TGG AGC ATC GGA CA-3′ [28] were synthesized and purified by high performance liquid chromatography (Sangon Biotechnology, Shanghai, Inc. http://www.sangon.com/). Zearalenone (ZEN), aflatoxin B1 (AFB1) and ochratoxin A (OTA) standard substances were purchased from Sigma Aldrich (St. Louis, MO, USA. http://www.sigmaaldrich.com). AuNPs were synthesized by sodium citrate reduction of HAuCl4 according to the previous literature [29]. UV-vis spectrophotometer (UV-6100S, MAPADA instruments, Shanghai, Inc. http://www.mapada.com.cn/) was used to characterize the absorption of AuNPs. The UV − vis spectrum of AuNP solution exhibited a characteristic absorption peak at 520 nm. The monodispersed and spherical AuNPs with an average size of 13 nm were confirmed by performing transmission electron microscopy (HT7700 TEM, Hitachi High Tech Co. Ltd., Tokyo, Japan. https://www.hitachi-hightech.com/global/). 3,3′,5,5′-tetramethylbenzidine (TMB)-H2O2 was purchased from Solarbio Co., Ltd. (Beijing, China, http://www.solarbio.bioon.com.cn/). Chloroauric acid (HAuCl4) and trisodium citrate were analytically pure and purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China, http://www.sinoreagent.com/). Corns and corn oil were purchased from the local supermarket. The organic solvents in the experiment were chromatographically pure. ELISA kit for ZEN was purchased from Quicking Biotech company (Shanghai, China, http://www.quicking.cn/). Spectra absorbance was obtained by using RT-6000 Microplate Reader (Rayto life science Co. Ltd., Shen Zhen, China, http://www.rayto.com).
Procedure for colorimetric assay
In a typical experiment, 50 μL of 1 μM aptamer solution, 50 μL of gold nanoparticles and 50 μL of zearalenone with different concentrations were added into the 96-well microplate and incubated for 5 min. Then 20 μL of 0.5 mol·L−1 H2O2 and 50 μL TMB were added and incubated for 15 min. Subsequently, the absorbance of reaction system at 630 nm was measured by a microplate reader. All reactions were performed at room temperature. Each reaction was repeated and detected three times. In addition, the possible distractions including AFB1, OTA and some common metal ions (Ca2+, Na+, Mg2+, Zn2+) were analyzed with the same strategy as described above.
Real sample detection
The ZEN contamination in corn and corn oil samples were analyzed by using the above colorimetric assay. Corns and corn oil samples were firstly prepared by spiking with different ZEN concentrations (50 ng·mL−1 and 100 ng·mL−1). The corn samples was mashed and dissolved in 60% methano-water solution with 1:5 m/v ratio and shaken for 3 min. The liquid was centrifuged at 10000 r/min for 10 min, and then the supernatant solution was diluted 100 times with phosphate buffer (2 mM, pH 7.0). For the analysis of corn oil samples, 2 g of spiked oil sample was mixed with 4 mL of n-hexane and 4 mL of 80% methanol-water solution. After being shaken for 1 min, the mixture was centrifuged at 4000 r/min for 5 min and the underlying solution was diluted 100 times with phosphate buffer. After that, 50 μL of each dilution was added into microplate and incubated with 50 μL of 1 μM aptamer solution and 50 μL of gold nanoparticles. 5 min later, 20 μL of 0.5 mol·L−1 H2O2 and 50 μL TMB were added into the plate. Then the absorbance of sample at 630 nm was measured after 15 min. In addition, each dilution was also detected by the commercially ELISA kit according to the specification. The results were compared and evaluated by the recovery and relative standard deviation (RSD).
Results and discussion
Optimization of the assay for ZEN
The colorimetric assay in this study is based on the underlying concept (Fig. 1). In the absence of ZEN, the ZEN aptamer can absorb on the surface of AuNPs by electrostatic interaction, thus inhibiting the peroxidase-like activity of AuNPs to oxidize TMB due to the shielding of AuNP surface [26]. However, in the presence of ZEN, the aptamer would bind with ZEN by the high specific affinity and can’t absorb on the AuNPs because of the structure change, thus promoting the restoration of peroxidase-like activity of AuNPs to oxidize the colorless TMB into blue oxTMB. The color change can be observed with bare eyes. In the general competitive process, an appropriate amount of aptamer and target is required to develop the sensitive detection method. In addition, the reaction environment and incubation period are also key parameters for the development of sensitive aptamer based assay. Several experimental parameters including: (a) sample pH value; (b) temperature; (c) H2O2 concentration; (d) incubation time; (e) aptamer concentration were optimized. Respective data and figures are given in the Electronic Supporting Material (ESM). The following experimental conditions were found to give best results: (a) Best sample pH value of 4; (b) Optimal temperature of 25 °C; (c) Optimal H2O2 concentration of 0.5 mol·L−1; (d) Optimal incubation time of 15 min; (e) Optimal aptamer concentration of 1 μM.
Quantitative detection of ZEN
Under the optimal detection conditions, the performance of the assay was evaluated by using the various concentration of ZEN. As can be seen in Fig. 2, the spectra absorbance of reaction gradually increased with the increase of the target concentration (ZEN). A good linearity between the absorbance of reaction system and the concentration of ZEN was observed at the concentration of 10–250 ng·mL−1 with a correlation coefficient of 0.9888 and a linear regression equation of y = 0.385 + 0.0003x. The detection limit (S/N = 3) of ZEN was found to be 10 ng·mL−1. The sensitivity of this assay for ZEN detection was compared with the recently reported aptasensors [14, 15, 30, 31] listed in Table 1. Although the sensitivity of this assay is lower in comparison with the aptamer-based fluorescence assay [15, 31], it still can meet the measuring requirement on account of the lowest tolerance limit (20–1000 μg·kg−1) of ZEN set by authorities. What’s more, the current method is rapid, low cost and visual without the need for expensive instruments, modification of aptamers and use of fluorescent dye. Therefore, the assay has great potential in the field test.
Specificity of the assay
The selectivity and specificity of the assay were also evaluated by determining and comparing ZEN and other non-targeted mycotoxins (AFB1 and OTA) as well as metal ions (Ca2+, Na+, Mg2+ and Zn2+) with the same procedure. As illustrated in Fig. 3, absorbance of the system with ZEN (50 ng·mL−1) obviously increased comparing with the blank (no analyte). While the absorbance of reaction systems with OTA and AFB1 were basically consistent with the blank control group even though their concentrations were 100 times higher than ZEN. What’s more, the absorbance of reaction system with a mixed solution including 50 ng·mL−1 of ZEN and 5 μg·mL−1 of interfere mycotoxins had no obvious change comparing with that of single ZEN solution. The similar absorbance change was also observed in the presence of various metal ions (0.01 M). These results demonstrated that this method was appropriate for the specific detection of ZEN.
Furthermore, in order to confirm the spectroscopic results were indeed caused by TMB oxidization rather than aggregation of AuNPs, the change of AuNP morphology and the absorbance spectra under different reaction systems were respectively checked by TEM and UV spectrophotometer. As can be seen in Fig. 4, comparing with the aggregation morphology of AuNPs under high salt, AuNPs in the assay always kept dispersion with the similar particle size no matter whether adding aptamer or target. The absorbance variation and color change of different solution systems are presented in Fig. 5. The absorption peak of pure AuNP solution, AuNP/aptamer compounds, as well as triple complex of AuNP, aptamer and ZEN all appeared in 520 nm. There is no obvious absorption appeared in around 630 nm (Fig. 5a). The color of above three solution system kept in purple red, though the spectra values decreased and the colors became shallow gradually after adding aptamer and ZEA in AuNP solution because of the dilution effect (Fig. 5b). The above results strongly demonstrated the reliability of the nanozyme-aptamer based colorimetric assay in this study. In addition, the obvious inhibition of aptamer on the peroxidase-like activity of AuNPs are also reflected in Fig. 5. Comparing with the pure AuNPs, the absorbance obviously decreased in 630 nm and color was fade in AuNP/aptamer compound after adding the H2O2 and TMB, while the absorbance reincreased and color was deepen after adding ZEN in AuNP/aptamer compound, followed by adding H2O2 and TMB. The observation is different from a report [32] that claims single stranded DNA or RNA enhance the activity of citrate-capped AuNPs. This maybe due to the nucleotide components of the oligonucleotide sequences and reaction parameters used in our work are different from the previous report, which can effect the multiplexing catalytic activity of AuNPs [32]. Nevertheless, these findings verify that the inhibition of aptamer on nanozyme activity of AuNPs can be employed to develop aptamer based assay for ZEN detection.
Actual sample analysis
The practicality of the assay was evaluated by detecting corns and corn oil samples spiked with different concentration of ZEN. The results of recoveries and relative standard deviation (RSD) are summarized in Table 2. The recovery ratio and RSD of ZEN concentration in spiked samples detected by the assay were 92–110% and 2.37–6.35%, respectively. These data had good agreement with that of ELISA analysis with the recovery and RSD being 92–115% and 1.03–6.72% respectively. The results demonstrated that the aptamer based colorimetric assay in this study is feasible for the rapid and specific detection of ZEN levels in the actual food samples.
Conclusion
In this work, a rapid and visual aptamer-based colorimetric assay for determining ZEN was developed on the basis of the inherent peroxidase-like activity of AuNPs. The high sensitivity of the assay mainly attribute to the enzyme mimic activity of AuNPs independent of the uncontrolled aggregation. The high specificity and feasibility for real-sample analysis of this colorimetric assay have displayed great potential in detection of ZEN. In addition, this method may also be applied to the determination of other food contaminants if appropriate aptamers are available. Overall, this work just provide the preliminary design of colorimetric assay by using the peroxidase-like activity properties. The high sensitivity of the assay is still the challenge. In future, the combination of AuNP and other nanomaterials or signal amplification strategies should be considered to improve the sensitivity and broaden the analytic range of the assay.
References
Liu J, Hu Y, Zhu G, Zhou X, Jia L, Zhang T (2014) Highly sensitive detection of zearalenone in feed samples using competitive surface-enhanced Raman scattering immunoassay. J Agric Food Chem 62:8325–8332. https://doi.org/10.1021/jf503191e
Chun HS, Choi EH, Chang HJ, Choi SW, Eremin SA (2009) A fluorescence polarization immunoassay for the detection of zearalenone in corn. Anal Chim Acta 639:83–89. https://doi.org/10.1016/j.aca.2009.02.048
Liu N, Nie D, Zhao Z, Meng X, Wu A (2015) Ultrasensitive immunoassays based on biotin–streptavidin amplified system for quantitative determination of family zearalenones. Food Control 57:202–209. https://doi.org/10.1016/j.foodcont.2015.03.049
Al-Taher F, Banaszewski K, Jackson L, Zweigenbaum J, Ryu D, Cappozzo J (2013) Rapid method for the determination of multiple mycotoxins in wines and beers by LC-MS/MS using a stable isotope dilution assay. J Agric Food Chem 61:2378–2384. https://doi.org/10.1021/jf304729f
Turner NW, Bramhmbhatt H, Szabo-Vezse M, Poma A, Coker R, Piletsky SA (2015) Analytical methods for determination of mycotoxins: an update (2009–2014). Anal Chim Acta 901:12–33. https://doi.org/10.1016/j.aca.2015.10.013
Li SJ, Sheng W, Wen W, Gu Y, Wang JP, Wang S (2018) Three kinds of lateral flow immunochromatographic assays based on the use of nanoparticle labels for fluorometric determination of zearalenone. Microchim Acta 185(4):238. https://doi.org/10.1007/s00604-018-2778-6
Liu N, Nie DX, Tan YL, Zhao ZY, Liao YC, Wang H et al (2017) An ultrasensitive amperometric immunosensor for zearalenones based on oriented antibody immobilization on a glassy carbon electrode modified with MWCNTs and AuPt nanoparticles. Microchim Acta 184:147–153. https://doi.org/10.1007/s00604-016-1996-z
Zhang XY, Eremin SA, Wen K, Yu XZ, Li CL, Ke YB et al (2017) Fluorescence polarization immunoassay based on a new monoclonal antibody for the detection of the zearalenone class of mycotoxins in maize. J Agric Food Chem 65:2240–2247. https://doi.org/10.1021/acs.jafc.6b05614
Nimjee SM, Rusconi CP, Sullenger BA (2005) Aptamers: an emerging class of therapeutics. Annu Rev Med 56:555–583. https://doi.org/10.1146/annurev.med.56.062904.144915
Liu LH, Zhou XH, Shi HC (2015) Portable optical aptasensor for rapid detection of mycotoxin with a reversible ligand-grafted biosensing surface. Biosens Bioelectron 72:300–305. https://doi.org/10.1016/j.bios.2015.05.033
Vasilescu A, Marty JL (2017) Aptasensors, an analytical solution for mycotoxins detection. Compr Anal Chem 77:101–146. https://doi.org/10.1016/bs.coac.2017.05.006
Ruchika CH, Singh J, Sachdev TS, Basu T, Malhotra BD (2016) Recent advances in mycotoxins detection. Biosens Bioelectron 81:532–545. https://doi.org/10.1016/j.bios.2016.03.004
Wu H, Liu R, Kang X, Liang C, Lv L, Guo Z (2018) Fluorometric aptamer assay for ochratoxin A based on the use of single walled carbon nanohorns and exonuclease III-aided amplification. Microchim Acta 185(1):27. https://doi.org/10.1007/s00604-017-2592-6
Taghdisi SM, Danesh NM, Ramezani M, Sarreshtehdar AE, Abnous K (2018) A novel colorimetric aptasensor for zearalenone detection based on nontarget-induced aptamer walker, gold nanoparticles and exonuclease-assisted recycling amplification. ACS Appl Mater Interfaces 10(15):12504–12509. https://doi.org/10.1021/acsami.8b02349
Goud KY, Hayat A, Satyanarayana M et al (2017) Aptamer-based zearalenone assay based on the use of a fluorescein label and a functional graphene oxide as a quencher. Microchim Acta 184(11):4401–4408. https://doi.org/10.1007/s00604-017-2487-6
Feng CJ, Dai S, Wang L (2014) Optical aptasensors for quantitative detection of small biomolecules: a review. Biosens Bioelectron 59:64–74. https://doi.org/10.1016/j.bios.2014.03.014
Lan LY, Yao Y, Ping JF, Ying YB (2017) Recent progress in nanomaterial-based optical aptamer assay for the detection of food chemical contaminants. ACS Appl Mater Interfaces 9:23287–23301. https://doi.org/10.1021/acsami.7b03937
Esfahani MR, Pallem VL, Stretz HA, Martha JM (2017) Extinction, emission, and scattering spectroscopy of 5–50 nm citrate-coated gold nanoparticles: an argument for curvature effects on aggregation. Spectrochim Acta A Mol Biomol Spectrosc 175:100–109. https://doi.org/10.1016/j.saa.2016.11.052
Comotti M, Della Pina C, Matarrese R, Rossi M (2004) The catalytic activity of naked gold particles. Angew Chem Int Ed 43:5812–5815. https://doi.org/10.1002/anie.200460446
Lin YH, Ren JS, Qu XG (2014) Catalytically active nanomaterials: a promising candidate for artificial enzymes. Acc Chem Res 47:1097–1105. https://doi.org/10.1021/ar400250z
Shah J, Purohit R, Singh R, Karakoti AS, Singh S (2015) ATP-enhanced peroxidase-like activity of gold nanoparticles. J Colloid Interface Sci 456:100–107. https://doi.org/10.1016/j.jcis.2015.06.015
Yun J, Li BX, Cao R (2010) Positively-charged gold nanoparticles as peroxidase mimic and their application in hydrogen peroxide and glucose detection. Chem Commun 46:8017–8019. https://doi.org/10.1039/c0cc02698k
Jiang X, Sun CJ, Guo Y (2015) Peroxidase-like activity of apoferritin paired gold clusters for glucose detection. Biosens Bioelectron 64:165–170. https://doi.org/10.1016/j.bios.2014.08.078
Wang L, Yang W, Li T et al (2017) Colorimetric determination of thrombin by exploiting a triple enzyme-mimetic activity and dual-aptamer strategy. Microchim Acta 184(9):3145–3151. https://doi.org/10.1007/s00604-017-2327-8
Hu L, Liao H, Feng L, Wang M, Fu W (2018) Accelerating the peroxidase-like activity of gold nanoclusters at neutral pH for colorimetric detection of heparin and heparinase activity. Anal Chem 90(10):6247–6252. https://doi.org/10.1021/acs.analchem.8b00885
Weerathunge P, Ramanathan R, Shukla R, Sharma TK, Bansal V (2014) Aptamer-controlled reversible inhibition of gold Nanozyme activity for pesticide sensing. Anal Chem 86:11937–11941. https://doi.org/10.1021/ac5028726
Yan J, Huang Y, Zhang C, Fang Z, Bai W, Yan M, Zhu C, Chen A (2017) Aptamer based photometric assay for the antibiotic sulfadimethoxine based on the inhibition and reactivation of the peroxidase-like activity of gold nanoparticles. Microchim Acta 184(1):59–63. https://doi.org/10.1007/s00604-016-1994-1
Le LC, Cruz-Aguado JA, Penner GA (2011) DNA ligand for aflatoxin and zearalenone. US, WO/2011/020198. http://www.freepatentsonline.com/WO2011020198.html
Li L, Li B (2009) Sensitive and selective detection of cysteine using gold nanoparticles as colorimetric probes. Analyst 134:1361–1365. https://doi.org/10.1039/b819842j
Wu S, Liu L, Duan N, Li Q, Zhou Y, Wang Z (2018) An aptamer-based lateral flow test strip for rapid detection of zearalenone in corn samples. J Agric Food Chem 66:1949–1954. https://doi.org/10.1021/acs.jafc.7b05326
Wu ZZ, Xu E, Muhammad FJC, Jin ZY, Irudayaraj J (2017) Highly sensitive fluorescence sensing of zearalenone using a novel aptasensor based on upconverting nanoparticles. Food Chem 230:673–680. https://doi.org/10.1016/j.foodchem.2017.03.100
Hizir MS, Top M, Balcioglu M, Rana M, Robertson NM, Shen F, Sheng J, Yigit MV (2016) Multiplexed activity of perAuxidase: DNA-capped AuNPs act as adjustable peroxidase. Anal Chem 88(1):600–605. https://doi.org/10.1021/acs.analchem.5b03926
Acknowledgments
This study was funded by Fundamental Research Funds for the Henan Provincial Colleges and Universities in Henan University of Technology (2016QNJH14) and Key Scientific and Technological Project of Henan Province (162102310084).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 1.83 kb)
Rights and permissions
About this article
Cite this article
Sun, S., Zhao, R., Feng, S. et al. Colorimetric zearalenone assay based on the use of an aptamer and of gold nanoparticles with peroxidase-like activity. Microchim Acta 185, 535 (2018). https://doi.org/10.1007/s00604-018-3078-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3078-x