Abstract
The authors describe a method for the preparation of orange-red emissive carbon dots (CDs) with excitation/emission peaks at 520/582 nm. The CDs were hydrothermally prepared by a one-pot strategy from trimesic acid and 4-aminoacetanilide. The fluorescence of the CDs is strongly quenched by hydrogen peroxide. The oxidation of glucose by glucose oxidase (GOx) produces H2O2 that quenches the fluorescence via static quenching. Based on this phenomenon, a fluorometric method was established for the determination of glucose. Under the optimum conditions, response is linear in the 0.5 to 100 μM glucose concentration range, with a 0.33 μM limit of detection. The method is selective for glucose over its analogues and was successfully applied to the determination of glucose in diluted human serum and in urine from diabetics and healthy individuals. Recoveries from spiked samples range from 98.7 to 102.5%.

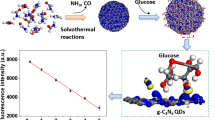
(a) One-step synthetic strategy of the CDs; (b) Schematic illustration of the CDs for glucose detection
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucose plays a key role in biological systems because it is acting not only as a source of energy of the living cells but also as a metabolic intermediate product in the formation of other complex molecules. Moreover, glucose level in blood and urine is also an indicator of human health conditions [1, 2]. Monitoring of blood and urine glucose levels is extremely essential, mostly because dysfunction of glucose uptake can lead to metabolic syndrome, hypertension, increased cardiovascular risk, and type-II diabetes [3]. Diabetes mellitus is one of the most common public health threats worldwide. According to the World Health Organization statistics, about 347 million people suffered from diabetes in the year 2013 and 3.8 million people are dying from it every year; this number is estimated to almost double in 2030 [4]. Even though blood glucose levels in diabetic patients and healthy individuals are both in the range of millimolar, the complex blood matrix are the source of grave interferences in glucose detection [5], resulting in the need for sample dilution processes preceding the analysis. Hence, for detecting low glucose concentrations, a highly sensitive method is needed.
Various methods for glucose determination have been reported such as Surface Enhanced Raman Scattering (SERS) [6], colorimetry [7], electrochemistry [8], mass spectrometry [9], chemiluminescence [10], and fluorescence [11]. Among them, fluorescence-based technologies have gained tremendous attention due to their good stability, excellent sensitivity, low cost, test rapidity and ease of handling [12]. Thus numerous fluorescent compounds have been used for accurate determination of glucose, including nanoclusters [13], organic dyes [14], and quantum dots (QDs) [15]. However, most of previous strategies usually need complicated modification, complex sample pretreatment, high experimental cost, unsatisfactory limits of detection and lack of suitability to clinical samples. Thus, it is of great importance to develop a modification-free, cost-effective, sensitive and selective fluorescence method for glucose detection to deal with complex biological samples.
Compared to molecular fluorescence, QDs, also called semiconductor nanocrystals have been intensively studied owing to their exceptional properties such as size dependent emission, broad absorption spectrum, narrow and symmetric emission peak, high quantum yield, high luminescence efficiency and resistance against photo-bleaching [16]. However, semiconductor QDs are associated with the inherent heavy metal toxicity, and noble metal materials involve high cost. In a comparison to QDs, CDs have shown distinct advantages owing to their excellent photostability, biocompatibility, low cytotoxicity, water dispersibility, highly tunable photoluminescence property, electrochemiluminescence and chemical inertness [17]. Therefore, CDs are continuously being explored as an alternative for dye-based probes and toxic QDs in many biological applications [18].
Herein, we have demonstrated an effective fluorescent platform for serum and urine glucose sensing with modification-free CDs as the signal output. The CDs were synthesized by one-step hydrothermal method using trimesic acid and 4-aminoacetanilide hydrochloride as nitrogen and carbon sources (Scheme 1a). The CDs have been successfully utilized as a fluorometric probe for sensitive and selective detection of glucose for the first time. The fluorescence method is mainly based on the enzymatic conversion of glucose by GOx to form H2O2 [11].
Compared to the known fluorescence-based H2O2-assisted glucose detection strategies [13, 19,20,21], our assay possesses some notable features: first, the assay results of this new type of nanoprobe can be read as soon as the probe-sample incubation was completed; second, glucose can be well distinguished from both its analogues and various potential interfering materials present in human serum and urine; most importantly, the CDs were directly functionalized by amino and carboxyl groups in the particle synthesis thus no any additional surface modification for introducing binding sites was needed which allows for detection of glucose without increasing the complexity and cost of the nanoprobes.
Experimental section
Reagents and materials
4-Aminoacetanilide hydrochloride was purchased from Alfa Aesar (China) Chemicals Co., Ltd. (https://www.alfa.com), H2O2 (30%) and CH3COOH were purchased from Tianjin Guangfu Chemical Reagent Factory (Tianjin, China) (https://guangfuhuashi.cn.china.cn/). Glucose was purchased from Sangon Biotech (Shanghai) Co., Ltd. (http://sangon.bioon.com.cn/). 2-[4-(2-Hydroxyethyl)-1-piperazine]ethanesulfonic acid (HEPES), 3-(N-Morpholino) propanesulfonic acid (MOPS) and glucose oxidase (GOx, 0.5 U/mg) were purchased from Sigma-Aldrich (https://www.sigmaaldrich.com/china-mainland.html) and stored in a refrigerator at −18 °C. Trimesic acid, arabinose, ribose, xylose, lactose, maltose, sucrose, mannose, fructose, galactose and other regents were purchased from Adamas Reagent Co., Ltd. (http://www.adamas-beta.com/). Tris(hydroxymethyl)aminomethane (Tris) was acquired from Sinopharm Chemical Reagent Co. Ltd. (http://www.sinoreagent.com/). In all experimental processes, the ultrapure water used was purified through an 18,202 V AXL water purification system (Chongqing, China). All chemicals used were of analytical reagent grade, obtained from commercial sources, and directly used without further purification.
Instrumentation
Transmission electron microscopy (TEM) observations were performed on a Hitachi-600 microscope (Hitachi, Japan). UV-visible (UV–Vis) absorption spectra of the samples were recorded on a TU-1901 double-beam UV − vis spectrophotometer with a 1 cm quartz cell (Purkine General Instrument Co., Ltd., Beijing, China). Fluorescence emission and excitation spectra were measured on a RF-5301PC spectrofluorometer equipped with a 1 cm quartz cell (Shimadzu, Kyoto, Japan). Fourier transform infrared (FT-IR) spectra were obtained on a Nicolet Nexus 670 FT-IR spectrometer with the KBr pellet technique. X-ray photoelectron spectroscopy (XPS) measurements were carried out using a PerkinElmer PHI-5702 multifunctional photoelectron spectrometer with an Al Kα exciting source.
Synthetic procedures for the CDs
The CDs were synthesized by one-pot hydrothermal route. In a typical experiment, 0.5 g of trimesic acid and 0.5 g of 4-aminoacetanilide hydrochloride were dissolved in 10 mL of ultrapure water, and stirred for several minutes. Then the mixture solution was transferred into 25 mL of Teflon lined stainless autoclave. The sealed autoclave was heated at 200 °C for 12 h. After the reaction, the autoclave was naturally cooled to room temperature. The final product solution was dialyzed in a dialysis bag (1000 Da, molecular weight cutoff) for about 24 h at room temperature. The CDs were stored at 4 °C for further use.
Fluorescence detection of glucose
In a typical experiment, 100 μL glucose solutions of varied concentration were incubated with GOx (0.5 U/mg) in Britton-Robinson (BR) buffer (pH 6.1) at 37 °C for 40 min to yield H2O2. 50 μL of the CDs was then added to the reaction mixture. After 5 min, the fluorescence spectrum was recorded using excitation at 520 nm.
Glucose detection in human urine and serum samples
The human urine samples of diabetic patients were obtained from the First Hospital of Lanzhou University and samples of healthy individuals were collected from healthy volunteers before and after 50 g Oral Glucose Tolerant Test (OGTT). Human serum sample was purchased from Beijing Solarbio Life Sciences Co., Ltd., (Beijing, China). Prior to analysis, the collected samples were stored at −18 °C and thawed at ambient temperature before further preparation. The preparation of samples was performed with the following two steps: Firstly, samples were centrifuged at 13000 rpm for 10 min. Secondly, the supernatants were diluted 10 times using BR buffer and finally, 100 μL of the diluted samples was used to detect glucose as described above.
Results and discussion
The design principle of the fluorescence method
A novel and efficient fluorescence method for selective glucose detection has been designed by using the synthetic fluorescent CDs as the fluorescence probe. The process of this approach is shown in Scheme 1b. Glucose was oxidized by oxygen (O2) in the presence of GOx to produce gluconic acid and H2O2. When the reaction mixture resulting from GOx catalyzed oxidation of glucose is added to the CDs solution, the generated H2O2 effectively quenches the fluorescence of the CDs due to static quenching effect and the quenched luminescence intensity of the CDs is proportional to the level of H2O2. Therefore, the concentration of glucose can be obtained by detecting the amount of the enzymatically generated H2O2. Thus, the CDs can be used as a fluorometric probe for detection of glucose.
Optimization of parameters in the synthesis of the CDs
The CDs were synthesized by one-step hydrothermal method using trimesic acid and 4-aminoacetanilide hydrochloride as both carbon source and surface functionalizing agents. As shown in Fig. S1, two other chemicals containing C, H, O and N were experimentally tested in pairs under hydrothermal conditions, and their products were observed under UV light illumination to judge whether or not they merited further investigation. The obtained solutions emitted blue and yellowish fluorescence. Trimesic acid and 4-aminoacetanilide hydrochloride were finally selected because their products yielded orange-red fluorescence. Subsequently, different amounts of trimesic acid and 4-aminoacetanilide hydrochloride were adjusted in parallel reactions (Fig. S2). The results demonstrate that there was no fluorescence when only trimesic acid was used to synthesis the CDs. Moreover, a weak fluorescence at 582 nm was observed when only 4-aminoacetanilide hydrochloride was used for carbon source and surface functionalization of the CDs. Interestingly, it was found that when the mass amount of the reactants is 0.5 g: 0.5 g, the CDs possess the optimum FL intensity. Similarly, the optimum temperature and reaction time were determined to be 200 °C and 12 h, respectively (Fig. S3 and S4).
Characterization of the CDs
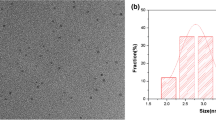
The morphology of the CDs was measured by TEM. As shown in Fig. 1a, the CDs are spherical morphology with homogeneous particle size range of 0.7–2.8 nm and an average diameter of around 1.6 nm (the inset in Fig. 1a). The FT-IR spectrum (shown in Fig. 1b) was measured to identify the surface functional groups present on the CDs, which displays numerous distinct absorption peaks in the range of 500–4000 cm−1. It is perceived that the CDs reveal a great number of carboxylic and amino groups [20, 22, 23]. The detailed description is provided in the supporting information. The remarkable optical properties of the CDs were confirmed by UV-vis absorption and fluorescence spectra. A series of emission spectra for the CDs were recorded at different excitation wavelength ranging from 420 to 570 nm at 10 nm intervals. The results shown in Fig. 1c indicate that the emission peaks of the CDs exhibit an excitation-wavelength-dependent property, a specific behavior to CDs [24]. This excitation-dependent fluorescence feature of the CDs can be ascribed to the size variation and the distinct emissive trap sites. The strongest fluorescence emission band is located at 582 nm with excitation at 520 nm. Figure 1d shows that the aqueous solution of the CDs exhibits two characteristic absorption bands centered at 231 and 281 nm in the UV-vis spectrum (green curve) which can be attributed to the π-π* and n-π* transitions of CDs [25], respectively. The black line and the red line present the optimal excitation (520 nm) and emission (582 nm) wavelength of the CDs. Moreover, the inset shows the photograph of the aqueous solution of the CDs, showing that the CDs are water-soluble and that, under 365 nm UV light, they exhibit a strong orange-red fluorescence originated from surface functional groups as well as the electronic conjugate structures [26]. The surface composition and elemental analysis for the CDs were determined by XPS. The full range of XPS analysis (Fig. S5) clearly shows four major peaks at 200.2, 285.3, 398.7, and 531.4 eV which are corresponded to Cl 2p (5.84%), C 1 s (73.96%), N 1 s (8.30%), and O 1 s (11.89%), respectively [27,28,29]. The detailed description is provided in the electronic supporting information (ESM). Furthermore, the influence of strong light exposure on the fluorescence of the CDs was investigated. As shown in Fig. S6, the fluorescence intensity of the CDs remained relatively stable after light illumination at 520 nm for 60 min, indicating the outstanding photostability of the CDs.
a TEM image of the CDs (Inset: size distribution histogram); b FTIR spectrum of the CDs; c Emission spectra of the CDs at various excitation wavelengths from 420 to 570 nm with an interval of 10 nm; and (D) UV − vis absorption (green line) and fluorescence spectra (black line for excitation and red line for emission) of the CDs (Inset: photograph of the CDs under UV light with excitation wavelength at 365 nm)
Enzymatic detection of glucose based on the CDs probe
As shown in Fig. 2a, the fluorescence intensity of the CDs gradually decreased with increasing H2O2 concentration from 0 to 1000 μM indicating that the correlation between fluorescence quenching and H2O2 concentration is dose-dependent. The calibration plot was also established by plotting the fluorescence quenching efficiency (I0-I)/I0 versus H2O2 concentration (Fig. 2b), where I0 and I are the fluorescence intensities in the absence and presence of H2O2, respectively. A good linearity concentration was raised from 10 to 300 μM, the regression equation is (I0-I)/I0 = 0.0016\( {c}_{{\mathrm{H}}_2{\mathrm{O}}_2} \)(μM) − 0.0152, with a correlation coefficient R2 of 0.995. These results suggest that the CDs are appropriate for highly sensitive quantification of H2O2 in aqueous solution. Since GOx can be used to catalyze the oxidation of glucose to release gluconic acid and H2O2 [11], therefore, the sensitive detection of H2O2 enables the CDs to detect glucose with aid of GOx.
Fluorescence emission spectra (λex = 520 nm) of the CDs solution upon the addition of various concentrations of H2O2 (from top to bottom, 0, 10, 20, 50, 70, 200, 300, 500, 700 and 1000 μM). b Plot of fluorescence quenching efficiency (I0 − I)/ I0 vs concentration of H2O2, where I0 and I are the fluorescence intensities in the absence and presence of H2O2, respectively. Error bars are the standard deviation of three independent experiments. Inset: plot of linear region from 10 to 300 μM
To improve the sensitivity of this method, the experiment condition was optimized, such as buffer, pH, temperature, incubation time as well as concentration of GOx. The performance of GOx and glucose was carefully assessed in five different buffers (20 mM, pH 7.4), including phosphate, Tris-HCl, HEPES, MOPS and BR buffer. As shown in Fig. S7, the highest response of fluorescence was achieved when the enzymatic reaction occurred in BR buffer. Similarly, the effect of pH on the CDs used for glucose detection was investigated because the catalytic activity of GOx is pH-dependent [15]. From Fig. S8 it can be concluded that a weak acid environment (around pH 6.1) is the optimum pH value for glucose detection. Moreover, the reaction temperature of GOx-mediated enzymatic reaction was investigated. Fig. S9 shows that the maximum fluorescence quenching efficiency was obtained at 37 °C. The incubation time of GOx-mediated enzymatic reaction was studied as well. Fig. S10 shows that the optimum fluorescence quenching efficiency was obtained at 40 min. Furthermore, the amount of GOx for GOx-mediated enzymatic reaction was investigated. Fig. S11 shows that the highest fluorescence quenching efficiency was reached using 500 μg/mL of GOx.
Using the above optimum conditions, the sensitivity of GOx-mediated glucose detection was assessed. The fluorescence quenching of the CDs was observed by increasing the glucose concentrations ranging from 0.5 to 1500 μM (Fig. 3a). As a result of H2O2 generated from the GOx-mediated oxidation of glucose, a gradual decrease of fluorescence intensity occurred with increasing the concentration of glucose, indicating that the fluorescence quenching was glucose dose-dependent. Figure 3b displays a plot of (I0-I)/I0 versus glucose concentration, where I0 and I are the fluorescence intensities in the absence and presence of glucose, respectively. The inset shows that the response is linear in the range 0.5–100 μM glucose. The linear equation is (I0-I)/I0 = 0.0029cglucose(μM) + 0.0062 with a correlation coefficient R2 of 0.998. The detection limit of glucose was calculated to be 0.33 μM (3 s/k, where s is the standard deviation of the blank samples and k is the slope of the calibration plot). In addition, we compared the analytical performance of our method to some previous reported approaches for glucose detection. Table 1 shows that the present method is more sensitive than numerous reported fluorescent approaches based on various nanomaterials including ZnO nanoparticles [30], nanoceria [31], MnO2-nanosheet [21], gold nanoparticles (AuNPs) and graphene oxide quantum dots (GOQD) [32], silicon nanoparticles [11], copper nanoparticles [19], and some other C-dots [24], except for the method based on MnO2-CD nanocomposite [20]. Nevertheless, the present method avoids the complex preparation of the probe in the MnO2-CD nanocomposite and most importantly, this method is one of few examples of the fluorescence-based H2O2-assisted glucose detection strategy to achieve the highest sensitive detection of glucose using modification free CDs.
Fluorescence emission spectra (λex = 520 nm) of the CDs solution upon the addition of various concentrations of glucose (from top to bottom, 0, 0.5, 6, 10, 40, 70, 100, 300, 500, 1000 and 1500 μM). b Plot of fluorescence quenching efficiency (I0 − I)/I0 vs concentration of glucose, where I0 and I are the fluorescence intensities in the absence and presence of glucose, respectively. Error bars are the standard deviation of three independent experiments. Inset: plot of linear region from 0.5 to 100 μM
Selectivity of the CDs for glucose detection
To evaluate the selectivity of the CDs probe toward glucose, the effects of several different sugars (arabinose, ribose, xylose, lactose, maltose, sucrose, mannose, fructose and galactose) and some potential interfering materials commonly existing in human urine (uric acid (UA), urea, ascorbic acid (AA), K+, Ca+, Mg+, Cl−, Na+ and Zn2+) were analyzed. As shown in Fig. 4a, the addition of these sugars to the CDs solution caused a negligible fluorescence quenching efficiency (I-I0)/I0 compared to the glucose results even at concentrations 100-times greater than that of glucose. The high selectivity is attributed to the specificity of GOx towards glucose. Similarly, the substances existing in urine showed no significant interference (Fig. 4b). These results indicate that this approach is extremely selective for differentiating glucose from its analogues and demonstrate high selectivity towards glucose sensing against other coexisting interferences.
Selectivity investigation of the present method for glucose detection: a Fluorescence quenching efficiency (I0 − I)/I0 response of the CDs to glucose (75 μM) and some carbohydrates (750 μM), where I0 and I are the fluorescence intensities in the absence and presence of glucose, respectively. b Some common substances as interferences (75 μM); (Note: the mixture stands for the anti-interference study in the coexistence of glucose). The error bars signify the standard error obtained from three repetitive measurements
Possible quenching mechanism
The quenching of fluorescent materials generally results from Förster resonance energy transfer (FRET), inner filter effect (IFE), dynamic quenching (DQE) or static quenching (SQE) [33]. To explore the sensing quenching mechanism between H2O2 and the CDs, corresponding experiments were assessed to study each of the above mentioned quenching effects.
When a spectral overlap occurred between the UV − vis absorption spectrum of a quencher and the emission spectrum of a fluorescence agent, the quenching mechanism is considered to be FRET or IFE. As shown in Fig. S12A, the emission spectrum of the CDs is not overlapped with the absorption spectrum of the quencher (H2O2), indicating that FRET or IFE probably did not occur in the fluorescence quenching mechanism. Furthermore, the changes of zeta-potential were performed to further discuss the mechanism. As shown in Fig. S12B, the zeta potential of the CDs was increased from −0.1025 mV to 12.4 mV on the addition of H2O2, indicating the tendency for the formation of aggregates. The formation of aggregated assembly was also confirmed by TEM images of the CDs before and after adding H2O2. Fig. S13 shows that the CDs were well dispersed in buffer and their average particle size was 1.6 nm. However, the addition of H2O2 into the CDs solution caused the aggregation of the CDs and their average particle size was increased to 8 nm (Fig. S14). Correspondingly, the color of the CDs solution changed from red to brown along with the dispersion state of the CDs turning into an aggregation state. (Fig. S15A and B). To further investigate the quenching mechanism of the CDs by H2O2, the interaction between the CDs and H2O2 was characterized by the UV/Vis spectrophotometry. The absorbance of the CDs showed a remarkable decrease upon addition of H2O2 (Fig. S12A), suggesting a static interaction between H2O2 and the CDs [34]. To confirm the quenching mechanism, quenching results were interpreted using the Stern-Volmer plot. Previous reports have demonstrated that, in a defined range of concentration, if the quenching type is single static or dynamic quenching then the plot of F0/F versus [Q] (Stern–Volmer plot) would be linear [35]. Whereas, an ascending curvature of the Stern–Volmer plot refers to combined (dynamic and static) quenching [36]. In this work, a good linear correlation was obtained with the Stern−Volmer plot in the range of 10–300 μM (Fig. S16A), indicating the occurrence of a single static or dynamic quenching. Considering the fact that there were no obvious changes of fluorescence lifetime for the CDs before and after adding different concentrations of H2O2 (Fig. S16B), the dynamic quenching effect can be excluded. Thus, it infers the presence of clear characteristics of a single static quenching process. These results suggest that the H2O2 induced fquenching of the CDs is considered to stem from static quenching caused by aggregation.
Application to real samples
To assess the feasibility of the CDs for glucose detection in biological samples, glucose concentrations in human serum and urine samples from diabetics and healthy individuals were analyzed. Unknown concentrations of glucose in serum were detected by the present method and compared to commonly used glucometer. As shown in Table 2, there is a good agreement between the results provided by two independent approaches which are in accordance with the fact that the general range of blood glucose concentration in healthy individual is about 3–8 mM [3]. To further verify the accuracy of our method, the recoveries were determined by spiking 2.5, 5.0 and 10.0 mM glucose in human serum samples (Table 2). The average glucose concentration recoveries of 98.7–102.5% were achieved with a relative standard deviation (RSD) of less than 5%, which is acceptable for quantitative analyses performed in biological samples.
Urine and blood glucose monitoring are two major techniques usually applied for evaluating diabetes control. Urine glucose monitoring cannot substitute blood glucose monitoring, but it can be used as an alternative or complement where blood glucose monitoring is not affordable, accessible, or desirable. To extend the applicability of the present fluorescence assay, glucose concentrations in urine samples from diabetics and healthy individuals before and after 2 h for taking 50 g oral glucose tolerant test were analyzed and the results were compared with the commonly used glucometer (Table 3). The effective glucose detection in both serum and urine samples has accentuated the potential for practical application of the CDs for the analysis of glucose in physiological real clinical samples.
Nonetheless, one major limitation of our method lies in the fact that the incubation time of GOx-mediated enzymatic reaction is a bit longer (40 min). Hence, future work should focus on improvements in accelerating the incubation time.
Conclusions
In conclusion, a novel CDs-based fluorescent probe was synthesized. The CDs display good water dispersibility, excellent photostability as well as strong orange-red fluorescence. Owing to the static quenching of the CDs caused by the H2O2, we developed a sensitive enzymatic assay for glucose determination. Moreover, this fluorometric probe showed a great potential for glucose detection in complex biological samples. Compared to the previous nanoprobe-based methods for glucose detection, this approach shows several advantages: Firstly, the approach does not involve complex modification of the CDs. Secondly; it has a lower detection limit than most of others. Thirdly, the orange-red emission of the CDs presages more opportunities for studying the biological systems in future applications. Furthermore, this method provides an alternative platform to detect other oxidase substrates which can catalyze the oxidation of their respective substrates to generate H2O2.
References
Wang GF, He XP, Wang LL, Gu AX, Huang Y, Fang B, Geng BY, Zhang XJ (2013) Non-enzymatic electrochemical sensing of glucose. Microchim Acta 180:161–186
Su L, Feng J, Zhou XM, Ren CL, Li HH, Chen XG (2012) Colorimetric detection of urine glucose based ZnFe2O4 magnetic nanoparticles. Anal Chem 84:5753–5758
Ferrannini E (1998) Insulin resistance versus insulin deficiency in non-insulin-dependent diabetes mellitus: problems and prospects. Endocr Rev 19:477–490
Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87:4–14
Zachariou M, Hearn MTW (2000) Adsorption and selectivity characteristics of several human serum proteins with immobilised hard Lewis metal ion–chelate adsorbents. J Chromatogr A 890:95–116
Bi XS, Du XZ, Jiang JJ, Huang X (2015) Facile and sensitive glucose sandwich assay using in situ-generated raman reporters. Anal Chem 87:2016–2021
Ortiz-Gómez I, Salinas-Castillo A, García AG, Álvarez-Bermejo JA, de Orbe-Payá I, Rodríguez-Diéguez A, Capitán-Vallvey LF (2017) Microfluidic paper-based device for colorimetric determination of glucose based on a metal-organic framework acting as peroxidase mimetic. Microchim Acta 185:47
Dhara K, Mahapatra DR (2017) Electrochemical nonenzymatic sensing of glucose using advanced nanomaterials. Microchim Acta 185:49
Tsao CW, Yang ZJ (2015) High sensitivity and high detection specificity of gold-nanoparticle-grafted nanostructured silicon mass spectrometry for glucose analysis. ACS Appl Mater Interfaces 7:22630–22637
Hallaj T, Amjadi M, Song ZL, Bagheri R (2017) Strong enhancement of the chemiluminescence of the Cu(II)-H2O2 system on addition of carbon nitride quantum dots, and its application to the detection of H2O2 and glucose. Microchim Acta 185:67
Ding LH, Gong ZJ, Yan M, Yu JH, Song XR (2017) Determination of glucose by using fluorescent silicon nanoparticles and an inner filter caused by peroxidase-induced oxidation of o-phenylenediamine by hydrogen peroxide. Microchim Acta 184:4531–4536
Steiner M-S, Duerkop A, Wolfbeis OS (2011) Optical methods for sensing glucose. Chem Soc Rev 40:4805–483925
Wang HB, Chen Y, Li N, Liu YM (2017) A fluorescent glucose bioassay based on the hydrogen peroxide-induced decomposition of a quencher system composed of MnO2 nanosheets and copper nanoclusters. Microchim Acta 184:515–523
Luo PG, Sahu S, Yang S-T, Sonkar SK, Wang J, Wang H, LeCroy GE, Cao L, Sun Y-P (2013) Carbon "quantum" dots for optical bioimaging. J Mater Chem B 1:2116–2127
Wang LL, Zheng J, Li YH, Yang S, Liu CH, Xiao Y, Li JS, Cao Z, Yang RH (2014) AgNP-DNA@GQDs hybrid: new approach for sensitive detection of H2O2 and glucose via simultaneous AgNP etching and DNA cleavage. Anal Chem 86:12348–12354
Li YL, Zhou J, Liu CL, Li HB (2012) Composite quantum dots detect cd(II) in living cells in a fluorescence "turning on" mode. J Mater Chem 22:2507–2511
Zuo PL, Lu XH, Sun ZG, Guo YH, He H (2016) A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim Acta 183:519–542
Zheng XT, Ananthanarayanan A, Luo KQ, Chen P (2015) Glowing graphene quantum dots and carbon dots: properties, syntheses, and biological applications. Small 11:1620–1636
Chen JY, Ji XH, He ZK (2017) Smart composite reagent composed of double-stranded DNA-templated copper nanoparticle and SYBR green I for hydrogen peroxide related biosensing. Anal Chem 89:3988–3995
Ma H, Liu XY, Wang XD, Li XR, Yang CD, Iqbal A, Liu WS, Li JP, Qin WW (2017) Sensitive fluorescent light-up probe for enzymatic determination of glucose using carbon dots modified with MnO2 nanosheets. Microchim Acta 184:177–185
Yuan J, Cen Y, Kong XJ, Wu S, Liu CLW, Yu RQ, Chu X (2015) MnO2-nanosheet-modified upconversion nanosystem for sensitive turn-on fluorescence detection of H2O2 and glucose in blood. ACS Appl Mater Interfaces 7:10548–10555
Hu CF, Liu YL, Yang YH, Cui JH, Huang ZR, Wang YL, Yang LF, Wang HB, Xiao Y, Rong JH (2013) One-step preparation of nitrogen-doped graphene quantum dots from oxidized debris of graphene oxide. J Mater Chem B 1:39–42
Wahab MA, Kim I, Ha CS (2004) Bridged amine-functionalized mesoporous organosilica materials from 1,2-bis(triethoxysilyl)ethane and bis[(3-trimethoxysilyl)propyl]amine. J Solid State Chem 177:3439–344739
Ma JL, Yin BC, Wu X, Ye BC (2017) Simple and cost-effective glucose detection based on carbon nanodots supported on silver nanoparticles. Anal Chem 89:1323–1328
Tang LB, Ji RB, Li XM, Teng KS, Lau SP (2013) Energy-level structure of nitrogen-doped graphene quantum dots. J Mater Chem C 1:4908–4915
Zhu S, Song Y, Zhao X, Shao J, Zhang J, Yang B (2015) The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano Res 8:355–381
Zhang HJ, Chen YL, Liang MJ, Xu LF, Qi SD, Chen HL, Chen XG (2014) Solid-phase synthesis of highly fluorescent nitrogen-doped carbon dots for sensitive and selective probing ferric ions in living cells. Anal Chem 86:9846–9852
Lu WB, Qin XY, Liu S, Chang GH, Zhang YW, Luo YL, Asiri AM, Al-Youbi AO, Sun XP (2012) Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury(II) ions. Anal Chem 84:5351–5357
Yang Z, Xu MH, Liu Y, He FJ, Gao F, Su YJ, Wei H, Zhang YF (2014) Nitrogen-doped, carbon-rich, highly photoluminescent carbon dots from ammonium citrate. Nanoscale 6:1890–1895
Sodzel D, Khranovskyy V, Beni V, Turner APF, Viter R, Eriksson MO, Holtz PO, Janot JM, Bechelany M, Balme S, Smyntyna V, Kolesneva E, Dubovskaya L, Volotovski I, Ubelis A, Yakimova R (2015) Continuous sensing of hydrogen peroxide and glucose via quenching of the UV and visible luminescence of ZnO nanoparticles. Microchim Acta 182:1819–1826
Liu BW, Sun ZY, Huang PJJ, Liu JW (2015) Hydrogen peroxide displacing DNA from nanoceria: mechanism and detection of glucose in serum. J Am Chem Soc 137:1290–1295
Na WD, Liu H, Wang MY, Su XG (2017) A boronic acid based glucose assay based on the suppression of the inner filter effect of gold nanoparticles on the orange fluorescence of graphene oxide quantum dots. Microchim Acta 184:1463–1470
Zu FL, Yan FY, Bai ZJ, Xu JX, Wang YY, Huang YC, Zhou XG (2017) The quenching of the fluorescence of carbon dots: a review on mechanisms and applications. Microchim Acta 184:1899–1914
Wu HM, Liang JG, Han HY (2008) A novel method for the determination of Pb2+ based on the quenching of the fluorescence of CdTe quantum dots. Microchim Acta 161:81–86
Gong A, Xiashi Z, Yanyan H, Suhai Y (2007) A fluorescence spectroscopic study of the interaction between epristeride and bovin serum albumine and its analytical application. Talanta 73:668–673
Yuming H, Zhang Z, Zhang D, Lv J (2001) Flow-injection analysis chemiluminescence detection combined with microdialysis sampling for studying protein binding of drug. Talanta 53:835–841
Acknowledgements
The authors are grateful for financial support from the National Natural Science Foundation of China (No. 21675068).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Research highlights
1. New orange-red emissive carbon dots (CDs) were prepared via one-pot strategy.
2. The fluorescence of the CDs was quenched by hydrogen peroxide (H2O2) via static quenching effect caused by aggregation.
3. The present fluorescence assay can detect glucose in the range of 0.5 μM to 100 μM, with a 0.33 μM limit of detection which makes it to be more sensitive than numerous reported fluorescent approaches based on various nanomaterials.
4. The fluorometric probe was successfully applied to detect glucose in diluted (spiked) human serum and urine samples from diabetic patients and healthy individuals.
5. The approach required no surface modification of the CDs which makes it to be more simple, rapid and cost-efficient than other reported methods that rely on complex preparation of nanoprobes.
Electronic supplementary material
ESM 1
(DOCX 4.81 mb)
Rights and permissions
About this article
Cite this article
Mutuyimana, F.P., Liu, J., Na, M. et al. Synthesis of orange-red emissive carbon dots for fluorometric enzymatic determination of glucose. Microchim Acta 185, 518 (2018). https://doi.org/10.1007/s00604-018-3041-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3041-x