Abstract
The authors describe a non-enzymatioc glucose assay that has three features: (a) The use of a boronic acid as the recognition element; (b) the aggregation of gold nanoparticles (AuNPs), and (c) the use of graphene oxide quantum dots (GOQD) whose fluorescence matches the absorption of the AuNPs and thereby results in an inner filter effect (IFE). The GOQD display an orange colored fluorescence that is filtered off by the AuNPs due to an IFE. In the presence of 3-aminobenzeneboronic acid, glucose is bound to form a cationic species that causes the aggregation of AuNPs to form large assemblies. This is accompanied by a color change from red to blue, and the IFE no longer does occur so that the orange fluorescence of the GOQD pops up again. These findings were used to design an assay witha linear response in the 2.5 to 75 μmol L−1 glucose concentration range, with a detection limit of 0.65 μmol L−1. The method was applied to the determination of glucose in spiked diluted serum and gave satisfactory results.

Schematic of the detection of glucose based on inner filter effect of dispersed AuNPs on the orange fluorescence of graphene oxide quantum dots.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucose is a key nutrient for biological metabolism and is also an essential compound for medical, biotechnological, nutritional, and environmental applications [1]. As a result, its measurement is of important significance in clinical diagnosis, health protection and control of diabetes or hypoglycemia [2, 3]. Despite the enormous progress achieved in the field of glucose assay during the last decade [4], most of the glucose assay employ electrochemical or photoelectrochemical [5, 6] type and these methods are generally based on the oxidation of glucose by the enzyme glucose oxidase or glucose dehydrogenase [7, 8]. This is due to their high sensitivity, reproducibility, and ease of handling as well as their low production and running cost [9]. However, the catalytic of activity enzyme can be easily affected by environmental conditions such as acidity, temperature and inhibitors [10]. Selective recognition is a prerequisite for selective detection of glucose. This can be accomplished by various means such as relying on unique peroxidase-like activity of some nanomaterials instead of enzymes or the capability of organic boronic acids to act as molecular receptors for glucose [11]. Fluorescence methods show great promise for the detection of trace amounts of analytes owing to their sensitivity, simplicity, fast response, and cost-effective instrumentation [12]. Thus, it is of great significance to develop a sensitive and selective non-enzymatic fluorescent method as alternative for the determination of glucose.
Graphene oxide (GO), as a direct derivative of graphene, consists of small graphene domains surrounded by carboxyl, epoxyl and hydroxyl groups. When the sheets are less than 100 nm lateral size and between 3 and 20 nm diameter size, they are referred to as graphene oxide quantum dots (GOQD) [13]. Quantum confinement and edge effect make them exhibit interesting properties such as fluorescent activity, robust chemical inertness, excellent photostability, high biocompatibility and low toxicity [14–16]. These features make GOQD especially useful for optical sensing and imaging and to be a potential candidate to replace traditional quantum dots. Tremendous attention has been paid to the covalent or noncovalent modification of GOQD with functional molecules or nanoparticles to facilitate target analyte sensing through energy or charge transfer from GOQD to the targets, which is often marked by fluorescence quenching of the GOQD [17]. However, the “turn off” sensing principle usually suffers from relatively low sensitivity and might be interfered with a variety of ligands or solvents, leading to false positives [18]. Moreover, the development and application of GOQD is still in its initial stage, and very little work has been done in the sensing system with “turn-on” model. To develop “turn on” fluorescence sensors, new approaches based on new GOQD architectures need to be established.
Gold nanoparticles (AuNP) have been broadly used in biochemical assays, owing to their straightforward synthetic/modification protocols and excellent properties such as high extinction coefficient for bare eye readout [19, 20] Moreover, AuNP can quench the fluorescence of organic dyes and QDs through the fluorescence resonance energy transfer (FRET) or the efficient inner filter effect (IFE). In particular, the IFE method does not require the intermolecular connection of fluorophores and absorbers at a particular distance, which provides a comparatively simple and facile approach for the analytical detection [21].
Considering the unique optical properties of GOQD and the high quenching efficiency of dispersed AuNP on the fluorescence of GOQD through the IFE, we develop a method for fluorescent detection of glucose. The detection strategy is shown in Scheme 1. An efficient IFE would occur between GOQD and AuNP, with GOQD acting as the donors and AuNP as the acceptors. So the fluorescence of GOQD can be significantly quenched by AuNP. The APBA functional groups react with the glucose molecule containing two pairs of cis-diols to form a new positively charged molecule named AGA. We find that AGA can induce the aggregation and color change of AuNP, whereas the formation of AGA prevents AuNP-triggered GOQD fluorescence quenching and restore the fluorescence. The specific interaction between AGA and AuNP can be exploited in the development of a simple and selective assay for glucose detection.
Experimental section
Materials
The water used in all experiments had a resistivity higher than 18 MΩ·cm. Graphite powder (100 mesh), H2SO4 (98%), H2O2 (30%) and KMnO4 were purchased from HuaCheng Biological Co., Ltd. (http://www.cchcsw.com). HAuCl4 was purchased from Acros Organics (http://www.acros.com). Glucose, 3-aminophenyl boronic acid (APBA) and some other chemicals used were purchased from Beijing Dingguo Changsheng Biotechnology Company (http://www.dingguo.com). Sodium dehydrogen phosphate (NaH2PO4) and disodium hydrogen phosphate (Na2HPO4) were purchased from Beijing Chemical Works (www.vvchem.com). All other chemicals were of analytical reagent grade. The 0.1 mol L−1 phosphate buffer (pH 6.2) was used as the medium for detection process.
Apparatus
The fluorescence spectra were obtained by using a Shimadzu RF-5301 PC spectrofluorophotometer (Japan, http://www.shimadzu.com) equipped with a xenon lamp using right-angle geometry. UV-vis absorption spectra were obtained by a Varian GBC Cintra 10e UV-vis spectrometer (Japan, http://www.shimadzu.com). In both experiments, a 1 cm path-length quartz cuvette was used. FT-IR spectra were recorded with a Bruker IFS66V FT-IR spectrometer equipped with a DGTS detector. All pH measurements were made with a PHS-3C pH meter (China, http://www.lei-ci.com).
The preparation of GO by the modified hummers method
Graphene oxide (GO) was prepared from graphite powder by the modified Hummers method. Typically, 1.0 g of graphite powder was added to 25 mL H2SO4 (98%) and stirred 30 min. Afterwards, 3.5 g KMnO4 was added and the mixture was stirred for 30 min in an ice bath, and then stirred at 35 °C for 1 h. Next, the mixture was diluted by 90 mL distilled water and the temperature was increased to 90 °C stirred for another 30 min. Subsequently, another 90 mL diluted water was added and then 10 mL of 30% H2O2 solution was added. The color of the mixture changed to bright yellow quickly. The resulting mixture was washed with distilled water several times and purified in a dialysis bag with a molecular weight cutoff of 10,000 Da against deionized water until the pH of the solution became neutral. After that, the solution was dried under vacuum to remove water. Finally, some black GO powder was obtained.
The preparation of GOQD by the chemical oxidation of GO
1.0 g GO was dissolved in 30 mL H2SO4 (98%) and stirred 15 min, Afterwards, 5.0 g KMnO4 was added and the mixture was stirred for 30 min in an ice bath, and then stirred at 40 °C for 1 h, Next, the mixture was diluted by slowly dropwise adding 120 mL distilled water, afterward, 10 mL of 30% H2O2 solution was added. The solution became transparent and color of the solution changed to bright yellow quickly. Finally, the solution was purified in a dialysis bag with a molecular weight cutoff of 1000 Da against deionized water to obtain a pure GOQD solution. Finally, the brightly orange GOQD solution was obtained. 300 μL GOQD was diluted to 1.6 mL with deionized water. The fluorescence spectra were recorded from 490 nm to 700 nm with the excitation wavelength of 470 nm. The slit widths of excitation and emission were both 10 nm.
Synthesis of AuNP
The AuNP were synthesized by the reduction of HAuCl4 with sodium citrate according to the previous reports [22]. In a typical experiment, 1 mL HAuCl4 solution (1%, V/V) and 99 mL deionized water were added into a 250 mL round-bottom three-necked flask. Then, the solution was heated to a boiling state under reflux condenser conditions with vigorous stirring. Subsequently, 5 mL trisodium citrate solution (1%) was quickly injected to the flask. When the color of the solution changed from pale yellow to deep red, the reaction was finished. The solution was cooled down to room temperature and then stored at 4 °C in the refrigerator for further use. The molar extinction coefficient (ε) around 520 nm for the AuNP is about 2.7*108 mol−1 cm−1 [23], thus the molar concentration of the AuNP was calculated to be nearly 4 nmol L−1 according to Lambert Beer’s law.
Glucose detection
A typical glucose detection process was performed as follow. 0.1 mol L−1 phosphate buffer (pH 6.2, 160 μL), 150 μmol L−1 APBA solution, different amounts of glucose and 1.50 nmol L−1 AuNP were added to a series of 2.0 mL calibrated test tubes followed by the thoroughly shaking and equilibrated for 40 min. Subsequently, 300 μL purified GOQD solution were placed in the above calibrated test tubes. Then, the mixtures were diluted to 1.6 mL with deionized water and mixed thoroughly. At last, the fluorescence spectra were recorded from 490 nm to 700 nm with the excitation wavelength of 470 nm. The slit widths of excitation and emission were both 10 nm.
Detection of glucose in serum samples
The fresh human blood samples were supplied by the local hospital and all the blood samples were obtained through venipuncture. Some necessary processes were conducted to remove large molecules and proteins to get the serum samples. The blood samples were segregated by adding acetonitrile (the volume of acetonitrile and blood was 1.5:1) and at 5364 g centrifugational force 5 min after stored for 2 h at room temperature. Then, all supernatant serum samples were subjected to a 200-fold dilution before analysis, and a certain concentration of glucose was added to prepare the spiked samples. These samples were detected with the fluorescence measurements under the optimal conditions.
Results and discussion
The inner filter effect between GOQD and non-aggregated gold nanoparticles
The GOQD was synthesized by a facile chemical oxidized method. The morphology and size of GOQD was recorded by TEM (Fig. S1). It reveals that GOQD are spherical with an average size of about 16 nm. The assay system consisted of AuNP and GOQD, where GOQD acted as fluorometric reporter, and AuNP served as fluorescence quencher. Compared to the fluorescence resonance energy transfer, IFE does not require chemical linkage between absorber and fluorophore. As shown in Fig. 1(a), the green emissive GOQD (black solid line) emitted strong fluorescence with the maximal emission wavelength at 544 nm. The elemental analysis of GOQD was performed and the data summed up in Table S1, we can see the oxygen ratio was 37.21 which indicated numerous oxygen functional group existed on GOQD. Figure 1(a) shows the absorption spectra of AuNP (red dash line) and the maximum absorption at 522 nm. The remarkable overlap between the fluorescence emission spectrum of GOQD and the absorption spectrum of the AuNP is an indispensable factor for IFE. The existence of surface functional groups of GOQD is studied using FT-IR spectra, which is shown in Fig. 1(b). Peaks at 3200–3550 cm−1 and 2800–2950 cm−1 are attributed to the C–OH and C–H stretching vibrations, respectively [24]. Peak at about 1600 cm−1 indicate the presence of C = O and the band at around 1080 cm−1 present the existence of C–O (hydroxyl, ester, epoxide or ether) groups [25]. Generally, the existence of these functional groups represent excellent water solubility of these nanomaterials without further surface modification. Furthermore, both GOQD and AuNP possess negative charges, there is no electrostatic attraction between the GOQD and AuNP. We conclude that quenching by AuNPs occurs via an IFE, not by FRET.
The mechanism for glucose assay
Boronic acid and various molecules containing the boronic acid have drawn an immense interest in the fabrication of monosaccharide sensors based on UV–vis absorption, fluorescence and surface plasmon resonance, due to the ability of forming five or six membered boronate esters through reversible covalent bonds with cis-1,2- or 1,3-diols [26, 27]. In this assay, APBA can react with glucose in the molar ratio 2:1 to produce a AGA, a five-ring boronate ester that carries two amino groups. AGA induces destabilization and aggregation of AuNP, phenomenon that can be ascribed to the electrostatic interaction between the positively charged AGA and the negatively charged AuNP. Consequently, as shown in Fig. S2, the optical properties of AuNP changed and the corresponding surface plasmon resonance induced absorption peak was shifted from 520 to 680 nm after the addition of AGA [28]. Moreover, AGA can trigger the color of AuNP solution change from wine-red to blue which depicted in Fig. S3. From the color of AuNP we can estimate the level of glucose via bare eye. More direct evidence for the AuNP aggregation comes from the TEM images (Fig. 2), we can see the AuNP is well dispersed while become aggregated after the addition of AGA molecule. Figure 2(c) shows the zeta potentials of AuNP, AuNP-AGA, GOQD, AuNP-GOQD and AuNP-GOQD-AGA system. The zata potential of AuNP-AGA is higher than that of AuNP, proves the AGA indeed absorb to the surface of AuNP via electrostatic interactions. The zeta potentials of AuNP (−21.58) and GOQD (−12.64) indicate that the former is more competitive to interact with AGA. The potential of the detection system become more positive than AuNP-GOQD and is near to GOQD after the addition of AGA, which indicates the AuNP aggregation formed while the GOQD still keep dispersed state.
Detection strategy for glucose
To study the detection mechanism of the GOQD based assay, the fluorescence emission spectra of GOQD, GOQD/Glucose, GOQD/APBA, GOQD/APBA/Glucose, GOQD/AuNP, GOQD/AuNP/APBA and GOQD/AuNP/APBA/Glucose system were studied and showed in Fig. 3. The fluorescence of the GOQD is obviously quenched with the addition of AuNP, which is due to the efficiently IFE between GOQD and AuNP. Subsequently, when introduction of APBA to the GOQD/AuNP system, it didn’t display a remarkable influence on the fluorescence of GOQD/AuNP system. However, a great enhancement in the fluorescence intensity can be observed by adding the glucose to the GOQD/AuNP/APBA system. Therefore, the observed fluorescence recovery of the GOQD/AuNP can be only due to the aggregation of AuNP, which is induced by AGA, the reactants of APBA and glucose. Based on the fluorescent signal changes of GOQD/AuNP/APBA/Glucose system, the detection of glucose is achieved. The fluorescence of GOQD can be partially quenched by the introduction of AGA or APBA, this phenomenon is caused by the weakly electrostatic interactions and hydrogen bond interaction between GOQD and AGA/APBA. It also can be seen that the fluorescence intensity of GOQD is not influenced by the addition glucose, which indicated that the interaction between glucose and GOQD is negligible.
Optimization of method
The following parameters were optimized: (a) concentration of gold nanoparticles; (b) concentration of APBA; (c); Sample pH value; (d) incubation time; Respective data and Figures are given in the Electronic Supporting Material. We found the following experimental conditions to give best results: (a) 1.5 nmol L−1 concentration of AuNP (Fig. S4, ESM); (b) 150 μmol L−1 concentration of APBA (Fig. S5, ESM); (c) A sample pH value of 6.2 (Fig. S6, ESM); (d) a sample reaction time of 40 min (Fig. S7, ESM).
Glucose detection
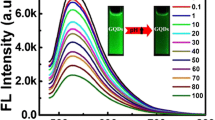
Under the optimized conditions, we further characterized the detection range of this sensing strategy. Figure 4(a) showed the fluorescence spectra of GOQD/AuNP/APBA system in the presence of different concentrations of glucose from 0 to 75 μmol L−1. It can be seen that the fluorescence intensity increased dramatically with the increase of glucose concentration from 2.5 to 75 μmol L−1. Figure 4(b) shows the calibration curve for the glucose detection and the linear range was found to be from 2.5 to 75 μmol L−1 with a linear equation of F/F0 = 1.01277 + 0.00997 [Glucose] (μmol L−1), where F and F0 is the fluorescence intensity of GOQD/AuNP/APBA system in the presence and absence of glucose, respectively. The corresponding regression coefficient is 0.995, and the limit of detection (LOD) for glucose is 0.65 μmol L−1. In addition, a comparison between the method and other reported methods for glucose detection in linear range and LOD were summed up in Table S2 [29–33]. Compared with other methods, our method shows a comparable or super quantification range and LOD for glucose detection. We also make a comparison between our method and other repoerted methods which used boronic acid as the recognition molecule (Table 1) [11, 16, 34–36]. It can be seen that other methods used various boronic acid as recognition molecule are almost all based on the electrochemistry assay mode. Moreover, our method based on fluorometry assay mode can offer a comparable or supper detection limit for glucose assay. So this method offer an alternative for the selective detection of glucose.
a The fluorescence spectrum of GOQD in the presence of 1.50 nmol L−1 AuNP and 150 μmol L−1 APBA upon the addition of glucose at different concentrations from 0 to 75 μmol L−1. The concentrations of glucose were 0, 2.5, 6.25, 18.75, 25, 37.5, 50, 62.5 and 75 μmol L−1, respectively. b The linear plot of F/F0 versus the concentration of glucose. F and F0 were the fluorescence intensity of GOQD/AuNP/APBA system in the presence and absence of glucose, respectively
Interference study
Selectivity is a very important parameter to evaluate the performance of a new detection method, especially for ones with potential applications in biomedical samples, a highly selective response to the target over other potentially competing species is necessary. Therefore, we further evaluated the selectivity of our assay system with various coexistence substances added. Table S3 shows the interference effect of some biological molecules and common inorganic ions on the determination of glucose, a relative error of 5.0% was considered to be tolerable. Tolerable concentration was defined as the concentrations of coexisting substances causing less than 5.0% relative error. As shown in Table S3, the tolerable concentration of lysozyme (lys), human serum albumin (HSA) is 1 μmol L−1 and GSH is 100 μmol L−1. The tolerable concentration of Na+ and K+ is 0.25 mmol L−1 while the concentration of Zn2+, Mg2+ and Ca2+ is 0.1 mmol L−1. The concentration of Fe2+ is 0.05 mmol L−1 and ATP is 103 μU mL−1. When the concentration of fructose and saccharose was 50 μmol L−1, the assay system does not obviously response to them. The results show that there is little interference from commonly existing substances. As the concentration of glucose in human serum is in mM levels [37], which is much higher than the coexistence substances. Thus, the present method is suitable for selective detection of glucose.
In order to demonstrate the feasibility of the present method, it was applied to the determination of glucose in human serum samples. Serum is what remains from whole blood after coagulation, the chemical composition is similar to plasma but does not contain coagulation protein. Since the glucose in human serum samples is at several mM level, we diluted the samples 200-fold. In this way, the concentrations of glucose in diluted samples are well within the linear detection range of our assay. All data were collected from three independent measurements. From Table 2, it can be seen that the recoveries based on our method in the real samples are between 95.6 and 99.5%. The relative standard deviations (RSD) are not higher than 3.8%, indicating that the accuracy and precision of the method are satisfactory.
Conclusion
In summary, a fluorescent glucose assay system have been constructed based on the efficiently IFE of AuNP on GOQD for the first time. AuNP serve as a dual function of fluorescence quencher and colorimetric reporter in the assay system. The presence of glucose can be observed via the color change of AuNP, and quantitated by the fluorescence of GOQD. Furthermore, the fluorescent assay system performs well in glucose sensing with a wide linear relationship and a low detection limit. The application of this glucose assay in real serum sample analysis shows satisfactory results.
References
Yang J, Cho M, Lee Y (2016) Synthesis of hierarchical Ni(OH)(2) hollow nanorod via chemical bath deposition and its glucose sensing performance. Sensors Actuators B Chem 222:674–681
Yu Z, Li H, Zhang X, Liu N, Tan W, Zhang X, Zhang L (2016) Facile synthesis of NiCo2O4@polyaniline core-shell nanocomposite for sensitive determination of glucose. Biosens Bioelectron 75:161–165
Chen J, Ge J, Zhang L, Li Z, Qu L (2016) Poly(styrene sulfonate) and Pt bifunctionalized graphene nanosheets as an artificial enzyme to construct a colorimetric chemosensor for highly sensitive glucose detection. Sensors Actuators B Chem 233:438–444
Steiner M-S, Duerkop A, Wolfbeis OS (2011) Optical methods for sensing glucose. Chem Soc Rev 40(9):4805–4839
Fan Y, Tan X, Liu X, Ou X, Chen S, Wei S (2015) A novel non-enzymatic electrochemiluminescence sensor for the detection of glucose based on the competitive reaction between glucose and phenoxy dextran for concanavalin a binding sites. Electrochim Acta 180:471–478
Gao ZF, Chen DM, Lei JL, Luo HQ, Li NB (2015) A regenerated electrochemical biosensor for label-free detection of glucose and urea based on conformational switch of i-motif oligonucleotide probe. Anal Chim Acta 897:10–16
Fang X, Wu X-M, Hu X-L, Li Z-J, Wang G-L (2016) Native carbon nanodots as a fluorescent probe for assays based on the use of glucose oxidase or horseradish peroxidase. Microchim Acta 183(10):2761–2770
Durán GM, Benavidez TE, Ríos Á, García CD (2016) Quantum dot-modified paper-based assay for glucose screening. Microchim Acta 183(2):611–616
Sodzel D, Khranovskyy V, Beni V, Turner APF, Viter R, Eriksson MO, Holtz P-O, Janot J-M, Bechelany M, Balme S, Smyntyna V, Kolesneva E, Dubovskaya L, Volotovski I, Ubelis A, Yakimova R (2015) Continuous sensing of hydrogen peroxide and glucose via quenching of the UV and visible luminescence of ZnO nanoparticles. Microchim Acta 182(9):1819–1826
Ding C, Yan Y, Xiang D, Zhang C, Xian Y (2016) Magnetic Fe3S4 nanoparticles with peroxidase-like activity, and their use in a photometric enzymatic glucose assay. Microchim Acta 183(2):625–631
Çiftçi H, Alver E, Çelik F, Metin AÜ, Tamer U (2016) Non-enzymatic sensing of glucose using a glassy carbon electrode modified with gold nanoparticles coated with polyethyleneimine and 3-aminophenylboronic acid. Microchim Acta 183(4):1479–1486
Tan L, Chen K, Huang C, Peng R, Luo X, Yang R, Cheng Y, Tang Y (2015) A fluorescent turn-on detection scheme for α-fetoprotein using quantum dots placed in a boronate-modified molecularly imprinted polymer with high affinity for glycoproteins. Microchim Acta 182(15):2615–2622
Adolfsson KH, Hassanzadeh S, Hakkarainen M (2015) Valorization of cellulose and waste paper to graphene oxide quantum dots. RSC Adv 5(34):26550–26558
He Y, Sun J, Feng D, Chen H, Gao F, Wang L (2015) Graphene quantum dots: highly active bifunctional nanoprobes for nonenzymatic photoluminescence detection of hydroquinone. Biosens Bioelectron 74:418–422
Du X, Jiang D, Liu Q, Zhu G, Mao H, Wang K (2015) Fabrication of graphene oxide decorated with nitrogen-doped graphene quantum dots and its enhanced electrochemiluminescence for ultrasensitive detection of pentachlorophenol. Analyst 140(4):1253–1259.S
Benitez-Martinez S, Valcarcel M (2015) Fluorescent determination of graphene quantum dots in water samples. Anal Chim Acta 896:78–84
Zhang L, Peng D, Liang R-P, Qiu J-D (2015) Graphene quantum dots assembled with Metalloporphyrins for "turn on" sensing of hydrogen peroxide and glucose. Chem Eur J 21(26):9343–9348
Pei H, Zhu S, Yang M, Kong R, Zheng Y, Qu F (2015) Graphene oxide quantum dots@silver core-shell nanocrystals as turn-on fluorescent nanoprobe for ultrasensitive detection of prostate specific antigen. Biosens Bioelectron 74:909–914
Abnous K, Danesh NM, Ramezani M, Emrani AS, Taghdisi SM (2016) A novel colorimetric sandwich aptasensor based on an indirect competitive enzyme-free method for ultrasensitive detection of chloramphenicol. Biosens Bioelectron 78:80–86
Xianyu Y, Xie Y, Wang N, Wang Z, Jiang X (2015) A dispersion-dominated chromogenic strategy for colorimetric sensing of glutathione at the Nanomolar level using gold nanoparticles. Small 11(41):5510–5514
Xia N, Zhou B, Huang N, Jiang M, Zhang J, Liu L (2016) Visual and fluorescent assays for selective detection of beta-amyloid oligomers based on the inner filter effect of gold nanoparticles on the fluorescence of CdTe quantum dots. Biosens Bioelectron 85:625–632
Liu Z, Liu H, Wang L, Su X (2016) A label-free fluorescence biosensor for highly sensitive detection of lectin based on carboxymethyl chitosan-quantum dots and gold nanoparticles. Anal Chim Acta 932:88–97
Zhao W, Brook MA, Li Y (2008) Design of Gold Nanoparticle-Based Colorimetric Biosensing Assays. Chembiochem 9(15):2363–2371
Lou J, Liu S, Tu W, Dai Z (2015) Graphene Quantums dots combined with endonuclease cleavage and bidentate chelation for highly sensitive Electrochemiluminescent DNA Biosensing. Anal Chem 87(2):1145–1151
Song L, Shi J, Lu J, Lu C (2015) Structure observation of graphene quantum dots by single-layered formation in layered confinement space. Chem Sci 6(8):4846–4850
Vasu KS, Sridevi S, Sampath S, Sood AK (2015) Non-enzymatic electronic detection of glucose using aminophenylboronic acid functionalized reduced graphene oxide. Sensors Actuators B Chem 221:1209–1214
Qian S, Liang Y, Ma J, Zhang Y, Zhao J, Peng W (2015) Boronic acid modified fiber optic SPR sensor and its application in saccharide detection. Sensors Actuators B Chem 220:1217–1223
Cayuela A, Soriano ML, Valcarcel M (2015) Reusable sensor based on functionalized carbon dots for the detection of silver nanoparticles in cosmetics via inner filter effect. Anal Chim Acta 872:70–76
Wu Q, Wang X, Liao C, Wei Q, Wang Q (2015) Microgel coating of magnetic nanoparticles via bienzyme-mediated free-radical polymerization for colorimetric detection of glucose. Nanoscale 7(40):16578–16582
Xiong Y, Zhang Y, Rong P, Yang J, Wang W, Liu D (2015) A high-throughput colorimetric assay for glucose detection based on glucose oxidase-catalyzed enlargement of gold nanoparticles. Nanoscale 7(38):15584–15588
Wang Y, Zhang S, Bai W, Zheng J (2016) Layer-by-layer assembly of copper nanoparticles and manganese dioxide-multiwalled carbon nanotubes film: a new nonenzymatic electrochemical sensor for glucose. Talanta 149:211–216
Fang L, Liu B, Liu L, Li Y, Huang K, Zhang Q (2016) Direct electrochemistry of glucose oxidase immobilized on Au nanoparticles-functionalized 3D hierarchically ZnO nanostructures and its application to bioelectrochemical glucose sensor. Sensors Actuators B Chem 222:1096–1102
Qu F, Guo X, Liu D, Chen G, You J (2016) Dual-emission carbon nanodots as a ratiometric nanosensor for the detection of glucose and glucose oxidase. Sensors Actuators B Chem 233:320–327
Lerner MB, Kybert N, Mendoza R, Villechenon R, Bonilla Lopez MA, Charlie Johnson AT (2013) Scalable, non-invasive glucose sensor based on boronic acid functionalized carbon nanotube transistors. Appl Phys Lett 102(18):183113
Zhao YQ, Luo HQ, Li NB (2009) Electrochemical characterization of in situ functionalized gold p-aminothiophenol self-assembled monolayer with 4-formylphenylboronic acid for recognition of sugars. Sensors Actuators B Chem 137(2):722–726
Badhulika S, Tlili C, Mulchandani A (2014) Poly(3-aminophenylboronic acid)-functionalized carbon nanotubes-based chemiresistive sensors for detection of sugars. Analyst 139(12):3077–3082
Tang X, Zhang B, Xiao C, Zhou H, Wang X, He D (2016) Carbon nanotube template synthesis of hierarchical NiCoO2 composite for non-enzyme glucose detection. Sensors Actuators B Chem 222:232–239
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 21075050 and 21275063), the Science and Technology Development project of Jilin province, China (No. 20150204010GX).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 6411 kb)
Rights and permissions
About this article
Cite this article
Na, W., Liu, H., Wang, M. et al. A boronic acid based glucose assay based on the suppression of the inner filter effect of gold nanoparticles on the orange fluorescence of graphene oxide quantum dots. Microchim Acta 184, 1463–1470 (2017). https://doi.org/10.1007/s00604-017-2090-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2090-x