Abstract
An immunosensor is described for the voltammetric determination of α-fetoprotein. It is making use of an AuNP-dendrimer conjugate and an ionic liquid. A gold electrode was first modified with chitosan. Then, the AuNP-dendrimer conjugate was covalently immobilized on the electrode. Following this, an ionic liquid was placed on the electrode via formation of a covalent bond between the amino groups of PAMAM and the aldehyde groups of an ionic liquid containing ferrocene. Thus, the redox probe ferrocene becomes immobilized on the electrode surface. PAMAM increases the amount of ferrocene immobilized on the electrode due to its globular shape and rich amino groups. The use of AuNPs improves the conductivity of the electrode. The modified electrode was applied to the determination of α-fetoprotein in human serum and has a linear response that covers the 0.05 to 30 ng mL−1 α-fetoprotein concentration range, with a detection limit of 0.02 ng mL−1. This assay is stable, selective and reproducible. It is perceived to provide a powerful tool for the early detection of cancer markers.

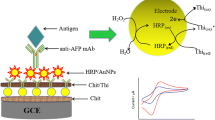
Schematic of a voltammetric immunoassay for α-fetoprotein based on a gold nanoparticle/dendrimer conjugate and ionic liquids anchored with both aldehyde and ferrocene. Chit: chitosan; GA: glutaraldehyde; PAMAM: G4 polyamidoaminic dendrimers; AuNP: Au nanoparticle; Fc: ferrocene; IL: ionic liquid. PB: phosphate buffer solution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Demand for high sensitivity of immunosensors has resulted in extensive research in improving the antibody immobilization. Dendrimers have been widely applied in the fabrication of biosensing due to their unique properties, such as a highly-branched dendritic structure and a high density of active groups [1, 2]. The G4 polyamidoaminic dendrimers (PAMAM) possesses 64 primary amine groups on the surface and has a globular shape with a diameter about 4.0 nm [3]. They can be closely packed on the substrate surface, and have multiple branch ends available for further conjugation [4,5,6]. Dendrimers have been recently reported to significantly increase the binding ability of biomolecules [7, 8]. However, the conductivity of dendrimer is not good, and that limits extensive application in electrochemical biosensors. Thus, the conductivity of electrode surface using the dendrimer must be increased. Metal-dendrimer nanocomposites can greatly improve the conductivity of the dendrimer. Various nanoparticles, including Fe3O4, ZnO, Pd and Au, have successfully formed at the surface of the dendrimer or been encapsulated in the dendrimer [9,10,11,12].
Ionic liquids (IL) have unique properties compared to conventional solvents, including low volatility, ionic conductivity, and low toxicity, which make them considered to be green media [13]. In order to obtain IL-containing conductive composite materials, IL was also incorporated into conventional matrixes such as biopolymers, cellulose, carbon nanomaterial, metal nanomaterials, gel-sol-based matrixes [14,15,16,17]. However, IL incorporated into conventional matrixes was easy to leach out from electrode surface to electrolyte solution. In order to solve this problem, we introduced IL on the electrode surface by covalent binding.
Ferrocene (Fc) is an organometallic compound consisting of two cyclopentadienyl rings, in which ferrous ion is sandwiched. It and its derivatives were also employed as redox species modified on the surface of electrode for the fabrication of immunosensors [18,19,20] due to their good electrochemical reversibility, low bio-toxicity and commercial availability. However, Fc and its derivatives can easily diffuse from the electrode surface into the bulk solution due to weak physical interaction and their low molecular weight. This event decreased the stability and performance of biosensor. In order to prevent the leakage, it is a good strategy that Fc or its derivatives were covalently attached to chitosan [21], dendrimer [22] and nanoparticles [23] to obtain redox-active hybrids.
α-Fetoprotein (AFP), a common and important cancer biomarker, is a major plasma protein produced by the yolk sac and the liver. The AFP expression is often associated with hepatoma. Thus, it was used as a diagnostic biomarker for hepatocellular carcinoma. Early detection of AFP is of great importance in clinical diagnosis. Although many immunosensors for AFP were reported [24,25,26,27,28], the detection signals of those immunosensor resulting from Fe(CN)63−/Fe(CN)64−. It is necessary to develop a new electrochemical immunsensor for AFP determination in electrolyte solutions without Fe(CN)63−/Fe(CN)64−.
In our previous work, a sensitive biosensing platform based on a new ionic liquid anchored with both Fc groups and aldehyde groups (Fc-IL-CHO) was fabricated. In order to enhance the signal resulting from Fc, AuNP-PAMAM conjugates were used as a “bridge” reagent to introduce more ionic liquids containing Fc groups on the electrode surface. The Au electrode was first modified with chitosan (Chit) which has abundant reactive amino groups. AuNP-PAMAM was attached to Chit using glutaraldehyde as a linking reagent. Then, functionalized ionic liquid was modified on the electrode surface by covalent bonding between aldehyde groups of functionalized ionic liquid and amino groups of PAMAM. Thus, redox species Fc was immobilized on the electrode surface, which ensure electrochemical measurement was carried out in phosphate buffered solution without Fe(CN)63−/Fe(CN)64−. In the same time, IL was immobilized on the electrode through covalent binding, which prevented it leached out from electrode surface to electrolyte solution. PAMAM can increase the amount of Fc immobilized on the electrode due to its globular shape and rich amino groups. AuNP adsorbed on Fc-IL-CHO/AuNP-PAMAM/GA/Chit-modified electrode was used to not only immobilize primary antibodies as the platform to construct the immunosensor, but also improve the conductivity of electrode surface. The novel strategy would provide a useful technology for the quantitative detection of AFP in human serum due to its high current response, a relatively wide linear range and a low detection limit.
Experimental
Reagents and apparatus
We purchased anti-AFP antibody (Ab), antigen-AFP (AFP), bovine serum albumin (BSA) from Beijing Dingguo Biotechnology Company (Beijing, China, http://www.dingguo.com). HAuCl4, sodium citrate and PAMAM G4 were purchased from Sigma-Aldrich (http://www.sigmaaldrich.com/china-mainland/promotions/new.html). Functionalized ionic liquid (Fc-IL-CHO) was synthesized as previously reported [29]. The structure of Fc-IL-CHO was shown in Electronic Supporting Material (ESM). We prepared AuNP-PAMAM conjugates according to previously reported method [30] (See ESM).
Cyclic voltammetric (CV), differential pulse voltammetry (DPV), electrochemical impedance spectra (EIS) were performed on a CHI 660E electrochemistry workstation (Shanghai CH Instruments, China). We prepared 0.1 M phosphate buffer solution (PB, pH 7.0) using Na2HPO4 and KH2PO4. Chitosan (Chit) solution (1%) was obtained by fully dissolving chitosan in acetic acid solution by sonication.
Preparation of immunosensor
Prior to fabricating immunosensor, the gold electrode (Au, 3 mm in diameter) was polished with 0.3 and 0.05 μm alumina slurries followed by washing with doubly distilled water three times. Then, a 10 μL of Chit solution was dropped on the cleaned Au electrode and then dried in the air. Subsequently, a 10 μL of glutaraldehyde (GA, 2.5%) solution was dropped on the Chit film and incubated for 1 h at room temperature, washed with double distilled water. After that, a 10 μL of AuNP-PAMAM conjugates was added on the electrode and incubated for 1 h, washed with double distilled water. Flowing that, a 10 μL of Fc-IL-CHO solution (2 mg mL−1) was added on the electrode and incubated for 1 h, washed with double distilled water. Then, a 10 μL of AuNPs soluiton was added on the electrode and dried in the air. A 10 μL of anti-AFP antibody solution (50 μg mL−1) was added on the electrode and incubated for 1 h at 37 °C, washed with PB solution. To eliminate nonspecific binding, a 10 μL BSA (2.0 wt%) solution was dropped on the electrode modified with anti-AFP antibody and incubated for 1 h at 37 °C. Finally, a 10 μL of AFP solution with different concentration was dropped on the surface of electrode and incubated for 40 min, followed by washing with double distilled water and then measuring the electrochemical signals. The whole process of the immunosensor fabrication is shown in Fig. 1.
Immunoassay protocol
Three-electrode system was used. The system includes a Pt electrode (counter electrode), a saturated calomel electrode (reference electrode), and a gold electrode (Au) (working electrode). After the electrode modified with anti-AFP antibody was blocked with BSA, a 10 μL of AFP solution with different concentration was dropped on the surface of the electrode, incubated for 40 min at 37 °C. Washed thoroughly with PB solution to remove the unbound antigens, the prepared immunosensor modified with AFP was monitored by the differential pulse voltammetric (DPV) in 0.1 M PB solution. The peak current of DPV will decrease with the increase of the AFP concentration because the redox probe Fc was blocked by AFP layer. Thus, the quantitative detection of AFP can be achievable. DPV measurements were carried out in PB solution (pH 7.0) under the following: the potential range was from 0.2 to 0.8 V, pulse amplitude was 0.05 V, pulse width was 0.05 s, and sample width was 0.02 s.
Results discussion
The characterization of AuNP-PAMAM conjugates
Transmission electron microscope (TEM) was used to characterize AuNP and AuNP-PAMAM conjugates (Fig. S2, ESM). UV-vis spectroscopy was also used to characterize the formation of AuNP-PAMAM nanocomposites (Fig. S2, ESM). Those results display the formation of AuNP-PAMAM conjugates.
The electrochemical behavior of electrodes covered with different coatings
The electrochemical behavior of electrodes covered with different coatings was investigated by cyclic voltammetry at Au electrode surface in PB solution. Figure 2 shows that no redox peak at AuNPs-PAMAM/GA/Chit-modified electrode is observed (Fig. 2, a). When the electrode was modified with Fc-IL-CHO/Chit film, the redox peak appeared due to the introduction of Fc on the electrode surface (Fig. 2, b). When the electrode was modified with Fc-IL-CHO/PAMAM/GA/Chit film, the peak current increased (Fig. 2, c), indicating more Fc-IL-CHO was immobilized on the electrode surface due to the rich amine groups of PAMAM. When the electrode was modified with Fc-IL-CHO/AuNP-PAMAM/GA/Chit film, the peak current further increased (Fig. 2, d), verifying that AuNP improved the conductivity of the electrode. The enhanced signal was due to the following reasons: (1) PAMAM possesses rich amine groups available for further conjugation of more Fc. (2) AuNP was introduced for improving the conductivity of the electrode. (3) IL is a high electric conductivity material and it can enhance the conductivity of the electrode.
CV profiles of electrodes modified by different coatings in PB solution (pH 7.0): (a) AuNP-PAMAM/GA/Chit modified electrode; (b) Fc-IL-CHO/Chit modified electrode; (c) Fc-IL-CHO/PAMAM/GA/Chit; (d) Fc-IL-CHO/AuNP-PAMAM/GA/Chit modified electrode. Potential range is −0.2 to +0.6 V (vs SCE reference). Scan rate is 100 mV s −1
The characterization of the modified electrode
Electrochemical impedance spectroscopy was used to characterize the stepwise modification process of electrode. The Faradaic impedance was recorded in 5 mM solution of [Fe(CN) 6] 3−/4−. The inset of Fig. 3 expresses the Randles model of equivalent circuit. Ret represent electron transfer resistance of the sensing interface. The semicircle diameter in the impedance spectrum is the value of Ret. As can be seen from Fig. 3, curve a shows a small semicircle domain, indicating the electron-transfer resistance at bare electrode surface was small. When the bare electrode was modified with GA/Chit, the Ret increased (curve b). After the GA/Chit-covered electrode was modified with AuNP-PAMAM, the Ret increased again (curve c). After AuNP-PAMAM /GA/Chit-covered electrode was modified with Fc-IL-CHO, the Ret further increased (curve d). However, after Fc-IL-CHO /AuNP-PAMAM /GA/Chit-covered electrode was modified with AuNP, the Ret decreased (curve e), indicating AuNP improved conductivity of electrode surface. After the Fc-IL-CHO /AuNP-PAMAM /GA/Chit-covered electrode was modified stepwise with BSA (curve f), anti-AFP antibody (curve g), and AFP (curve h), the Ret increased successively. It is because these proteins obstructed the electron transfer and resulted in the increase of Ret. Values of Ret obtained from the fitting of the Nyquist plots for each step of the modifying process are shown in Table S1.
Nyquist plots of EIS profiles of the different modified electrodes: (a) bare electrode; (b) GA/Chit modified electrode; (c) AuNP-PAMAM/GA/Chit modified electrode; (d) Fc-IL-CHO/AuNP-PAMAM/GA/Chit modified electrode; (e) AuNP/Fc-IL-CHO/AuNP-PAMAM/GA/Chit modified electrode. When anti-AFP antibody, BSA, AFP was stepwise immobilized on the (e) electrode, EIS profile was (f), (g), (h), respectively. The inset is Randle equivalent circuit for the impedance spectra. The concentration of AFP is 15 ng mL−1
The detection of AFP
The performance of the proposed immunosensor was evaluated by assaying AFP. After the immobilization of anti-AFP antibody onto the electrode surface, AFP antigen was captured to form antibody-antigen immunocomplex, resulting in the decreasing of response current of redox probe Fc. The DPV of the immunosensor to different concentration of AFP was measured in PB solution and the results were ascribed in Fig. 4. As can be seen, the peak current of DPV decreases with the increasing of the concentration of AFP (inset). The concentration of AFP and the peak current possesses a good linear relationship within the range from 0.05 to 30 ng mL−1. The limit of detection is 0.02 ng mL−1 determined by 3 σ rule (where σ is the standard deviation of a blank solution). The immunosensor was compared with other AFP immunosensors. As can be seen from Table 1, the analytical performance of this immunosensor is exhibited a good performance compared with other immunosensors reported for the detection of AFP.
Calibration curve of the immunosensor to different concentrations of AFP. Insert: DPV responses of the immunosensor to different concentrations of AFP (from a to g: 0.05. 1, 5, 10, 15, 25, 30 ng mL−1). Error bars represent standard deviation, n = 3. Potential range is −0.2 to +0.6 V (vs SCE reference). Scan rate is 100 mV s −1
Specificity, reproducibility and stability of the immunosensor
A satisfied immunosensor should have a good specificity. The specificity of this immunosensor was examined by carcinoembryonic antigen (CEA), bovine serum albumin (BSA), human immunoglobulin G (IgG). The electrode modified with capture anti-AFP antibody was respectively incubated with these nonspecific species solution. The concentration of these nonspecific species was 100 ng mL−1. Figure 5 shows that the responses obtained in the presence of nonspecific species are close to the response of blank solution. However, the response in the presence of 15 ng mL−1 of AFP is the lowest. These results indicate that the specificity of the prepared immunosensor is acceptable.
Reproducibility of the proposed immunosensor was estimated by calculating the coefficient of variation (CV) for both intra-assay and inter-assay at 20 ng mL−1 AFP (n = 3). The CV for inter-and intra-assay was 8.9 and 4.6%, respectively. This indicates that the immunosensor for AFP are reproducible.
In order to study the stability of the fabricated immunosensor, the modified electrode was stored at 4 °C when it wasn’t in use. DPV was recorded every one week. After four weeks, the peak current had no obvious changes, indicating the immunosensor had a good long-time stability.
Real sample analysis
Using three human serum samples, we studied the feasibility of the proposed immunosensor. Serum samples measured were diluted with PB solution (1:20, v/v). The assay results were compared with those obtained by Enzyme-linked Immunosorbent Assay (ELISA). Table 2 demonstrates that the proposed strategy was suitable for real sample analysis.
Conclusion
In summary, Fc-IL-CHO /AuNP-PAMAM-based platform was developed for the detection of AFP. Fc-IL-CHO was covalently bound to AuNP-PAMAM conjugates which were modified on the electrode in advance. The combination of them avoided the leakage of Fc from the matrix and introduced redox species Fc on the electrode. The use of PAMAM increased the amount of redox species Fc on the electrode surface. The immunosensor proved to have a good sensitivity, specificity and reproducibility. It can be used for the detection of AFP and also for other cancer biomarkers. But, the immunosensor is still promising for a wide linear range and high sensitivity.
References
Xiong C, Wang H, Yuan Y, Chai Y, Yuan R (2015) A novel solid-state Ru(bpy) 3 2+ electrochemiluminescence immunosensor based on poly(ethylenimine) and polyamidoamine dendrimers as co-reactants. Talanta 131:192–197
Akter R, Jeong B, Lee YM, Choi JS, Rahman M (2017) Femtomolar detection of cardiac troponin I using a novel label-free and reagent-free dendrimer enhanced impedimetric immunosensor. Biosens Bioelectron 91:637–643
Tsukruk VV, Rinderspacher F, Bliznyuk VN (1997) Self-assembled multilayer films from dendrimers. Langmuir 13:2171–2176
Kim DM, Rahman M, Do MH, Ban C, Shim YB (2010) An amperometric chloramphenicol immunosensor based on cadmium sulfide nanoparticles modified-dendrimer bonded conducting polymer. Biosens Bioelectron 25:1781–1788
Giannetto M, Mori L, Mori G, Careri M, Mangia A (2011) New amperometric immunosensor with response enhanced by PAMAM-dendrimers linked via self- assembled monolayers for determination of alpha-fetoprotein in human serum. Sensors Actuators B Chem 159:185–192
Dong J, Zhao H, Xu M, Ma Q, Ai S (2013) A label-free electrochemical impedance immunosensor based on AuNPs/PAMAM-MWCNT-chi nanocomposite modified glassy carbon electrode for detection of Salmonella typhimurium in milk. Food Chem 141:1980–1986
Gao Q, Han J, Ma Z (2013) Polyamidoamine dendrimers-capped carbon dots/au nanocrystal nanocomposites and its application for electrochemical immunosensor. Biosens Bioelectron 49:323–328
Kavosi B, Salimi A, Hallaj R, Amani K (2014) A highly sensitive prostate-specific antigen immunosensor based on gold nanoparticles/PAMAM dendrimer loaded on MWCNTS/chitosan/ionic liquid nanocomposite. Biosens Bioelectron 52:20–28
Erdem A, Congur G, Mese F (2015) PAMAM dendrimer functionalized magnetic particles developed for voltammetric DNA analysis. J Electroanal Chem 741:51–55
Jiang W, Wu L, Duan J, Yin H, Ai S (2018) Ultrasensitive electrochemiluminescence immunosensor for 5-hydroxymethylcytosine detection based on Fe3O4@SiO2 nanoparticles and PAMAM dendrimers. Biosens Bioelectron 99:660–666
Jiang X, Wang H, Yuan R, Chai Y (2015) Sensitive electrochemiluminescence detection for CA15-3 based on immobilizing luminol on dendrimer functionalized ZnO nanorods. Biosens Bioelectron 63:33–38
Kavosi B, Hallaj R, Teymourian H, Salimi A (2014) Au nanoparticles/PAMAM dendrimer functionalized wired ethyleneamine–viologen as highly efficient interface for ultra-sensitive α-fetoprotein electrochemical immunosensor. Biosens Bioelectron 59(2014):389–396
Park S, Kazlauskas RJ (2003) Biocatalysis in ionic liquids-advantages beyond green technology. Curr Opin Biotechnol 14:432–437
Wei Y, Li X, Sun X, Ma H, Zhang Y, Wei Q (2017) Dual-responsive electrochemical immunosensor for prostate specific antigen detection based on au-CoS/graphene and CeO2/ionic liquids doped with carboxymethyl chitosan complex. Biosens Bioelectron 94:141–147
Dong S, Tong M, Zhang D, Huang T (2017) The strategy of nitrite and immunoassay human IgG biosensors based on ZnO@ZIF-8 and ionic liquid composite film. Sensors Actuators B Chem 251:650–657
Fei J, Dou W, Zhao G (2015) A sandwich electrochemical immunosensor for Salmonella pullorum and Salmonella gallinarum based on a screen-printed carbon electrode modified with an ionic liquid and electrodeposited gold nanoparticles. Microchim Acta 182:2267–2275
Shen G, Zhang X, Shen Y, Zhang S, Fang L (2015) One-step immobilization of antibodies for α-1-fetoprotein immunosensor based on dialdehyde cellulose/ionic liquid composite. Anal Biochem 471:38–43
Sung D, Yang S (2014) Facile method for constructing an effective electron transfer mediating layer using ferrocene-containing multifunctional redox copolymer. Electrochim Acta 133:40–48
Liang R, Fan L, Huang D, Qiu J (2011) A label-free amperometric immunosensor based on redox-active ferrocene-branched chitosan/multiwalled carbon nanotubes conductive composite and gold nanoparticles. Electroanal 23:719–727
Wei Z, Sun X, Li Z, Fang Y, Ren G, Huang Y, Liu J (2011) Highly sensitive deoxynivalenol immunosensor based on a glassy carbon electrode modified with a fullerene/ferrocene/ionic liquid composite. Microchim Acta 172:365–371
Qiu JD, Wang R, Liang RP, Xia XH (2009) Electrochemically deposited nanocomposite film of CS-fc/au NPs/GOx for glucose biosensor application. Biosens Bioelectron 24:2920–2925
Senel M, Nergiz C, Cevik E (2013) Novel reagentless glucose biosensor based on ferrocene cored asymmetric PAMAM dendrimers. Sensors Actuators B Chem 176:299–306
Feng T, Qiao X, Wang H, Sun Z, Hong C (2016) A sandwich-type electrochemical immunosensor for carcinoembryonic antigen based on signal amplification strategy of optimized ferrocene functionalized Fe3O4@SiO2 as labels. Biosens Bioelectron 79:48–54
Stobiecka M, Hepel M (2011) Effect of buried potential barrier in label-less electrochemical immunodetection of glutathione and glutathione-capped gold nanoparticles. Biosens Bioelectron 26:3524–3530
Niu Y, Yang T, Ma S, Peng F, Yi M, Wan M, Mao C, Shen J (2017) Label-free immunosensor based on hyperbranched polyester for specific detection of α-fetoprotein. Biosens Bioelectron 92:1–7
Li N, Ma H, Cao W, Wu D, Yan T, Du B, Wei Q (2015) Highly sensitive electrochemical immunosensor for the detection of alpha fetoprotein based on PdNi nanoparticles and N-doped graphene nanoribbons. Biosens Bioelectron 74:786–791
Zhang P, Huang H, Wang N, Li H, Shen D, Ma H (2017) Duplex voltammetric immunoassay for the cancer biomarkers carcinoembryonic antigen and alpha-fetoprotein by using metal-organic framework probes and a glassy carbon electrode modified with thiolated polyaniline nanofibers. Microchim Acta 184(10):4037–4045
Jia H, Yang T, Zuo Y, Wang W, Xu J, Lu L, Li P (2017) Immunosensor for a-fetoprotein based on a glassy carbon electrode modified with electrochemically deposited N-doped graphene, gold nanoparticles and chitosan. Microchim Acta 184(10):3747–3753
Shen YM, Shen GY, Zhang YY (2018) A versatile matrix of ionic liquid functionalized with aldehyde and ferrocene for label-free electrochemical immunosensors. Anal methods, in press https://doi.org/10.1039/c8ay00108a
Shen G, Xu H, Gurung AS, Yang Y, Liu G (2013) Lateral flow immunoassay with the signal enhanced by gold nanoparticle aggregates based on polyamidoamine dendrimer. Anal Sci 29:799–804
Xu T, Chi B, Wu F, Ma S, Zhan S, Yi M, Xu H, Mao C (2017) A sensitive label-free immunosensor for detection α-fetoprotein in whole blood based on anticoagulating magnetic nanoparticles. Biosens Bioelectron 95:87–93
Yuan Y, Li S, Xue Y, Liang T, Cui L, Li Q, Zhou S, Huang Y, Li G, Zhao Y (2017) A Fe3O4@au-based pseudo-homogeneous electrochemical immunosensor for AFP measurement using AFP antibody-GNPs-HRP as detection probe. Anal Biochem 534:56–63
Wang H, Li X, Mao K, Li Y, Du B, Zhang Y, Wei Q (2014) Electrochemical immunosensor for α-fetoprotein detection using ferroferric oxide and horseradish peroxidase as signal amplification labels. Anal Biochem 465:121–126
Qi T, Liao J, Li Y, Peng J, Li W, Chu B, Li H, Wei Y, Qian Z (2014) Label-free α-fetoprotein immunosensor established by the facile synthesis of a palladium–graphene nanocomposite. Biosens Bioelectron 61:245–250
Wang L, Gan X (2009) Antibody-functionalized magnetic nanoparticles for electrochemical immunoassay of α-1-fetoprotein in human serum. Microchim Acta 164:231–237
Acknowledgments
The authors gratefully acknowledge the financial supports from Hunan Provincial Natural Science Foundation of China (2016JJ6105) and Foundation of Hunan University of Arts and Science (17ZD03).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 98 kb)
Rights and permissions
About this article
Cite this article
Shen, Y., Shen, G. & Zhang, Y. Voltammetric immunoassay for α-fetoprotein by using a gold nanoparticle/dendrimer conjugate and a ferrocene derived ionic liquid. Microchim Acta 185, 346 (2018). https://doi.org/10.1007/s00604-018-2886-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2886-3