Abstract
A simple and sensitive electrochemical immunoassay protocol for the detection of α-1-fetoprotein (AFP) has been designed based on antibody-functionalized core–shell–shell nanocomposite particles. The core–shell CoFe2O4/(3-mercaptopropyl) trimethoxysilane (CoFe2O4–MPS) was initially synthesized by covalent conjugation, then gold nanoparticles were adsorbed onto the surface of CoFe2O4–MPS via an Au–S bond, and then anti-AFP antibodies were immobilized onto the nanogold surface. With the aid of the external magnet, the biomolecules-doped magnetic nanoparticles were attached to the electrode surface. The electrochemical response was based on the modification of potential (potentiometric method) due to the interfacial change of charges as a consequence of the antigen-antibody reaction. Under optimal conditions, the immunosensor exhibited rapid response and a linear range from 0.8 to 120 ng mL−1 AFP with a detection limit of 0.3 ng mL−1 (S/N = 3). Moreover, the precision, reproducibility, selectivity and stability of the immunosensor were acceptable. Analytical results, obtained for the clinical serum specimen by the immunosensor, were in accordance with those assayed by the standard ELISA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Tumor markers are substances produced by tumor cells or by other cells of the body in response to cancer or certain benign (noncancerous) conditions [1–5]. Different tumor markers are found in different types of cancer, and levels of the same tumor marker can be altered in more than one type of cancer [2]. To date, various immunoassay methods have been developed for the detection of tumor markers, such as piezoelectric, chemiluminescence, capillary electrophoretic, electrochemical, immunoaffinity chromatograph and surface plasmon resonance [3, 4]. These methods could not still meet the requirements for the rapid and sensitive diagnosis of tumor markers, however, it is still a challenge to find new approaches that could improve the simplicity, selectivity, and sensitivity of clinical immunoassays. A preferable approach is to utilize electrochemical immunosensor in combination with immunochemical technique [5].
For the design and implementation of the electrochemical immunosensors, the target-specific ligands must be effectively immobilized on the electrode surface, and the analytes must be able to access their immobilized recognition couple under satisfactory frequency without severe steric hindrance and nonspecific binding [6, 7]. Recently, gold nanoparticles with good biocompatibility and high volume-to-surface ratio have been extensively applied for the immobilization of biomolecules [8–11]. Especially, when the (magnetic) core–shell nanoparticles were used for the immobilization of biomolecules, the ligands functionalized magnetic beads could be rapidly separated, concentrated and purificated with an external magnet, which could reduce the time of the assay [12]. Tang et al. synthesized magnet core–shell NiFe2O4/SiO2 nanoparticles and fabricated an electrochemical magnetic controlled microfluidic device for the detection of four tumor markers [13]. Zhu and Han found that CdSe–ZnS–SiO2 core–shell–shell nanoparticles could improve their stability in biological buffers and biocompatibility in fluorescence imaging [14]. Shi and Asefa synthesized size tunable and structure tailored core–shell–shell nanospheres containing silica cores, gold nanoparticle shells, and controlled thickness of smooth, corrugated, or porous silica shells over the gold nanoparticles [15]. The synthesis involved the deposition of gold nanoparticles on silica cores, followed by sol–gel processing of tetraethoxysilane (TEOS) or sodium silicate to form dense or porous silica shells, respectively, over the gold nanoparticles. To the best of our knowledge, no report has been focusing on the external effect of magnetic core–shell–shell gold nanocomposite particles on the electrochemical immunosensors.
Alpha-fetoprotein (AFP) is a normal fetal serum protein synthesized by the liver, yolk sac, and gastrointestinal tract that shares sequence homology with albumin. It reaches a peak concentration of 3 mg ml−1 at 12 weeks of gestation. Following birth, it clears rapidly from the circulation, having a half life of 3.5 days, and its concentration in healthy adult serum is less than 20 ng ml−1. Elevation occurs in certain liver diseases, especially acute viral or drug induced hepatitis and conditions associated with hepatic regeneration. In general, the elevations are under 500 ng ml−1 and do not denote hepatocellular carcinoma. The aim of this study is to fabricate an magnetic-controlled electrochemical immunosensor for the detection of AFP based on the core–shell–shell CoFe2O4–MPS/nanogold magnetic beads. The synthesized procedure of the magnetic beads was characterized by UV–vis absorption spectroscopy. The performance and factors influencing the proposed immunosensor were examined. The potential application of the developed immunosensor was explored in the following experiments.
Experimental and method
Reagents
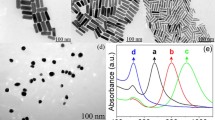
α-1-Fetoprotein (AFP) and anti-AFP antibody were purchased from AutoBio Bioeng. Ltd Co. (www.autobio.com.cn, China). Bovine serum albumin (BSA, 96–99%), (3-mercaptopropyl) trimethoxysilane (MPS), gold chloride (HAuCl4) and sodium citrate were obtained from Sigma (www.sigmaaldrich.com, USA). Phosphate buffer (0.1 mol l−1) solutions (PBS, pH 7.4) were prepared with NaCl 8.0 g, Na2HPO4 1.15 g, KH2PO4 0.2 g, KCl 0.2 g dissolved in 1,000 ml water. All chemicals and solvents used were of analytical grade and were used as received. All solutions were made up with twice-distilled water. Gold colloids were prepared according to the literature [16] by adding 1.0 ml of 1% sodium citrate solution to 100 ml boiling solution of 0.01% HAuCl4. The size of the prepared gold colloids was about 16 nm, which was estimated from transmission electron microscopy (www.hitachi.com, TEM, H600, Hitachi Instrument Co., Japan; Fig. 1).
Preparation of core–shell–shell CoFe2O4–MPS/nanogold nanoparticles
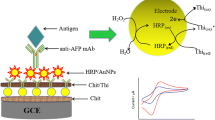
The fabrication procedure of the core–shell–shell CoFe2O4–MPS/nanogold composite nanoparticles was illustrated in Scheme 1a. At the first step, the magnetic CoFe2O4 particles (40 nm in diameter) were synthesized according to the literature [17]. Briefly, Fe(NO3)3·9H2O, Co(NO3)3·6H2O and Glycine (Gly) were dissolved in distilled water (note: Fe3+/Co2+ = 2/1, Gly/nitrate = 4/1, in molar ratio). After filtration, the red precursor solutions obtained were concentrated by heating, forming mixtures of amorphous melted salts. Then combustion reaction fragmentarily appeared and rapidly diffused. Finally, the black loose powders (i.e. CoFe2O4 nanoparticles) were obtained after combustion for several seconds. Following that, the dendrimer-functionalized CoFe2O4 sol was prepared by mixing (3-mercaptopropyl) trimethoxysilane (MPS) with 10% (v/v) of methanol, water at a 1:4 ratio, 10% (w/v) CoFe2O4, and 3.3% (v/v) of 0.1 mol l−1 hydrochloric acid via hydrolyzed reaction. The mixture was sonicated for 50 min until a clear and homogeneous solution resulted and was stored at room temperature for 2–3 h. At the third step, appropriate gold colloids with 16 nm were added into the homogeneous solution, and slightly shaken for 30 min at room temperature to make gold nanoparticles assemble the thiol groups of the sol–gel network. Finally, the formed magnetic core–shell–shell nanocomposite particles were obtained by magnetic separation, and stored at 4 °C when not in use. For comparison, the core–shell CoFe2O4–nanogold composite nanoparticles were also prepared by adding 1 ml of 1% sodium citrate solution to 100 ml boiling solution containing 20 mg of CoFe2O4 and 1 ml of 1% HAuCl4.
Antibody immobilization
Two grams of the core–shell–shell CoFe2O4–MPS/nanogold composite nanoparticles was initially sonicated for 5 min, and then the mixture was centrifugated, and the precipitates were washed three times with pH 7.4 PBS. Afterwards, 300 μl of anti-AFP (200 ng ml−1) was added into the composite nanoparticles solution with the total volume of 4 ml, and slightly stirred for 24 h at 4 °C. The synthesized bionanoparticles were collected from the solution by magnetic separation. Moreover, the anti-AFP-functionalized core–shell CoFe2O4–nanogold composite nanoparticles were synthesized by using the same method.
Immunosensor fabrication
An magnetic carbon paste electrode (CPE) was fabricated as the following description (Scheme 1). Briefly, 500 mg of graphite powder and 400 mg of the melted paraffin were initially blended thoroughly, and the homogeneous paste was then deposited into a glass tube with 5-mm diameter and 10-mm depth. Meanwhile, the external magnet was placed the middle of the glass tube. A nut was turned to extrude ~1 mm thick outer paste layer to achieve enough magnetic field on the electrode surface. The CPE was polished with ultra-fine emery paper until a smooth surface was obtained, the electrode surface was then cleaned with doubly distilled water. Fifty microliters of bionanoparticles was dipped onto the CPE surface. With the aid of a permanent magnet, the magnetic bionanoparticles were attached onto the electrode surface via the magnetic force between the external magnet and the magnetic bionanoparticles.
Measurement methods
Potentiometric measurement was carried out with a two-electrode system comprising a saturated calomel electrode (SCE) as reference and the CPE as working electrode in 1.0 ml PBS (pH 7.4) at room temperature (25 ± 1.0 °C; Scheme 1b). Each sample to be analyzed was introduced into the detection cell after stabilization of response (shift less than 1 mV min−1) by a digital ion analyzer (Model PHS-3C, Shanghai, China). To avoid the possible error resulting from different additions of samples and deduct the responses induced by nonspecific adsorption, the response of each sensor was recorded as the immunoreaction proceeded from 30 s (after the addition of samples) until equilibrium was reached ~6 min. The control tests with normal (negative) samples and the evaluations for clinical specimens were performed accordingly. The responses in all experiments were referred to the average responses of immunoreaction with corresponding standard deviations of triplicate measurements, unless otherwise indicated.
After each immunoassay run, the regeneration of the proposed immunosensors was performed by dipping into a versatile solution consisting of glycine–HCl (0.2 mol l−1, pH 2.8) and NaCl (0.25 mol l−1) to dissociate the antigen-antibody complex, and peel the captured antigens off the immunosensor [16]. The contaminated surface was regenerated by turning the nut to extrude a 0.1 mm thick outer paste layer and then polishing with an alumina paper wetted with water to produce a smooth shiny surface.
Results and discussion
Characteristics and construction of the protein assay system
The anti-AFP-functionalized magnetic beads were characterized by UV–vis absorption spectrometry. The absorption spectra of pure CoFe2O4 and CoFe2O4–MPS magnetic beads were complicated in the range of 600–200 nm. When gold nanoparticles were adsorbed onto the CoFe2O4–MPS magnetic beads, 520 nm of the absorption peak was remarkably observed, suggested that gold nanoparticles have been formed on the CoFe2O4–MPS surface. Moreover, 276 nm of the absorption peak was obtained after mixed anti-AFP with the nanogold/MPS–CoFe2O4 particles for 12 h. Thus, we might conclude that anti-AFP antibodies could be assembled onto the core–shell–shell CoFe2O4–MPS/nanogold composite nanoparticles.
To further investigate the activity of the immobilized antibody on the surface of the core–shell–shell CoFe2O4–MPS/nanogold composite nanoparticles, FT-IR (Spectrum GX, Perkin-Elmer, Co. USA) spectrum was used. The bands of the core–shell–shell CoFe2O4–MPS/nanogold nanoparticles were observed at 1,625, 1,402, 1,131 cm−1, which were mainly attribute to the –SH groups and –O– groups on the MPS. As is well known, the shapes of the infrared absorption bands of amide I groups at 1,610–1,690 cm−1 corresponding to the C=O stretching vibration of peptide linkages and amide II groups around 1,500–1,600 cm−1 from a combination of N–H bending and C–N stretching can provide detailed information on the secondary structure of proteins [18,19]. When mixing anti-AFP with the core–shell–shell CoFe2O4–MPS/nanogold nanoparticles for 12 h, the FT-IR spectrum of antibody-functionalized nanoparticles shows two absorption peaks at 1,656 and 1,545 cm−1, which corresponded to the amide I and II groups of the antibody [20]. The weak bands occurred in the region of 1,200–1,400 cm−1 were assigned to the wagging and twisting vibrations of the –CH2 group in these proteins and were commonly referred to as the progression bands, suggesting that the antibody molecules, adsorbed on the core–shell–shell CoFe2O4–MPS/nanogold nanoparticles, retained their native characterizations.
Magnetic measurements were made using Nanjing University Instruments on vibrating sample magnetometer (VSM) at room temperature that produces fields of up to 6 T on the sample. The hysteresis loops for one of the representative sample (d = 300 nm) are plotted in Fig. 2. It was found that the magnetization for the core–shell–shell CoFe2O4–MPS/nanogold composite nanoparticles was 43.3 emu g−1 while remanent magnetization was 28.1 emu g−1 for anti-AFP-functionalized CoFe2O4–MPS/nanogold composite nanoparticles. The magnetization of anti-AFP-functionalized composite nanoparticles is significantly lower as compared to the bare CoFe2O4–MPS/nanogold composite nanoparticles, indicating the anti-AFP molecules have been assembled onto the surface of the core–shell–shell CoFe2O4–MPS/nanogold composite nanoparticles. Moreover, the magnetization could still remain after modification.
Comparison of different immunosensors
To investigate the effect of MPS on the electrochemical properties of the proposed immunosensor, two types of magnetic beads, such as core–shell CoFe2O4–nanogold nanoparticles and core–shell–shell CoFe2O4–MPS/nanogold nanoparticles, were used for the fabrication of the immunomagnetic beads, respectively. Figure 3 displays the potential responses of various immunomagnetic beads-modified immunosensors to different AFP concentrations under the same conditions. It is found that the core–shell–shell immunomagnetic beads-functionalized immunosensor exhibited higher potentiometric responses than that of core–shell CoFe2O4–nanogold nanoparticles. The reason might be attributed to the following issues: (1) the three-dimensional network of MPS could amplify the coverage of gold nanoparticles on the CoFe2O4 surface, in contrast with the direct immobilization of gold colloids; (2) the core–shell–shell nanocomposite particles could enhance the immobilized amount of AFP. These advantages are likely to originate from a structural feature of the core–shell–shell CoFe2O4–MPS/nanogold nanoparticles such as a surface–surface-exposure of derivatized ligands and a corrugated surface.
Potentiometric responses of the developed immunoassay toward various analytes
Since the charge of a protein was mainly determined by pH, we investigated the effect of the pH of detection solution on potentiometric response of the immunosensor between 5.5 and 8.5 in PBS. The immunosensor potentiometric response increases with increasing pH value from 5.5 to 7.4 and the decreases as the pH increases further (data not shown). It is well known that at relatively high pH, the activity of the antibody or antigen is inhibited. The experimental results show that the maximum response occurs at pH 7.4 of the physiological pH and most immunoreactions exhibit optimal binding at this pH. Therefore, PBS of pH 7.4 is used as the medium for the immunoreaction.
Figure 4 shows the typical responses monitored in situ for evaluating various AFP concentrations. The output voltage increased rapidly with the elapse of time and converged after 6 min. The result indicates that the reaction between immobilized antibody and free antigen is an equilibrium process. Either antibodies or antigens in aqueous solution have a net electrical charge polarity, which is associated with the isoelectric points of the species and the ionic composition of the solution. If antibodies are immobilized on the electrode, the surface charge of the electrode will rely on the net charge of the immobilized antibody. When antigen molecules are present in the solution, the immunochemical reaction will take place at the interface with a resulting change of the surface charge. This change can be measured potentiometrically against the reference electrode immersed in the same solution. Since the isoelectric points of anti-AFP and AFP are less than 7.0, they are negatively charged species at pH 7.4. In pH 7.4 PBS, the density of negative electrical charges on the immunosensor surface was increased with the antigen-antibody reaction. Therefore, the output voltage increased (curves e–f in Fig. 4).
To further relegate the differences in response between probes to interference degree or crossing recognition level, three types of antigen, such as CEA, CA 15-3, and HBsAg, were injected into the detection cell. The potential changes to each antigen between probes were recorded, and the results are described in Fig. 4 (curves a–c). The interference degree of variability between lineage-different immunological markers was <6%, indicating acceptable cross-recognition of the proposed immunosensor. When normal (negative) serum samples were analyzed using the developed immunosensor, the immunosensor showed substantially low signals (ΔE <5 mV, curve d in Fig. 4), in contrast to the results obtained when the corresponding positive serum was assayed, revealing a significant difference between the lineage-specific recognition and the nonspecific adsorption.
Dose curve of the developed immunosensor
Figure 5 a shows the relationship between the potential shift (ΔE) of immunoagglutination and the logarithm of AFP concentration in pH 7.4 PBS. The potential changes of the resulting immunosensor were proportional to the logarithm of the concentration of AFP in the range of 0.8 to 120 ng ml−1, and the linear equation was ΔE (mV) = 5.346 + 30.79 × lgC [AFP] (ng ml−1) with a correlation coefficient of 0.992. The detection limit was 0.3 ng ml−1 (estimated according to 3 × the standard deviation of zero-dose response). For comparison, the core–shell CoFe2O4–nanogold conjugating immunosensor was also evaluated. As shown in Fig. 5 and Table 1, the immunosensor with core–shell–shell CoFe2O4–MPS/nanogold nanocomposite particles exhibited higher sensitivity, wider linear range and lower detection limit than that of core–shell CoFe2O4–nanogold nanocomposite particles.
Precision and stability of the developed immunoassay
The intra-assay precision of the developed immunoassay method was examined by using the same batch immunomagnetic beads toward five concentrations of AFP. The relation standard deviations (RSDs) of the intra-assay with this method were 4.3, 3.6, 5.2, 4.7 and 4.1% for 1.5, 5.0, 20, 40 and 80 ng ml−1AFP, respectively. The batch-to-batch reproducibility of immunomagnetic beads was also studied toward five concentrations of AFP. The mean RSDs of the inter-assay were 5.9, 6.3, 6.7, 5.2 and 4.7% for 1.5, 5.0, 20, 40 and 80 ng ml−1AFP, respectively. Thus, the precision and reproducibility of the proposed immunoassay method was satisfactory. When the as-prepared magnetic bionanoparticles were not in use, it was stored into pH 7.4 PBS at 4 °C. No obvious change was observed after storing for 7 days, and the signal response was 89.66% of initial response on the 17th day. The slow decrease of response seemed to be related to the gradual deactivation of anti-AFP incorporated in the composite nanoparticles.
Comparison of the developed immunoassay with standard ELISA
To monitor the effect of composite substrate on the immunoassay system, routine samples of various AFP were added into pH 7.4 PBS and analyzed. The recovery was from 95.8% to 105.4%. To further investigate the technique’s application for clinical analysis, we examined 22 serum specimens, which were diluted fivefold with pH 7.4 PBS, on the proposed immunoassay and the standard chemiluminescence ELISA. The regression equation (linear) for these data is as follows: y = 1.448 (±5.3) + 0.954 (±0.102) x(R 2 = 0.978; x-axis, by the as-prepared immunosensor; y-axis, by ELISA). These data shows that there is no significant difference between the results given by both methods. The developed immunoassays may give a promising pathway for determining AFP in clinical immunoassays.
Conclusions
A rapid, sensitive, regenerate and label-free electrochemical immunosensor for the detection of AFP in human serum was developed using the core–shell–shell magnetic beads. The advantage of immunomagnetic bead is their use in complex matrix to avoid interference and, after the immunological reaction with the antigen, the magnetic beads are captured to perform the detection. In contrast to the core–shell CoFe2O4–nanogold conjugating immunosensors, the presence of three-dimensional MPS could enhance the immobilized amount of nanogold particles, and improve the sensitivity of the proposed immunosensor. Significantly, the core–shell–shell nanoparticles could be promising in genosensors and enzymosensors on the basis of taking advantages of magnetism of the beads.
References
Rai AJ (2007) Biomarkers in translational research: focus on discovery, development and translation of protein biomarkers to clinical immunoassays. Expert Review of Molecular Diagnostics 7:545
Tang D, Yuan R, Chai Y (2008) Ultrasensitive electrochemical immunosensor for clinical immunoassay using thionine-doped magnetic gold nanospheres as labels and horseradish peroxidase as enhancer. Anal Chem 80:1582
Liu G, Wang J, Wu H, Lin Y, Lin Y (2007) Nanovehicles based bioassay labels. Electroanalysis 19:777
Schneider J (2006) Tumor markers in detection of lung cancer. Adv Clin Chem 42:1
Daniels J, Pourmand N (2007) Label-free impedance biosensors: opportunities and challenges. Electroanalysis 19:1239
Bakker E, Qin Y (2006) Electrochemical sensors. Anal Chem 78:3965
Marquette C, Blum L (2006) State of the art and recent advances in immunoanalytical systems. Biosens Bioelectron 21:1424
Huo Q, Worden J (2007) Monofunctional gold nanoparticles: synthesis and applications. J Nanoparticle Res 9:1013
Banin U (2007) Nanocrystals: tiny seeds make a big difference. Nat Mater 6:625–626
Tang D, Xia B, Zhang Y (2008) Direct electrochemistry and electrocatalysis of hemoglobin in a multilayer {nanogold/PDDA}n inorganic–organic hybrid film. Microchim Acta 160:367
Edwards P, Thomas J (2007) Gold in a metallic divided state—from Faraday to present-day nanoscience. Angew Chem Int Ed 46:5480
Smith J, Wang L, Tan W (2006) Bioconjugated silica-coated nanoparticles for bioseparation and bioanalysis. Trends Anal Chem 25:848
Tang D, Yuan R, Chai Y (2007) Magnetic control of an electrochemical microfluidic device with an arrayed immunosensor for simultaneous multiple immunoassays. Clin Chem 53:1323
Zhu M, Han J (2007) CdSe/CdS/SiO2 core/shell/shell nanoparticles. J Nanosci Nanotechnol 7:2343
Shi Y, Asefa T (2007) Tailored core–shell–shell nanostructures: sandwiching gold nanoparticles between silica cores and tunable silica shells. Langmuir 23:9455
Tang D, Yuan R, Chai Y, Dai J, Liu Y, Zhong X (2004) A novel immunosensor based on immobilization of hepatitis B surface antibody on platinum electrode modified colloidal gold and polyvinyl butyral as matrices via electrochemical impedance spectroscopy. Bioeletrochemistry 65:15
Tang D, Yuan R, Chai Y, An H (2007) Core–shell magnetic CoFe2O4/SiO2 composite nanoparticles as immobilized affinity supports for clinical immunoassay. Adv Funct Mater 17:976
Saguer E, For N, Alvarez P, Sedman J, Ismail A (2008) Structure–functionality relationships of porcine plasma proteins probed by FTIR spectroscopy and texture analysis. Food Hydrocolloids 22:459
Fernández-Vidal M, Rojo N, Herrera E, Gómara M, Haro I (2008) Liposome destabilization induced by synthetic lipopeptides corresponding to envelope and non-structural domains of GBV-C/HGV virus. Conformational requirements for leakage. Biophys Chem 132:55
Channasanon S, Graisuwan W, Kiatkamjornwong S, Hoven V (2007) Alternating bioactivity of multilayer thin films assembled from charged derivatives of chitosan. J Colloid Interface Sci 316:331
Acknowledgements
This work is supported by the Specialized Research Funds for the Excellent Young Teachers from Yibin University, China. The authors gratefully acknowledge the financial support from Postgraduate Science and Technology Innovation Program of YBU, and NSF of Sichuan, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Gan, X. Antibody-functionalized magnetic nanoparticles for electrochemical immunoassay of α-1-fetoprotein in human serum. Microchim Acta 164, 231–237 (2009). https://doi.org/10.1007/s00604-008-0059-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-008-0059-5