Abstract
It is reported that a mixture of WS2 nanosheets (WS2 NS) and silver nanoclusters (AgNCs) displays strongly enhanced peroxidase-mimicking activity. The catalytic effect of the mixture was studied by colorimetry, fluorometry, chemiluminescence (CL) and electrochemistry. The effect is interpreted in terms of a difference between the Fermi energy level of the two nanomaterials. This leads to the formation of charge separation regions which act as active sites for enzyme mimetic interaction with the substrates. The mixture of WS2 NS and AgNCs was exploited for the non-enzymatic determination of H2O2 and glucose. A stopped-flow method was applied as a sensitive CL detection system using the bicarbonate-H2O2 reaction. The mixture has a powerful peroxidase mimicking activity on the bicarbonate-H2O2 CL reaction, and this effect is much larger than that of any single constituent. In addition, the CL emission is improved several times by using the stopped-flow technique. Under optimum condition, H2O2 can be determined in the 2.5–1500 nM concentration range. Moreover, glucose levels in human serum can be quantified via glucose oxidase based oxidation which leads to the generation of H2O2. Using this CL assay, a linear relationship was obtained between the intensity of the CL emission and glucose concentration in the range of 0.03–20 μM, with a limit of detection (3S) of 13 nM.

An enhanced peroxidase-like catalytic activity for WS2 nanosheets (WS2 NS) was revealed in the presence of silver nanoclusters (AgNCs), and was exploited for the non-enzymatic determination of H2O2, and of glucose (via glucose oxidase; GOx) using a stopped-flow CL method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemiluminescence (CL) is an interesting detection system in analytical tools, due to its intrinsic advantages, such as high sensitivity, lengthy dynamic region and setup simplicity [1]. CL assays are generally applied as friendly flow injection mode [2, 3], or other efficient states such as stopped-flow procedure, which can practically provide the advantages of both flow and batch CL processes [1]. Moreover, different nanomaterials with their outstanding features have been applied in CL assays providing improved systems for the analysis aims [1, 4]. Also, some nanomaterials represent excellent mimetic properties to act as a suitable candidate for natural enzymes (for example horseradish peroxidase, HRP), with considerable advantages including cost-effective preparation process, simple preservation, higher stability, diverse composition or structure selections, and flexible catalytic properties [5]. These new catalysts have been appeared as perfect enzyme-mimics in bioassays especially with colorimetric detection [6,7,8]. Fe3O4 nanoparticles were studied as first peroxidase-like catalyst [9] and then, inherent peroxidase catalytic activity was also recognized for other metal oxide and also carbon nanomaterials, such as Co3O4 nanoparticles, graphene oxide, and carbon nanodots.
Tungsten disulfide nanosheets (WS2 NS) have been applied in numerous areas including biosensors [10, 11], biomedicine [12], energy harvesting [13], optoelectronics [14] and hydrogen evolution reactions [15]; due to its outstanding electronic and optical features. Changing of indirect-gap transitions in bulk WS2 to direct-gap in its monolayer state causes a notable improvement in the photoluminescence efficiency [16]. High carrier mobility and strong spin-orbit coupling are other advantages of transition metal dichalcogenides. So, WS2 NS can be a good nomination for investigation its efficient peroxidase activity with considerable advantages over natural enzyme; like higher stability, low preparing cost, simple preservation and excellent catalytic activity [17, 18]. The intrinsic peroxidase-like activity of WS2 NS was previously reported by Lin’s research group [17]. They applied WS2 NS as a catalyst for 3,3′,5,5′-tetramethylbenzidine (TMB)-H2O2 reaction to yield the blue colored product. This colorimetric method was used for the measurement of H2O2 and glucose. Moreover, hemin-modified WS2 NS was introduced as highly active peroxidase-like catalyst for the similar colorimetric system in the detection of H2O2 and glucose [18].
On the other hand, noble metal nanoclusters (NCs), with a usual size less than 1 nm which is close to the Fermi-wavelength of conduction electrons, show distinct and size-tunable electronic transitions. They show a strong fluorescence emission, due to the strong quantum-confinement effect [19]. Silver nanoclusters (AgNCs) with defined electronic transition peak in UV − vis area have been extensively considered in biosensing [20]. Owing to the strong absorption and emission and high stability in ambient circumstances, AgNCs have been applied as attractive fluorescence probes in both single-molecular and bulk fluorescence [19, 21]. Also, the catalytic activity of AgNCs have been frequently reported in literatures [19].
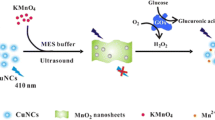
The synergetic catalytic effect of hybrid or mixed nanomaterials can be an interesting subject [22]. To the best of our knowledge, no comprehensive study has been reported for the synergetic mimetic properties of WS2 NS and AgNCs. In this study, an improved peroxidase-like activity for WS2 NS was presented in the presence of AgNCs using various optical or electrochemical detection systems. The reason for the observed synergetic effect was studied using the Fermi energy levels. Furthermore, the enhanced catalytic action of mixed WS2 NS-AgNCs was exploited for reliable measurement of H2O2 and glucose, using stop-flow CL method as a high sensitive detection system. WS2 NS-AgNCs mixture had a remarkable intensifying effect on the ultra-weak CL emission of bicarbonate-H2O2 reaction. The generated CL emission intensity was proportional with H2O2 concentration. Based on this observation, an advanced method was developed for glucose determination in human blood. After the oxidation of glucose in the presence of glucose oxidase, the produced H2O2 was detected by CL reaction in the presence of WS2 NS-AgNCs mixture as catalyst (Scheme 1).
Experimental
Instruments and materials
CL experiments were performed using a Berthold FB12 luminometer (Germany, www.berthold.com). Florescence investigations were done by LS-45 PerkinElmer spectrofluorimeter (USA, www.perkinelmer.com). In order to study the mechanism of CL reaction, CL spectra were achieved by the same device in a continuous flow state and with switched off radiation source. UV-visible absorption data were obtained by means of S2000 spectrophotometer (WPA Lightwave, England, www.biochrom.co.uk). The morphological assessments of synthesized nanomaterials were accomplished with JEOL high resolution transmission electron microscope (HR-TEM, JEM-2200FS, acting at 200 kV, www.jeol.co.jp). Nanosurf Mobile S atomic force microscope (AFM, Swiss, www.nanosurf.com) was employed for estimation of thickness of WS2 nanosheets. IviumStat potentiostat/galvanostat (Netherland, www.ivium.nl) was employed for electrochemical studies. X-ray diffraction (XRD) patterns were obtained by Siemens D5000 X-ray diffractometer (Germany, www.siemens.com) using a Cu Kα exciting source (λ = 1.54056 Å). Tensor 27 FT-IR spectrometer (Bruker, Germany, www.bruker.com) was applied to achieve Fourier transform infrared (FT-IR) spectra.
H2O2, glucose, sodium bicarbonate, NaOH, Albumin and silver nitrate were acquired from Merck (Darmstadt, Germany, www.merck.com). WS2, glucose oxidase (GOx) and 3,3′,5,5′-tetramethylbenzidine (TMB) were bought from Sigma (www.sigmaaldrich.com). All chemicals were obtained in analytical grade and used without any preparation step. All experimental solutions were prepared by double deionized (DI) water.
Synthesis of silver nanoclusters
Synthesis of AgNCs was carried out by rapid process reported by Mathew research group [23]. Details are provided in the Electronic Supporting Material (ESM).
Synthesis of WS2 nanosheets
Monolayer WS2 nanosheets were created via exfoliation of bulk WS2 powder using an ultrasonication process. For a typical synthesis aim, 1 g bulk WS2 powder was dispersed in 100 mL DMF and sonicated (power: 300 W) for 3 h. The dispersion was settled for 24 h and the supernatant was centrifuged (30 min at the speed of 1000 rpm and centrifugational force of 110 g) to collect bulk WS2. The dispersant was again centrifuged (30 min at the speed of 6000 rpm and 4000 g) to precipitate WS2 nanosheets.
Investigation of peroxidase-like activity of WS2 NS, AgNCs and their mixture
The peroxidase-like activity of prepared nanomaterials were investigated by colorimetry, fluorometry, chemiluminescence and electrochemical techniques. It is worth mentioning that all of investigations for each technique were done in optimized condition (including the concentration of reagents, catalyst, buffer type and pH). Details are presented in ESM.
Chemiluminescence detection of H2O2 and glucose
Glucose detection
50 μL glucose standard solution (with different concentration in the range of calibration curve) or prepared serum sample, and 50 μL GOx solution (60 U·mL−1) were added to 0.2 mL of 10 mM acetate buffer (pH 4.4) in a 3 mL Eppendorf tube. The solution was incubated at 37 °C for 5 min to complete the enzymatic oxidation of glucose with O2. The resulted H2O2 solution was appropriately diluted by DI water and subsequently applied for the CL analysis, described in the following part.
H2O2 determination
A flow mode CL system was employed for H2O2 detection using H2O2-bicarbonate reaction catalyzed by WS2 NS-AgNCs (Fig. S1). The setup was assembled by three polytetrafluoroethylene (PTFE) channels (inner diameter of 1.0 mm) to direct the reagents towards the CL cell. Sodium bicarbonate solution (0.8 M) together with WS2 NS (80 mg·L−1) and AgNCs (8 mg·L−1) were directly transferred via line c to CL cell in the front of detector. Carrier (DI water, in line b) was transported through a six-port valve having a 150 μL loop and, H2O2 (as oxidant or analyte in line a) was gathered in the loop. By changing the valve state to injection mode, the carrier stream carried H2O2 solution in loop to emission region. After observation of initial CL emission, the streams were instantly stopped by switching off the peristaltic pump. The CL emission was caused by insertion of H2O2 solution to the flow cell and its reaction with bicarbonate in the presence of catalyst. The maximum CL intensity was considered as analytical signal.
Determination of blood glucose
In order to measure glucose level in human blood, the samples were collected from healthy human volunteers and centrifuged for 30 min (10,000 rpm and 11,000 g). The serums were suitably diluted by phosphate buffer (5 mM, pH 7). Finally, suitable amounts of diluted samples were used for glucose measurement as mentioned above.
Results and discussion
WS2 NS and its various composites have been frequently applied as enzyme-like catalysts. The high surface of 2D WS2 NS as well as its high stability have resulted to great attentions to this nanomaterial. On the other hand, the synergistic catalytic effect of metal nanoclusters in the presence of some other nanomaterials (for example MoS2 NS, graphene, etc.) have been reported. But, there is no report about the synergetic effect of nanoclusters with WS2 NS. So, the investigation of mimetic activity of WS2 NC-metal nanoclusters can be an interesting subject.
Characterization of the nanomaterials
WS2 nanosheets
The TEM image for synthesized WS2 NS is indicated in Fig. 1a which showed clearly their mono-layered structure. Also, HR-TEM image (Fig. 1b) verified the crystal lattice of WS2 NS with a layer separation of 0.264 nm related to the (100) plane of WS2 [17, 24]. AFM analysis was applied for the investigation of morphology and thicknesses of the nanosheets (Fig. 1c). The results confirmed the synthesis of nanosheets with sizes of some hundreds of nanometers and a thickness of 2–4 nm. The high thickness of some sheets was related to the two or multi-layered structures. UV-vis absorption, FT-IR and XRD spectra are presented in Fig. S2 indicating the proper synthesis process. Diluted WS2 NS dispersed solution exhibit characteristic absorption peak at about 624 nm (Fig. S2a), which were not observed for bulk WS2. It indicated changing the indirect bandgap of bulk WS2 to direct bandgap after exfoliation step. FT-IR spectrum showed clear absorption peak of W-S at about 546 cm−1 (Fig. S2b). XRD pattern showed characteristic diffraction peaks of WS2 nanosheets (Fig. S2c). The peaks at 33.5° and 58.4° corresponded to the refection of (100) and (110) planes indicated the ab plane which is related to a 2D nanosheet construction of WS2 [25].
Silver nanoclusters
The results of HR-TEM analysis for prepared AgNCs are showed in Fig. 2. The size of AgNCs was in the range of 0.5–2 nm with an average of 1.6 ± 0.4 nm (calculated using about 50 particle). The crystal lattice of AgNCs was also confirmed by HR-TEM and a layer separation of about 0.232 nm was obtained which is agreed with the (111) plane of Ag. Other specific characterization of AgNCs was performed by their absorption, fluorescence emission, FT-IR and XRD spectra which are represented in Fig. S3. BSA capped AgNCs indicated a weak and broad absorption peak without any specific surface Plasmon resonance peak at 400–500 nm wavelengths (Fig. S3a). So, the formation of small nanoclusters was strengthened instead of lager nanoparticles. Also, the obtained absorption spectrum was different from BSA absorption band which is indicated in Fig. S3a. An intense fluorescence emission was obtained for BSA capped AgNCs at about 650 nm (λex = 420 nm) (Fig. S3b). As can be seen in Fig. S3b, the emission intensity of pure BSA is very weak in this range. The quantum yield (QY) for the fluorescence emission of AgNCs was obtained 5.9% using rhodamine B as reference material. The FT-IR spectrum of AgNCs indicated the characteristic bonds of protein amide I and II in the region 1600–1700 cm−1 (corresponding to C=O stretch) and 1548 cm−1 (C–N stretch coupled with N–H bending mode), respectively. Moreover, the pure peaks observed at about 3260 and 2920 cm−1 were ascribed to primary N-H and C–H stretching vibration (Fig. S3c). These bonds are very similar to the BSA FT-IR absorption spectrum and indicate the accurate modification of AgNCs by BSA. The XRD patterns for the synthesized AgNCs (Fig. S3d) showed the special diffraction peaks of AgNCs at 2θ values of about 38.1°, 44.3°, 64.4° and 77.5° assigning to the (111), (200), (220) and (311) planes of AgNCs crystals, respectively.
Investigation of peroxidase-like activity of WS2 NS, AgNCs, and their mixture
The exfoliated WS2 NS indicated an intrinsic catalytic activity toward the dissociation of H2O2 to hydroxyl radicals. In other words, WS2 NS acts as a peroxidase mimetic that offers many advantages over natural peroxidase enzyme. Also, the AgNCs had a weak catalytic activity on same reactions. The main achievement of this study is based on the promoting effect of AgNCs on the catalytic performance of WS2 NS. In next parts, it will be indicated that the AgNCs can be placed adjacent to the surface of WS2 NS and an electron transferring phenomenon occur between them due to the difference of their Fermi energy levels. The resulted charge separation was the basis of synergetic catalytic activity of WS2 NS-AgNCs mixture. This process was investigated by various detection systems including colorimetry, fluorometry, chemiluminescence and electrochemical techniques.
Colorimetric studies
In order to colorimetric investigation of the mimetic action of mixed WS2 NS-AgNCs, TMB was applied as peroxidase substrate to react with H2O2. The reaction was followed by blue colored product or its absorption spectra in the presence of WS2 NS and AgNCs (Fig. 3). The simultaneous adding of both WS2 NS (80 mg·L−1) and AgNCs (8 mg·L−1) efficiently enhances the rate of this reaction. As can be seen from Fig. 3, the change in the absorbance of TMB-H2O2 solution in the presence of the WS2 NS/AgNCs mixture was greater than the sum of changes caused by alone WS2 NS (80 mg·L−1) and AgNCs (8 mg·L−1). So, the synergistic catalytic activity of AgNCs and WS2 NS is obvious. It is noted that the comparison between the effects of WS2 NS, AgNCs and their mixture was performed by their maximum catalytic activity using optimal concentrations (not the same amounts). On the other hand, the absence of catalyst or H2O2 did not lead to sensible color changing in TMB-H2O2 solution; which means no reaction was occurred between them.
In order to study steady-state kinetic for applied catalyst, oxidation of TMB by H2O2 in the presence of WS2 NS, AgNCs or their mixture was followed using a constant condition and only concentration of one substrate (TMB or H2O2) was changed. In other words, a series of experiments were performed by constant amount of TMB, but different concentrations of H2O2. Also, another sequence of experiments was carried out by different concentrations of TMB, but constant H2O2 (To more details, see “ESM”). The primary rates of reaction for each series were fitted to the double reciprocal of the Michaelis–Menten equation (Fig. S4):
Where υ, Vmax (in 10−8 M·s−1), [S], and K m (mM) show the primary and highest reaction rate, reagent concentration and Michaelies-Menten constant, respectively. K m and V max values for each catalyst were calculated using the intercept and slope of linear Lineweaver-Burk graphs (Fig. S4c, S4d). The obtained values are indicated in Table S1. The lower K m shows a higher tendency of mixed catalyst to the substrates. As can be seen from the data in Table S1, the simultaneous presence of WS2 NS and AgNCs led to the lowest K m values for both TMB and H2O2. Consequently, very small amounts of substrates were required to obtain maximum effectiveness. On the other hand, the data were compared with obtained values for HRP (Table S1); the lower K m value for mixed mimetic catalyst indicated their superior performance in TMB oxidation by H2O2.
Fluorescence studies
The fluorometric investigations were performed by well-known TA–H2O2 reaction. H2O2 can oxidize TA molecules to generate its hydroxylated product (2-hydroxy terephthalic acid, HTA) which has a high fluorescence emission at 425 nm (λex = 315 nm). The performance of reaction is very low and an efficient peroxidase catalyst is necessary to obtain a high emission. Here, it was found that WS2 NS had a great accelerating effect on the system. In the presence of WS2 NS, H2O2 oxidized TA molecules and a high fluorescence emission was achieved which increased during the reaction time, assigning to HTA production (Fig. 4). Moreover, the catalytic activity of WS2 NS was remarkably promoted by addition of AgNCs to reaction medium. The detected fluorescence signal from TA-H2O2 solution using WS2 NS (80 mg·L−1)-AgNCs (8 mg·L−1) mixture was stronger than AgNCs (8 mg·L−1) or WS2 NS (80 mg·L−1) alone. The performance of two catalyst was compared using their optimized amounts to consider their highest effect. Furthermore, in the absence of H2O2 or applied catalyst, an insignificant emission intensity was obtained (Fig. 4).
The fluorescence spectra of TA (2 mM)-H2O2 (1 mM) solution in the absence (a) and presence of (b) AgNCs (8 mg·L−1), (c) WS2 NS (80 mg·L−1), and (d) WS2 NS (80 mg·L−1) + AgNCs (8 mg·L−1); [the pH was adjusted to 7 by 0.02 M phosphate buffer and the spectra were plotted after a time period of 10 min, λex = 315 nm]
Moreover, the experiments using TA-H2O2 solution in the presence of bulk WS2 and Ag indicated relatively lower fluorescence signals. This showed that the nanostructure of applied catalyst is responsible for their high performance.
Electrochemical studies
Electrochemical experiments were also applied to additional investigation of the mimetic behavior of WS2 NS, AgNCs or their mixture. The modified GCEs were prepared by mentioned process in “Experimental” section. The response of electrodes was assessed for H2O2 reduction using CV and amperometry techniques (Fig. 5). No obvious current was observed without H2O2 addition. In the presence of H2O2, a notable current was achieved which verified the effectual transferring of electron from electrode to H2O2 molecules demonstrating the electro-catalytic activity of applied catalyst in the reduction of H2O2. The obtained current for the modified GCE by mixed WS2 NS-AgNCs (3 mg containing 2 mg WS2 NS and 1 mg AgNCs) was greater than others (each 3 mg) by several times, indicating the synergetic catalytic activity of two applied nanomaterials. It is worth mentioning that the optimum amounts for electrochemical experiments were different from colorimetric or fluorescence methods, maybe due to their different practical condition. The experiments for optical analysis were done in a solution containing the dispersed catalyst. However, in electrochemical processes, the catalyst was immobilized on the electrode.
Also, the experiments by bulk WS2 and Ag showed a very low effect on observed current. So, the electrocatalytic effect in modified GCEs should be referred to nanostructures of WS2 NS, AgNCs and also their interactions.
Chemiluminescence studies
This H2O2-bicarbonate system is well-known as an ultra-weak CL system which was frequently studied in the presence of different nano-catalyst [26, 27]. Preliminary experiments indicated the enhancing effect of WS2 NS on the CL emission intensity of H2O2-bicarbonate reaction. The further investigation revealed the synergetic effect of AgNCs on the catalytic activity of WS2 NS in CL system, too. Time profiles of CL emission (Fig. 6a) confirmed the catalytic action of WS2 NS and AgNCs. In the presence of applied nanomaterials, the maximum emission intensity was reached after about 20–25 s. In contrast, the maximum CL emission without any catalyst was achieved in longer times (75 s). Also, the CL experiments using H2O2-bicarbonate reaction in the presence of precursor reagents of synthesized nanomaterials showed no practical enhancing effect on emission intensity (Table S2). Therefore, the observed catalytic activity was ascribed to the presence of WS2 NS and AgNCs.
a) Kinetic profiles of H2O2-NaHCO3 CL system in the absence (a) and presence of (b) AgNCs (8 mg·L−1), (c) WS2 NS (80 mg·L−1) and (d) WS2 NS (80 mg·L−1) + AgNCs (8 mg·L−1); the inset shows the a in magnified scale [NaHCO3 (0.8 M) and H2O2 (50 μM)]. b) CL emission spectra for bicarbonate-H2O2 system in the (a) absence and (b) presence of WS2 NS and AgNCs; the spectra obtained in a flow mode using a spectrofluorimeter with turned off excitation source and emission slit width of 20 nm. Bicarbonate (1 M), WS2 NS (120 mg·L−1) and AgNCs (15 mg·L−1) in one line and H2O2 (0.5 mM) in second line were pumped (12 mL·min−1) into a flow-cell
In order to recognize the emitting species in the offered H2O2-bicarbonate-WS2 NS-AgNCs CL system, CL intensities were recorded in different wavelengths using a flow-based spectrofluorimeter. Bicarbonate (1 M) and catalyst (120 mg·L−1 WS2 NS and 15 mg·L−1 AgNCs) in one line and H2O2 (0.5 mM) in second line were pumped (12 mL·min−1) into a flow-cell in the sample holder of spectrofluorimeter (Because of a different flow system to plot CL spectra by spectrofluorimeter, different optimal amounts of reagents were used). The emission slit width was 20 nm and excitation source was turned off. The obtained CL spectra are indicated in Fig. 6b which consisted of a peak with maximum intensity at about 495 nm. On the other hand, the mechanism of H2O2-bicarbonate to produce CL emission has been frequently described by various catalysts [26, 27]. A similar mechanism for the emission of H2O2-bicarbonate-WS2 NS-AgNCs system can be suggested based on (O2)2* emitter species [28]. As previously reported, H2O2 oxidize the bicarbonate to peroxymonocarbonate ion (HCO4−) (Eq. 1). HCO4−can be decomposed and produce O2•- and •OH radicals (Eqs. 2, 3, and 6) which are key species in CL emission generation. Other method to create these radicals is the decomposition of H2O2 to •OH radicals which then reacted with other H2O2 molecules to yield HO2• radicals (Eqs. 4 and 5). HO2• releases its proton and HCO3− promotes this reaction to produce O2•- (Eqs. 6 and 7). High reactivity of O2•- and •OH radicals can cause the generation of singlet oxygen molecular pair (O2)2* (Eq. 8), and excited (CO2)2* (Eq. 9a, b) which have been believed as the main CL emitters (Eq. 10) [28].
As it can be observed in Fig. 6b, WS2 NS and AgNCs did not alter the maximum wavelength of CL emission. Therefore, the emitter species were the same (O2)2* and (CO2)2* and a similar path was passed to generate CL. On the other hand, the static experiments showed the high affinity of WS2 NS and AgNCs to H2O2. Consequently, it can be considered that WS2 NS and AgNCs catalyzed the H2O2 decomposition in Eq. 4 or its reaction with bicarbonate (Eq. 1) by their adsorption that enriched the •OH and O2•- radicals in the reaction medium. It produced a high CL emission in the presence of WS2 NS, AgNCs or both of them.
Synergistic effect of WS2 NS and AgNCs
The synergistically peroxidase-like catalytic activity of WS2 NS and AgNCs can be discussed using their great effectiveness for the decomposition of H2O2 molecules to produce a high number of OH• radicals which increase the rate of oxidation process. It has been reported that the combination of transition metal dichalcogenides with a noble metal nanostructure leads to the charge separation in their interface area [29, 30]. This is because of a given difference between the Fermi energy levels of WS2 and AgNCs [29], which cause a band bending and electron transferring between them. In this state, a free charge space is generated at the interface area of two nanomaterial. In other words, a charge separation is occurred between them so that, WS2 nanosheets carry the negative charges on their surface and positive charges are on the surface of AgNCs [29]. This phenomenon can improve the electronic structure of WS2 leading to increase its catalytic activity. It means that AgNCs enhanced the creation of hydroxyl radicals by its supporting effect on the electron transferring process on the surface of WS2 NS. The interaction of WS2 NS with AgNCs was resulted to the quenching effect of WS2 NS on the fluorescence emission of AgNCs (Fig. S5a). Furthermore, the TEM image of WS2 NS-AgNCs solution verified their interaction (Fig. S5b).
Chemiluminescence system for the determination of H2O2 and glucose
In order to applicate the introduced peroxidase nano-mimetics in analytical area, it was used for the determination of H2O2 and glucose. The above mentioned CL system was selected as detection method due to the higher sensitivity of stop-flow CL techniques comparing to other investigated methods. The glucose detection is also based on its enzymatic oxidation by free O2 in the presence of glucose oxidase (GOx) to produce gluconic acid and H2O2. The process was followed by injection of produced H2O2 to CL flow-cell on bicarbonate solution in the presence of WS2 NS and AgNCs as catalysts. As the first CL emission was observed, the reagents streams were stopped by switching off the peristaltic pump. The emitted CL intensity was recorded against time and its maximum intensity was used as detection signal.
On the other hand, the optimization of key factors, either in the enzymatic process or chemiluminescence detection step, were performed to achieve highest sensitivity (See supporting information, Fig. S6). As can be seen from the results, the optimum signal was related to 10 U·mL−1 GOx at 6.6 mM acetate buffer and pH 4.4. Also, the CL detection process is better to be done by 0.8 M bicarbonate and 80 mg·L−1 WS2 NS and 8 mg·L−1 AgNCs. The higher amounts of AgNCs caused a decrease in signal maybe because of its tendency to react with hydroxyl radicals to produce AgOH.
Analytical figures of merit
After the optimization process, the emitted CL intensity was plotted against the concentration of H2O2 (Fig. S7a) as calibration graph. A linear relationship was obtained for the determination of H2O2 in the concentration range of 2.5–1500 nM (Fig. S7b) with detection limit (3Sb/m, m: slope of calibration graph, and Sb: standard deviation of signals for repetitive determination of 5 blank solutions) of 0.6 nM. An overview on some reported nanomaterial-based methods for the quantitation of H2O2 is provided in Table 1 which show the great features of presented method. Also, in order to compare the sensitivity of different techniques (described above), their linear ranges and detection limits for the detection of H2O2 are listed in Table S3. The comparison disclosed the high sensitivity and wide linear range of CL method. Consequently, it was applied for glucose measurement, which got a linear calibration graph over the concentration range of 0.03–20 μM glucose (Fig. S8). The linear relationship was obtained as ICL = 52,251 C + 3798 (R2 = 0.9997), where ICL is the CL emission intensity recorded for injection of different concentrations (C) of glucose in nM. The limit of detection was calculated for glucose which was equal to 13 nM. The comparison of analytical figures of merit for the introduced CL-based method with some other reported approaches is indicated in Table S4. It shows that the presented method provides a comparatively good sensitivity with a lower detection limit. On the other hand, this method is a reliable and rapid method for the determination of glucose which can be developed for other biomolecules.
In order to investigate the precision of introduced method, relative standard deviation (RSD) was calculated for the repeated measurement of five standard solutions containing a similar concentration of glucose. The RSD values were obtained 2.8 and 4.1% for the 0.2 and 15 μM glucose solutions, respectively. The stability of CL solution was also studied during the time (See ESM).
Selectivity
The selectivity of the manufactured bio-assay, was studied by the determination of a constant concentration (1 μM) of glucose, in the presence of possible interfering compounds in different concentrations. The maximum concentration of each species with an interfering effect of less than 5% in the signal of glucose, was considered as its tolerance limit (Fig. S9). As can be seen from the data, the tested compounds with indicated concentration in Fig. S9 showed no significant interfering effect on the response of bio-assay. Moreover, a 1:500 dilution of real samples was helpful in more elimination of interfering compound’s effects, so that the most of tested species did not show any considerable interfering effect, even in a higher concentration (5 mM).
On the other hand, the experiments showed that the presence of relatively higher concentrations of usual cations or anions caused no considerable effect on the probe signals (Table S5). These results verified the applicability of designed assay for recognizing the glucose level in real blood samples which is described in the next section.
Analysis of real samples
The reliability and validation of introduced probe was investigated by its application for the analysis of glucose level in human blood. The analysis was performed using standard addition process (Tables S6). For more validation, the method was used for the analysis of spiked samples which were prepared by adding definite volumes of glucose standard solution into the real sample, before any pretreatment process.
The calculated recoveries (Table S6) revealed the good reliability of method for the determination of glucose in real samples. Also, for a more validation of here-described probe, a standard reference material (SRM 1952a-2, frozen serum) was analyzed (Table S7). The achieved result (5.79 ± 0.09 mM) showed a good agreement with certified value (5.93 ± 0.10 mM) for SRM which allotted by the National Institute of Standards and Technology (NIST).
Conclusions
In summary, the improving effect of Ag nanoclusters on the peroxidase-like catalytic activity of WS2 nanosheets was proved by colorimetry, electrochemical, fluorescence and chemiluminescence techniques. It was showed that the simultaneous adding of WS2 NS and AgNCs caused a synergetic mimetic effect on the H2O2 reduction-based reactions. They provided a good activity compared to HRP, leading to a high sensitivity in the determination of H2O2. Based on this observation, a rapid and ultrasensitive assay for the determination of H2O2 was designed using efficient H2O2-bicabonate-WS2 NS/AgNCs chemiluminescence detection system. Furthermore, the system was applied for the analysis of glucose level in plasma, after its oxidation by glucose oxidase. The method had good specificity and showed satisfying results for a SRM sample.
References
Su Y, Deng D, Zhang L, Song H, Lv Y (2016) Strategies in liquid-phase chemiluminescence and their applications in bioassay. TrAC Trends Anal Chem 82:394–411
Khataee A, Lotfi R, Hasanzadeh A, Iranifam M, Joo SW (2016) Flow-injection chemiluminescence analysis for sensitive determination of atenolol using cadmium sulfide quantum dots. Spectrochim Acta A 157:88–95
Trojanowicz M, Kołacińska K (2016) Recent advances in flow injection analysis. Analyst 141:2085–2139
Su Y, Xie Y, Hou X, Lv Y (2014) Recent advances in analytical applications of nanomaterials in liquid-phase chemiluminescence. Appl Spectrosc Rev 49:201–232
Nasir M, Nawaz MH, Latif U, Yaqub M, Hayat A, Rahim A (2017) An overview on enzyme-mimicking nanomaterials for use in electrochemical and optical assays. Microchim Acta 184:323–342
Wei H, Wang E (2013) Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev 42:6060–6093
Yang B, Li J, Deng H, Zhang L (2016) Progress of mimetic enzymes and their applications in chemical sensors. Crit Rev Anal Chem 46:469–481
Shin HY, Kim B-G, Cho S, Lee J, Na HB, Kim MI (2017) Visual determination of hydrogen peroxide and glucose by exploiting the peroxidase-like activity of magnetic nanoparticles functionalized with a poly (ethylene glycol) derivative. Microchim Acta 184:2115–2122
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2:577–583
Hu Y, Huang Y, Tan C, Zhang X, Lu Q, Sindoro M, Huang X, Huang W, Wang L, Zhang H (2017) Two-dimensional transition metal dichalcogenide nanomaterials for biosensing applications. Mater Chem Front 1:24–36
Li BL, Wang J, Zou HL, Garaj S, Lim CT, Xie J, Li NB, Leong DT (2016) Low-dimensional transition metal dichalcogenide nanostructures based sensors. Adv Funct Mater 26:7034–7056
Cheng L, Liu J, Gu X, Gong H, Shi X, Liu T, Wang C, Wang X, Liu G, Xing H (2014) PEGylated WS2 nanosheets as a multifunctional theranostic agent for in vivo dual-modal CT/photoacoustic imaging guided photothermal therapy. Adv Mater 26:1886–1893
Cao X, Tan C, Zhang X, Zhao W, Zhang H (2016) Solution-Processed Two-Dimensional Metal Dichalcogenide-Based Nanomaterials for Energy Storage and Conversion. Adv Mater 28:6167–6196
Wang QH, Kalantar-Zadeh K, Kis A, Coleman JN, Strano MS (2012) Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat Nanotechnol 7:699–712
Lu Q, Yu Y, Ma Q, Chen B, Zhang H (2016) 2D transition-metal-dichalcogenide-nanosheet-based composites for photocatalytic and electrocatalytic hydrogen evolution reactions. Adv Mater 28:1917–1933
Zhao W, Ghorannevis Z, Chu L, Toh M, Kloc C, Tan P-H, Eda G (2012) Evolution of electronic structure in atomically thin sheets of WS2 and WSe2. ACS Nano 7:791–797
Lin T, Zhong L, Song Z, Guo L, Wu H, Guo Q, Chen Y, Fu F, Chen G (2014) Visual detection of blood glucose based on peroxidase-like activity of WS2 nanosheets. Biosens Bioelectron 62:302–307
Chen Q, Chen J, Gao C, Zhang M, Chen J, Qiu H (2015) Hemin-functionalized WS2 nanosheets as highly active peroxidase mimetics for label-free colorimetric detection of H2O2 and glucose. Analyst 140:2857–2863
Tao Y, Li M, Ren J, Qu X (2015) Metal nanoclusters: novel probes for diagnostic and therapeutic applications. Chem Soc Rev 44:8636–8663
Sheng Y, Yang H, Wang Y, Han L, Zhao Y, Fan A (2017) Silver nanoclusters-catalyzed luminol chemiluminescence for hydrogen peroxide and uric acid detection. Talanta 166:268–274
Huang S, Pfeiffer C, Hollmann J, Friede S, Chen JJ-C, Beyer A, Haas B, Volz K, Heimbrodt W, Montenegro Martos JM (2012) Synthesis and characterization of colloidal fluorescent silver nanoclusters. Langmuir 28:8915–8919
Hassanzadeh J, Khataee A (2018) Ultrasensitive chemiluminescent biosensor for the detection of cholesterol based on synergetic peroxidase-like activity of MoS2 and graphene quantum dots. Talanta 178:992–1000
Patel AS, Mohanty T (2014) Silver nanoclusters in BSA template: a selective sensor for hydrogen peroxide. J Mater Sci 49:2136–2143
Yang Z, Gao D, Zhang J, Xu Q, Shi S, Tao K, Xue D (2015) Realization of high Curie temperature ferromagnetism in atomically thin MoS2 and WS2 nanosheets with uniform and flower-like morphology. Nano 7:650–658
Jw S, Yw J, Sw P, Nah H, Moon T, Park B, Kim JG, Kim YJ, Cheon J (2007) Two-dimensional nanosheet crystals. Angew Chem Int Ed 46:8828–8831
Chen H, Lin L, Lin Z, Lu C, Guo G, Lin J-M (2011) Flow-injection analysis of hydrogen peroxide based on carbon nanospheres catalyzed hydrogen carbonate–hydrogen peroxide chemiluminescent reaction. Analyst 136:1957–1964
Chen H, Li R, Li H, Lin J-M (2012) Plasmon-assisted enhancement of the ultraweak chemiluminescence using Cu/Ni metal nanoparticles. J Phys Chem C 116:14796–14803
Liang S-X, Zhao L-X, Zhang B-T, Lin J-M (2008) Experimental studies on the chemiluminescence reaction mechanism of carbonate/bicarbonate and hydrogen peroxide in the presence of cobalt (II). J Phys Chem A 112:618–623
Cheah A, Chiu W, Khiew P, Nakajima H, Saisopa T, Songsiriritthigul P, Radiman S, Hamid MAA (2015) Facile synthesis of a Ag/MoS2 nanocomposite photocatalyst for enhanced visible-light driven hydrogen gas evolution. Catal Sci Technol 5:4133–4143
Yang L, Zhong D, Zhang J, Yan Z, Ge S, Du P, Jiang J, Sun D, Wu X, Fan Z (2014) Optical properties of metal–molybdenum disulfide hybrid nanosheets and their application for enhanced photocatalytic hydrogen evolution. ACS Nano 8:6979–6985
Yu H, Long D (2016) Highly chemiluminescent metal-organic framework of type MIL-101 (Cr) for detection of hydrogen peroxide and pyrophosphate ions. Microchim Acta 183:3151–3157
Li M, Gao H, Wang X, Wang Y, Qi H, Zhang C (2017) A fluorine-doped tin oxide electrode modified with gold nanoparticles for electrochemiluminescent determination of hydrogen peroxide released by living cells. Microchim Acta 184:603–610
Muhr V, Buchner M, Hirsch T, Jovanović DJ, Dolić SD, Dramićanin MD, Wolfbeis OS (2017) Europium-doped GdVO4 nanocrystals as a luminescent probe for hydrogen peroxide and for enzymatic sensing of glucose. Sens Actuat B 241:349–356
Cui M, Zhou J, Zhao Y, Song Q (2017) Facile synthesis of iridium nanoparticles with superior peroxidase-like activity for colorimetric determination of H2O2 and xanthine. Sens Actuat B 243:203–210
Wu S, Kong X-J, Cen Y, Yuan J, Yu R-Q, Chu X (2016) Fabrication of a LRET-based upconverting hybrid nanocomposite for turn-on sensing of H2O2 and glucose. Nano 8:8939–8946
Acknowledgements
We thank University of Tabriz and Near East University for the support provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
Electronic Supplementary Material (ESM): FTIR and XRD spectra of synthesized nanostructures, static study of peroxidase-like activity of nanostructures, optimizations graphs, Interfering results, calibration graph for H2O2. (DOCX 13749 kb)
Rights and permissions
About this article
Cite this article
Khataee, A., Haddad Irani-nezhad, M. & Hassanzadeh, J. Improved peroxidase mimetic activity of a mixture of WS2 nanosheets and silver nanoclusters for chemiluminescent quantification of H2O2 and glucose. Microchim Acta 185, 190 (2018). https://doi.org/10.1007/s00604-018-2727-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2727-4