Abstract
The authors describe a lateral flow assay (LFA) for the antibody against the infectious bacterium Clostridium tetani. Gold nanoparticles (AuNPs) were linked to tetanus antigen and are captured in the test line via the formation of a sandwich structure composed of AuNP-labeled tetanus antigen, tetanus antibody, and tetanus antigen. This leads to the formation of a characteristic red line due to the accumulation of AuNPs. The formation of the color line allows for a highly sensitive and selective detection of tetanus antibody, both with bare eyes and by smartphone-based quantitative analysis. This assay offers a wide detection range from 0 to 0.5 IU·mL−1 and has a linear relationship from 0.01 to 0.1 IU·mL−1 with an experimental detection limit of 0.01 IU·mL−1. This assay is simple, fast, inexpensive and highly selective. When applied to the detection of tetanus antibody in spiked whole blood, it provided reliable results that compared well to those obtained with a commercial ELISA kit.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tetanus is a fatal disease caused by the anaerobic Gram-positive bacterium named Clostridium tetani [1]. The bacterium can easily infect human with injuries, fractures, rusty nails or wood stab wounds and induce tetanus neurotoxin [2]. At present, the most effective means against the disease is vaccination of human tetanus immunoglobulin [3,4,5]. The level of tetanus antibody in human body indicates the individual immunity [6,7,8], and with lower-than-normal levels people may easily be infected with tetanus particularly in the case of injury [9]. Thus, quantification of the tetanus antibody level in human body is important in the assessment of individual immunity for early diagnosis and therapy. Currently, efforts have been made to design some methods for detecting tetanus antitoxin in vitro, such as enzyme-linked immunesorbent assay (ELISA) [10, 11], gel electrophoresis-based immunoassay [12], fluorometric immunoassay [13, 14], radioimmunoassay [15, 16], and microfluidic platform [17, 18]. Although these techniques obtained successful results, there are still some hindrances including the utilization of radioactive substances, specialized equipment, time-consuming analytical procedure, and tedious purification steps before analysis. Therefore, in order to effectively detect tetanus infection in developing countries, it is highly desirable to develop a low-cost, simple and highly sensitive method for tetanus antibody detection.
Lateral flow test strip (LFTS) assays [19, 20] have received increasing attention due to their excellent performance. The strip usually consists of four parts: a sample pad, a conjugate pad, a nitrocellulose (NC) membrane and an absorbent pad, which minimizes the requirements for highly qualified personnel and eliminates complex analysis procedures with expensive equipment. Therefore, LFTS has been widely used in medical test [21], food quality monitor [22], poison detection [23] and environment supervision [24]. Colloidal gold nanoparticles with a typical absorption spectrum at the range of 400–800 nm are frequently used as reporters for colorimetric detection [25]. In the past several years, various colorimetric immunochromatographic test strips based on AuNPs have been developed for ions, toxins and biomolecules including antibodies and antigens [26,27,28]. By using monoclonal antibody (MAb)-functionalized AuNPs as signal reporters, Wang et al. [29] reported a rapid immunochromatographic lateral flow test strip in a sandwich format for the rapid detection of the food allergen glycinin in powdered Milk and the detection limit was as low as 0.69 mg·kg−1. Zhang et al. [30] proposed the AuNP-based immunochromatographic test strip assay for simultaneous detection of total aflatoxins in peanuts. Ge et al. [31] developed an enhanced strip biosensor with AuNP-DNA for the rapid detection of H3K9me3 in HeLa cells. To the best of our knowledge, few AuNP-based strips were reported for tetanus antibody up-to-date and it has a great potential to be used in this research/application field.
In this study, we established an AuNP-based test strip platform for quantification of tetanus antibody. Test line and control line were modified by human tetanus and standard human tetanus antibody, respectively. Then, samples that contained tetanus antibody were added onto the sample pad and subsequently a characteristic red band can be observed on the test zone because of the accumulation of AuNPs. A red band also can be observed on the control zone which indicated the feasibility of the test strip. The color intensity of test line deepened with the increase of target antibody concentrations, which can be observed very easily by bare eyes. Furthermore, quantitative detection can also be realized by analyzing the intensity of color through Image J. This assay also has been successfully used for the detection of tetanus antibody in human whole blood samples. We anticipate that this AuNP-based test strip can be utilized as a novel effective individual immunity method for tetanus antibody detection in household and clinical diagnosis.
Experimental
Reagents and materials
HAuCl4·3H2O and trisodium citrate were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China, www.sinoreagent.com). Tetanus antigen was obtained from Fapon Biotech Inc. (Shenzhen, China, faponbiotech.bioon.com). The standard human tetanus antibody, human diphtheria antibody, human pertussis antibody, human measles antibody and rabies virus antibody were obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China, www.bzwzzx.com). Tween-20, Bovine Serum Albumin (BSA), poly (vinyl pyrrolidone) (PVP) and PEG-2000 were purchased from J&K Chemical Ltd. (Shanghai, China, www.jkchemical.com). Tetanus antibody ELISA kit was obtained from Zhengzhou Etebio Technology Co., Ltd. (Zhengzhou, China, www.etebio.com). All buffers and reagent solutions were prepared with ultrapure water generated from a Millipore Milli-Q water purification system (Billerica, MA, USA, www.merckmillipore.com) with an electric resistance of 18.2 MΩ·cm.
Instruments
XYZ-3030 dispenser and CT 200 Cutting System were purchased from Kinbio Tech. Co., Ltd. (Shanghai, China, kinbio.bioon.com.cn). UV-visible absorption spectra were recorded on a TU-1810 ultraviolet and visible spectrophotometer (Beijing Persee Co., Ltd., China, www.pgeneral.com). A circular quartz cell with a path length of 1 cm was used in all experiments.
Preparation of test strip
Both the sample pad (33 mm × 30 cm) and conjugate pad (7 mm × 30 cm) were made from glass fiber. The conjugation pad was prepared by dispensing a desired volume of AuNP-labeled tetanus antigen (AuNP-Ag) onto the glass fiber pad using an XYZ Biostrip Dispenser, followed by drying at 37 °C for 1 h and then stored at 4 °C. The NC membrane (20 mm × 30 cm) was spotted using the same dispenser with the optimal tetanus and standard tetanus antibodies applied in the test and control lines, leaving a 0.5 cm space between the two lines. The absorbent pad was cut to dimensions of 20 mm × 30 cm. The sample pad, conjugate pad, NC membrane and absorbent pad were assembled on a plastic backing support board (60 mm × 300 mm) sequentially with a 1–2 mm overlap. The master card was cut into 3 mm wide strips using a Cutting System CT 200 Cutter. The strip was then sealed in a plastic bag with desiccant gel and stored at 4 °C.
Detection of tetanus antibody
The detection of tetanus antibody was carried out by dropping appropriate tetanus antibody solution on the sample pad of the strip. After 16 min, the results of test line were observed by bare eyes. For quantitative measurements, the optical intensities of the test line and control line were obtained by using Image J. The digital images of the LFTS were captured with a Smartphone. After opened in Image J, the images were converted to 32-bit formats using Image/Type 32-bit command and exaggerated the contrast with Process/Enhance contrast command. In the image window, the test line was amplified using the magnifying glass and outlined with the rectangular selection tool, and then the signal was obtained through the Analyze/Measure command. The signal difference was realized by dragging the rectangular selection to blank position and using the measure command again.
Results and discussion
The design principle of LFTS
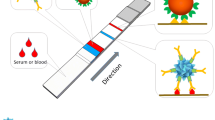
Scheme 1 illustrates the principle of AuNP-based LFTS for the detection of tetanus antibody. Typically, the sample solution containing target antibody is dropped onto the sample pad and then migrates along the pad and NC membrane by capillary action. Target antibody binds to tetanus on the surface of AuNPs by forming complex (AuNP-tetanus-tetanus antibody), and the complex continues to migrate along the strip. When reaching the test zone, the complex is captured by the tetanus immobilized on the test zone and a characteristic red band appears because of the accumulation of gold nanoparticles. In addition, once the sample solution passes through the control zone, the excess AuNP-Ag conjugates are captured by the standard tetanus antibodies, and thus a second red band appears on the control line. In the absence of target antibody, the red band only can be observed on the control line. Based on this response principle, tetanus antibody analysis can be simply performed by observing the color change of the test zone with bare eyes and/or Smartphone.
Optimization of the LFTS parameters
AuNPs were prepared according to the previous report [32], and the image of AuNPs was taken by a digital camera (Canon, EOS 6D, Japan). In order to investigate the morphological structure of the as-prepared AuNPs, AuNPs were characterized by Transmission electron microscopy (TEM) and UV-vis spectroscopy. As shown in Fig. 1a, the TEM image shows that AuNPs are spherical shape and possess a uniform size distribution. Figure 1b displays the UV-vis absorption spectrum of AuNPs, the as-prepared AuNPs have a typical absorption peak at 524 nm and the AuNP solution appears red and transparent. These properties indicated that the size of AuNPs was well-distributed and can provide the best basis for preparation of AuNP-labeled antigen probe for the test strip. In order to investigate the effect of acidity on the conjugation between AuNPs and tetanus, the following parameters were optimized: (a) volume of K2CO3; (b) concentration of antigen; (c) diluted ratios of AuNP-Ag on the conjugate pad; (d) amount of tetanus on the test line (Respective data and figures are given in the Electronic Supporting Material). The following experimental conditions were found to give best results: (a) volume of K2CO3 is 20 μL; (b) concentration of tetanus is 28 μg; (c) the optimal diluted ratios of AuNP-Ag on the conjugate pad is 1:3 (d) the optimal concentration of tetanus on the test line is 1.4 mg·mL−1.
Feasibility of the strip assay
Different concentrations (0 IU·mL−1, 0.04 IU·mL−1, 0.08 IU·mL−1, and 0.2 IU·mL−1) of tetanus antibody were added to the sample pad and the results was recorded by typical photo images (Fig. 2). From the results we can see that there is no test band observed without tetanus antibody. Whereas, in the presence of tetanus antibody, an obvious red band immediately appears on test line and a dramatic deepen in color intensity is observed as the tetanus antibody concentration increases, indicating that this AuNP-based LFTS can be used for the detection of tetanus antibody.
Sensitivity
The effect of immunoreaction time on the performance of the sensing strip was further investigated by recording the intensity of the test line at each time point with Image J. As shown in Fig. S3, the intensity reaches plateau after 16 min. Consequently, 16 min was chosen as the optimal assay time for the following experiments. Under optimized detection conditions, the sensitivity of the test strip sensor was investigated. The image of the test line was captured by a Smartphone and the color intensity was quantified by the software Image J. The error bars were calculated with three duplicated measurements of analysis. As shown in Fig. 3a, with the increasing of tetanus antibody concentrations from 0 IU·mL−1 to 0.5 IU·mL−1, more and more sandwich structures of AuNP-labeled tetanus-tetanus antibody-tetanus are formed and the red band on the test line can be obviously observed by bare eyes. Fig. 3b describes the relationship between the color intensity and the concentrations of tetanus antibody, and inset of Fig. 3b shows a good linear correlation in the range of 0.01 ~ 0.1 IU·mL−1 with a correlation coefficient square of 0.9916. These results show that this assay can be reliably employed for highly sensitive tetanus antibody detection down to 0.01 IU·mL−1, which is well qualified for the detection standard from the World Health Organization.
a The image of test strip with the increasing concentrations of tetanus antibody in the range from 0 to 0.5 IU·mL−1. b Linear relationship between color intensity and target antibody concentrations, where the value was obtained directly by quantifying the color intensity of test zone images through Image J
Specificity of the test strip
Besides sensitivity, specificity is another important parameter for a new test strip with potential applications in practical biological samples. The selectivity experiments were extended to various antibodies, including diphtheria, pertussis, measles and rabies. As shown in Fig. 4, only tetanus antibody can produce a significant red band on the test line, while other antibodies result in no red band appeared on the test line even at 5-fold concentration of target antibody. These results demonstrated that this test strip assay has a high specificity to tetanus antibody and can be applied to sensing tetanus antibody in real samples.
Stability of the assay
Stability of a lateral flow test strip is critical to its sensing applications in biological system. Therefore, to evaluate the stability of the LFTS, test strips were stored in dry environment at 4 °C for 0 day, 1 week, 1 month and 6 months, respectively. Subsequently, the stored strips were tested with different concentrations of tetanus antibody (0、0.01、 0.02、0.04、0.08、0.1 IU·mL−1). As shown in Fig. S4, the color density of the test line maintains almost the same as the freshly fabricated strip even after 6 months, indicating its great stability and promise in commercial applications in the near future.
Application to human blood samples
In order to further verify the practicality of this test strip in complicated biological samples, tetanus antibody in 10 human whole blood samples without any pretreatment was detected. The whole blood samples were collected by the first affiliated hospital of Zhengzhou University and informed consent was obtained for the use of human blood. All experiments were performed in compliance with the relevant laws and institutional guidelines and approved by Life-Science Ethics Review Committee of Zhengzhou University. Standard commercial ELISA kit was utilized as comparison. Experimental results showed that the response of the strips to human whole blood samples coincided well with commercial ELISA kit (Table 1). These results demonstrated that this strip assay has good reliability and can be applied to the practical application of tetanus antibody detection in real samples. In order to analyze the performance of this test strip assay, we compared our strategy with other nanomaterial-based methods for the determination of tetanus antibody (Table S5). According to World Health Organization, tetanus antitoxin levels of 0.01 IU·mL−1 and below is considered to be non-protective antibody level [33]. As can be seen from Table S5, detection limit of this strip assay is 0.01 IU·mL−1, which is comparable to other nanomaterial-based methods and indicates its ability to distinguish positive and negative samples with some advantages including rapid, convenient, cost-effective, portable, and no requirement of any complicated instruments, etc.
Conclusion
In summary, an AuNP-based colorimetric LFTS assay has been used for tetanus antibody detection. This assay showed short duration of sensing and high selectivity for tetanus antibody with detection limit of 0.01 IU·mL−1, indicating its ability to distinguish positive and negative samples, whereas it should be noted that this sensitivity is not the highest compared with the reported assay and highly desirable to be further improved. This system was also successfully used to quantify tetanus antibody in human whole blood and obtains a reliable result compared with standard commercial ELISA kit, which demonstrates its great potential to become a useful tool for analysis of tetanus antibody in clinical diagnostics, individual immunity evaluations and other biomedical applications.
References
Blow N (2008) Lab automation: tales along the road to automation. Nat Methods 5:109–112
Duerden BI, Brazier JS (2009) Tetanus and other clostridial diseases. Medicine 37:638–640
Gall SA, Myers J, Pichichero M (2011) Maternal immunization with tetanus–diphtheria–pertussis vaccine: effect on maternal and neonatal serum antibody levels. Am J Obstet Gynecol 204:334.e1–334.e5
Shirkey HC (1965) Tetanus immune globulin (human) in prophylaxis against tetanus. J Pediatri 67:643–646
Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, Holland LA, Weir S, Noah TL, Beck MA (2012) Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes 36:1072–1077
Vollman KE, Acquisto NM, Bodkin RP (2014) A case of tetanus infection in an adult with a protective tetanus antibody level. Am J Emerg Med 32:392.e3–392.e4
Jain S, Chattopadhyay S, Jackeray R, Abid CKVZ, Kumar M, Singh H (2010) Detection of anti-tetanus toxoid antibody on modified polyacrylonitrile fibers. Talanta 82:1876–1883
Zarei H, Ghourchian H, Eskandari K, Zeinali M (2012) Magnetic nanocomposite of anti-human IgG/COOH–multiwalled carbon nanotubes/Fe3O4 as a platform for electrochemical immunoassay. Anal Biochem 421:446–453
King CA, Spellerberg MB, Zhu D, Rice J, Sahota SS, Thompsett AR, Hamblin TJ, Radl J, Stevenson FK (1998) DNA vaccines with single-chain Fv fused to fragment C of tetanus toxin induce protective immunity against lymphoma and myeloma. Nat Med 4:1281–1286
Ramakrishnan G, Pedersen K, Guenette D, Sink J, Haque R, Petri WA Jr, Herbein J, Gilchrist CA (2015) Utility of recombinant fragment C for assessment of anti-tetanus antibodies in plasma. Diagn Microbiol Infect Dis 82:11–13
Gajdos V, Vidor E, Richard P, Tran C, Sadorge C (2015) Diphtheria, tetanus and poliovirus antibody persistence 5 years after vaccination of pre-schoolers with two different diphtheria, tetanus and inactivated poliomyelitis vaccines (td-IPV or DT-IPV) and immune responses to a booster dose of DTaP-IPV. Vaccine 33:3988–3996
Herr AE, Throckmorton DJ, Davenport AA, Singh AK (2005) On-chip native gel electrophoresis-based immunoassays for tetanus antibody and toxin. Anal Chem 77:585–590
Schroder JP, Kuhlmann WD (1991) Detection of tetanus antitoxin using Eu3+ −labelled anti-human immunoglobulin G monoclonal antibodies in a time-resolved fluorescence immunoassay. J Clin Microbiol 29:1504–1507
Maple PAC, Jones CS, Andrews NJ (2001) Time resolved fluorometric immunoassay, using europium labelled antihuman Ig G, for the detection of human tetanus antitoxin in serum. J Clin Pathol 54:812–815
Stiffier-Rosenberg G, Fey H (1975) Radioimmunological measurement of tetanus antitoxin. Schwiz Med Wochenschr 105:804–810
Layton GT (1980) A micro-enzyme-linked immunosorbent assay (ELISA) and radioimmunosorbent technique (RIST) for the detection of immunity to clinical tetanus. Med Lab Sci 37:323–329
Golberg A, Yarmush ML, Konry T (2013) Picoliter droplet microfluidic immunosorbent platform for point-of-care diagnostics of tetanus. Microchim Acta 180:855–860
Xu Y, Liu M, Kong N, Liu J (2016) Lab-on-paper micro-and nano-analytical devices: fabrication, modification, detection and emerging applications. Microchim Acta 18:1521–1542
Quesada-González D, Merkoçi A (2015) Nanoparticle-based lateral flow biosensors. Biosens Bioelectron 73:47–63
Li ZH, Wang Y, Wang J, Tang ZW, Pounds JG, Lin YH (2010) Rapid and sensitive detection of protein biomarker using a portable fluorescence biosensor based on quantum dots and a lateral flow test strip. Anal Chem 82:7008–7014
Zhang GP, Wang XN, Yang JF, Yang YY, Xing GX, Li QM, Zhao D, Chai SJ, Guo JQ (2006) Development of an immunochromatographic lateral flow test strip for detection of beta-adrenergic agonist Clenbuterol residues. J Immuno Methods 312:27–33
Li YS, Zhou Y, SY L, Guo DJ, Ren HL, Meng XM, Zhi BH, Lin C, Wang Z, Li XB, Liu ZS (2012) Development of a one-step test strip for rapid screening of fumonisins B1, B2 and B3 in maize. Food Control 24:72–77
Bidmanova S, Steiner MS, Stepan M, Vymazalova K, Gruber MA, Duerkop A, Damborsky J, Prokop Z, Wolfbeis OS (2016) Enzyme-based test strips for visual or photographic detection and quantitation of gaseous sulfur mustard. Anal Chem 88:6044–6049
Tang Y, Zhai YF, Xiang JJ, Wang H, Liu B, Guo CW (2010) Colloidal gold probe-based immunochromatographic assay for the rapid detection of lead ions in water samples. Environ Pollut 158:2074–2077
Huang X, Aguilar ZP, Xu H, Lai W, Xiong Y (2016) Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: a review. Biosens Bioelectron 75:166–180
Zhong Y, Chen Y, Yao L, Zhao D, Zheng L, Liu G, Ye Y, Chen W (2016) Gold nanoparticles based lateral flow immunoassay with largely amplified sensitivity for rapid melamine screening. Microchim Acta 183(6):1989–1994
Wang L, Cai J, Wang Y, Fang Q, Wang S, Cheng Q, Du D, Lin Y, Liu F (2014) A bare-eye-based lateral flow immunoassay based on the use of gold nanoparticles for simultaneous detection of three pesticides. Microchim Acta 181:1565–1572
Chen J, Li ZH, Ge J, Yang R, Zhang L, LB Q, Wang HQ, Zhang L (2015) An aptamer-based signal-on bio-assay for sensitive and selective detection of kanamycin a by using gold nanoparticles. Talanta 139:226–232
Wang Y, Deng R, Zhang G, Li Q, Yang J, Sun Y, Li Z, Hu X (2015) Rapid and sensitive detection of the food allergen glycinin in powdered milk using a lateral flow colloidal gold immunoassay strip test. J Agric Food Chem 63:2172–2178
Zhang D, Li P, Zhang Q, Zhang W (2011) Ultrasensitive nanogold probe-based immunochromatographic assay for simultaneous detection of total aflatoxins in peanuts. Biosens Bioelectron 26:2877–2882
Ge C, Yu L, Fang Z, Zeng L (2013) An enhanced strip biosensor for rapid and sensitive detection of histone methylation. Anal Chem 85:9343–9349
Zhao W, Ali MM, Aguirre SD, Brook MA, Li Y (2008) Paper-based bioassays using gold nanoparticle colorimetric probes. Anal Chem 80:8431–8437
Sonmez C, Coplu N, Gozalan A, Akin L, Esen B (2017) Comparison of in-house biotin-avidin tetanus IgG enzyme-linked-immunosorbentassay (ELISA) with gold standard in vivo mouse neutralization test for the detection of low level antibodies. J Immunol Methods 445:67–70
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (21205108, 21605038), the Outstanding Young Talent Research Fund of Zhengzhou University (1421316038), and the Scientific and Technological Project of Henan Province, China (152102410006). We are also thankful to the software Image J for imaging analysis.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 779 kb)
Rights and permissions
About this article
Cite this article
Liu, J., Wang, J., Li, Z. et al. A lateral flow assay for the determination of human tetanus antibody in whole blood by using gold nanoparticle labeled tetanus antigen. Microchim Acta 185, 110 (2018). https://doi.org/10.1007/s00604-017-2657-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2657-6