Abstract
Tetanus still possesses a high infection risk and leads a number of human deaths in poor nations. Point-of-care and ultrasensitive detection of tetanus antibody levels in serum is the key to decrease the risk of tetanus infection and improve the health of people. In this work, by using ultra bright fluorescent nanospheres (FNs) and portable lateral flow test strip (LFTS), a point-of-care and ultrasensitive sensing method has been developed for the detection of tetanus antibodies in human serum. This assay works quite well for tetanus antibodies in the concentration range from 0.0002 to 0.0220 IU/mL with a low detection limit of 0.00011 IU/mL, which is 100-fold lower than conventional gold-based LFTSs. The high sensitivity makes this method suitable for use to detect the low-abundant target in real samples. Besides, this cost-effective FN-based LFTS assay possesses good selectivity, high accuracy, and satisfactory reliability, which holds great potential as a robust candidate for routine medical diagnosis and rapid home testing.

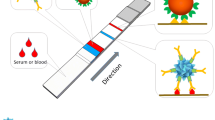
Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tetanus is a fatal disease caused by anaerobic Gram-positive bacterium named Clostridium tetani, which implant in injuries, fractures, rusty nails or wood stab wounds, etc. Clostridium tetani can produce tetanus exotoxin that can block inhibitory synapses on motor neurons in the central nervous system (CNS) [1]. At present, the most effective strategy against tetanus disease is vaccination of human tetanus antibody immunoglobulin [2, 3]. The level of tetanus antibody after vaccination in human body indicates the individual immunity [4], and people with lower-than-minimum protected level (0.01 IU/mL) has lethal risk to be infected with tetanus, particularly in the case of injury [5]. Therefore, it is of great significance to develop highly sensitive and convenient method for the detection of tetanus antibody level in human serum. Currently, many efforts have been made to detect tetanus antibody in vitro, such as enzyme-linked immunosorbent assay (ELISA) [6, 7], fluorometric immunoassay [8, 9], indirect haemagglutination assay [10,11,12], gel electrophoresis–based immunoassay [13], radioimmunoassay [14], and microfluidic platform [15]. Although these conventional strategies exhibit promising results for sensitive detection of tetanus antibody, they are not suitable for point-of-care monitoring in economically lagging regions.
Lateral flow test strips (LFTSs), as the most well-known point-of-care testing (POCT) diagnostic strategy, are particularly attractive for assaying targets due to their fast response ability, portable equipment, and straightforward signal readout [16,17,18]. Taking advantages of the LFTS, our group has constructed a gold nanoparticle (AuNP)–based test strip platform for semi-quantitative and point-of-care testing of tetanus antibody in human whole blood samples [19]. Recently, we also further developed a new quantum dot–based lateral flow test strip for the selective and quantitative detection of tetanus antibody [20]. However, the sensitivity of these methods is not high enough. Moreover, in order to reduce the matrix effect and interference of non-specific response, the real sample often needed diluted many times [21]. So, a highly sensitive, specific, and predictive test strip method is desirable.
Compared with single quantum dot, fluorescent nanospheres (FNs) integrated with QDs have some unique advantages of strong luminescence, durable light excitation, high stability in complex matrix, and easy modification [22]. FNs have been successfully used to detect mycotoxins, proteins, and bacteria. For example, Ren et al. used FNs as immunochromatographic competition assay (ICA) signal amplification signal for ultrasensitive detection of aflatoxin B1 (AFB1) in maize [23]. Pang’s group developed FN-based sandwich lateral flow test strip for sensitive detection of C-reactive protein and Ebola virus glycoprotein [24, 25]. Therefore, these properties indicate that FNs can be used as a robust candidate for ultrasensitive detection of tetanus antibody in complex matrixes.
Herein in this work, by employing the benefits inherited from both LFTS and FNs, we proposed an ultrasensitive assay for rapid detection of tetanus antibody. Based on the formation of the FNs-goat anti-human IgG (Fc)/tetanus antibody/tetanus antigen sandwich structure, the FN-based strip was easily prepared. As shown in Scheme 1, in the presence of the tetanus antibody, the sample was dropped onto the sample pads and migrated along the strip under the capillary effect. The FNs-Fc/tetanus antibody complexes were captured on the test line by tetanus antibody antigen via interaction between antigen and antibody and generated a positive signal. As the sample continued migrating, the residual FNs-Fc conjugate was captured by goat anti-human IgG on the control line and produced a control signal. Without tetanus antibody, the FNs-Fc conjugates only can be bound to goat anti-human IgG on the control line with a negative result. The fluorescence intensity on the test line and control line were then recorded by a portable strip reader for quantitative assay of tetanus antibody. This FNs-LFTS possessed high sensitivity for tetanus antibody detection, with a detection limit of 0.00011 IU/mL. This platform was further used for the detection of tetanus antibody in real serum samples with satisfactory results. More importantly, the results of FNs-LFTS in human blood coincided well with a commercial ELISA kit. These all results indicate that this FNs-LFTS has a great potential to be used as the POCT for tetanus antibody detection in hospitals, communities, and even homes.
Materials and methods
Chemicals and materials
Fluorescent nanospheres, which were pre-modified by polystyrene maleic-anhydride copolymer, were provided by Shanghai Kundao Biotech Co., Ltd. (Shanghai, China). The PcAb Goat Anti-Human IgG (Fc) and Human IgG were obtained from Baiaotong Experimental Materials Center (Luoyang, China). Tetanus antigen was obtained from Fapon Biotech Inc. (Shenzhen, China). The standard human tetanus antibody, human diphtheria antibody, human pertussis antibody, human measles antibody, and rabies virus antibody were obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). 1-Ethyl-3-(3-dimethyllaminopropyl)-carbodiimide hydrochloride (EDC), saccharose, and Tween 20 were obtained from J&K Scientific Co., Ltd. (Beijing, China). Bovine serum albumin (BSA), 2-(N-morpholine) ethane sulfonic acid (MES) powders, boric acid and borax were obtained from Aladdin reagent Co., Ltd. (Shanghai, China). Tetanus antibody kit was purchased in Ruixin Biological Technology Co., Ltd. (Quanzhou, China). All other chemicals were ordered from Sigma-Aldrich Reagent Co., Ltd. (St. Louis, MO, USA). Ultrapure water was generated from a Millipore Milli-Q water purification system (Billerica, MA, USA) with an electric resistance ≥ 18.2 MΩ cm.
Instruments
A scanning electron microscope (SEM) and transmission electron microscope (TEM) (FEI-Tecnai G2, USA) were applied to photograph the morphology of FNs. Fluorescence measurements were carried out on an F-4600 spectrophotometer (Hitachi, Japan) and the emission spectra were recorded from 550 to 700 nm at room temperature with an excitation wavelength at 365 nm. Fourier transform infrared (FTIR) spectrum was collected in the range of 4000–400 cm−1 by using a Bruker Tensor 27 spectrophotometer (Bruker, Germany) with KBr pellets. The amount of antibody conjugated on the surface of FNs was evaluated by the Spark™ Multimode Microplate Reader (Tecan, Switzerland) at 280 nm. Strip cutter-CTS300 and XYZ three-dimensional films spraying gold instrument-HM3035 (Shanghai Kinbio Tech Co., Ltd.) were used for the preparation of test strips. An ESEQuant lateral flow reader was used to record the fluorescence intensity of the strips (Qiagen GmbH, Stockach, Germany). The photos of FNs and the test strips under a UV lamp were taken by a digital camera (Canon, EOS 6D, Japan).
Preparation of the FNs-goat anti-human IgG conjugates
The FNs-goat anti-human IgG (Fc) conjugates were prepared by using a previously published procedure with minor modification [26]. First, carboxyl-functionalized FNs were activated by EDC. The reaction was carried out in MES buffer solution (0.05 M, pH 6.0) with continuous shaking for 30 min and the product was separated by centrifugation at 15,000 rpm for 10 min at 4 °C. Then, the activated FNs were dispersed in 100 μL borate buffer solution (0.05 M, pH 7.4) to react with desired concentration of Fc for about 1 h at room temperature under shock at darkroom. Subsequently, the obtained FNs-Fc was blocked with 100 μL of 2% BSA at room temperature for another 1 h. After that, the mixture was separated by centrifugation at 15,644×g for 10 min and the precipitates were dissolved with 100 μL of borate buffer solution (0.05 M, pH 7.4) containing 0.01% Tween 20. The FNs-Fc solution was stored at 4 °C for further use.
Fabrication of FNs-Fc-based test strips
The FNs-Fc strips comprised four parts: sample pad, nitrocellulose membrane (NC membrane), absorbent pad, and plastic backing card. The sample pad with 33 mm wide was pretreated with block buffer containing 5% (w/v) Tween 20 and dried at 60 °C for 2 h. The optimal tetanus antigen (1.8 mg/mL) and human IgG (1 mg/mL) were dispensed on the NC membrane at a rate of 1 μL/cm as the test line and control line, respectively, and then dried in a vacuum oven at 37 °C for 2 h. Then, the sample pad, NC membrane, and absorbent pad were sequentially assembled on a polyvinyl chloride (PVC) adhesive plate with a 2-mm overlap on top of each other, which allowed the solution migrating on the entire strip. Then, the assembled strips were cut into 3.9-mm-wide strips with the strip cutter-CTS300 and stored at 4 °C.
Detection of tetanus antibody using FNs-Fc-based test strip assay
The standard tetanus antibody with different concentrations and the desired amounts of FNs-Fc were pre-mixed with borate buffer solution (0.05 M, pH 7.4) containing 2% Tween 20. Then, 90 μL of mixture solution was added onto the sample pad of the LFTS and flowed along the long axis of the strip due to the effect of chromatography. After 10 min, the cassette containing prepared test strip was inserted into a fluorescence strip reader, and fluorescence intensity of the test line and the control line were recorded. At last, these strips were put under a UV light, and the corresponding fluorescence images were captured by a Sony DSLR-A300 digital camera.
Assay of tetanus antibody in human serum sample
Different tetanus positive serum samples were diluted 100 times and mixed with the FNs-Fc, and then, the mixture was dropped on the sample pad. After 10 min, the test strip was inserted into a fluorescence strip reader to monitor the fluorescence intensity. For the commercial ELISA kit, firstly, 50 μL of standard samples with different concentrations was added into the standard wells and the optical density (O. D.) values were recorded. The relationship between the O. D. values and the concentration of TT was obtained (R = 9981). Then, different serum samples were added into the sample wells and the O. D. value of each well was measured at the wavelength of 450 nm. At last, the O.D. value of the samples was substituted into the equation to calculate the concentration of the sample. Recovery experiments were also performed in the tetanus negative serum sample. Spiked samples were prepared by adding a desired amount of standard tetanus antibody solution in tetanus negative serum sample. The detection procedure was the same as that described in the aforementioned experiment for tetanus antibody detection. Each experiment was performed for three times.
Results and discussion
Characterization of FNs and FNs-Fc
The size and morphology of the FNs were characterized via scanning electron microscope (SEM) and transmission electron microscope (TEM). From Fig. 1a, it can be seen that the size of FNs is uniform and the diameter of FNs is about 110 nm (the inset of Fig. 1A). FNs-Fc conjugates are obtained by coupling goat anti-human IgG (Fc) onto the surface of the FNs. Fourier transform infrared (FTIR) experiments were carried out to investigate the formation of FNs-Fc conjugates. As shown in Fig. 1B, compared with FNs (curve a), the FTIR spectra of the FNs-Fc (curve b) show characteristic absorption peaks corresponding to protein amide bands I (1650 cm−1) and II (1533 cm−1). Then, a multimode microplate reader was applied to monitor the protein coupling. As shown in Fig. 1C, comparing with the bare FNs, the characteristic absorption peak of protein is observed at the 280 nm, which is consistent with that previously reported [27]. The concentration of the Fc in the FNs-Fc conjugates is about 55 μg/mL. Photoluminescence properties of FNs and FNs-Fc conjugates were also investigated. As shown in Fig. 1D, FNs exhibit a narrow and bright red fluorescence peak at 614 nm under the excitation of 365 nm, and there is no obvious change of the fluorescence position and intensity after the conjugation between FNs and Fc [23, 28], indicating that Fc has no effect on the fluorescence properties of FNs. These all results demonstrate that the Fc have been successfully coupled on the surface of FNs.
(A) SEM image of FNs, inset: high-resolution TEM image and diameter distribution of FNs. (B) The FTIR of (a) FNs and (b) FNs-Fc. (C) Quantitative spectrogram of (a) FNs and (b) FNs-Fc by multimode microplate reader. (D) Fluorescence spectra of FNs and FNs-Fc, inset: the photographic picture of FNs (tube a) and FNs-Fc (tube b) under the UV lamp
Feasibility of the strip assay for tetanus antibody
To investigate the feasibility of FNs-Fc-based lateral flow test strip assay, the fluorescence intensity and images of the strips under different conditions were recorded. As shown in Fig. 2A, there is no obvious fluorescence signal on test line (position = 46 mm) in the absence of target. However, after addition amount of tetanus antibody in the above mixture solution, the fluorescent intensity increases rapidly. Simultaneously, we also can see an obvious enhancement of fluorescence intensity on the test line by the typical photo images (Fig. 2B). These all results indicate that our system can be used for detection of tetanus antibody.
Optimization of assay conditions
To achieve the best performance of the FNs-Fc test strips, some parameters are optimized: (a) the volume of goat anti-human IgG (Fc) labeled to the FN; (b) blocking buffer; (c) running buffer; (d) immunoreaction time; (e) the amount of the tetanus antigen on the test line and the goat anti-human IgG (Fc) on the control line. Experimental results showed that the following experimental conditions could give maximum signal (see Electronic Supplementary Material (ESM), Figs. S1–S4): (a) the optimal volume of goat anti-human IgG (Fc) (5 mg/mL) labeled to the FNs is 5 μL; (b) the blocking buffer is 5% Tween 20; (c) the amount of Tween 20 in running buffer is 2%; the optimal running buffer is boric acid buffer (0.05 M, pH 7.4); (d) immunoreaction time is 10 min; (e) the amounts of the tetanus antigen on the test line and the goat anti-human IgG (Fc) on the control line are 1.8 mg/mL and 1 mg/mL, respectively.
Analytical performance of the assay
To evaluate the sensitivity of the FNs-Fc test strip tetanus antibody, tetanus antibody at a concentration gradient of 0–0.022 IU/mL diluted in boric acid buffer was dropped onto the sample pad under optimal conditions. As shown in Fig. 3a, we only observe an obvious fluorescence emission on the control line in the absence of tetanus antibody. However, in the presence of tetanus antibody, both the test line and control line can be observed bright fluorescence signal, and the fluorescence intensity of the test line gradually increases with the increasing of tetanus antibody concentration. A linear relationship is obtained in a wide range from 0.0002 to 0.018 IU/mL (Fig. 3b). The correlation equation is intensity = 118.3[tetanus antibody] + 27.44 (R2 = 0.9969). Based on the 3σ/slope rule, the limit of detection (LOD) for tetanus antibody is 0.00011 IU/mL. Comparing with conventional methods for tetanus antibody detection [29,30,31,32], e.g., AuNP-based lateral flow test strip and the enzyme-linked immunosorbent assay method, the FNs-Fc-based test strip can increase the sensitivity by 100-fold [9, 19] (Table 1). This property make this method to be used to the many times diluted sample, and thus can reduce the matrix effect and interference of non-specific response. In addition, brightness of the test zone also has increased with the increasing of tetanus antibody concentrations under irradiation by a 365-nm handheld ultraviolet lamp (Fig. 3c). These results indicate that this FNs-Fc-based test strip can meet point-of-care requirements in low-abundant tetanus antibody detection.
a Fluorescence spectra of tetanus antibody detection with the different concentrations; the concentrations of tetanus antibody from bottom to top are 0, 0.0002, 0.0005, 0.001, 0.003, 0.005, 0.007, 0.009, 0.01, 0.012, 0.014, 0.018, 0.020, and 0.022 IU/mL. b Scatter plot of fluorescence intensity as a function of the concentrations of tetanus antibody. Inset: linear relationship between fluorescence intensity and tetanus antibody concentration (in the range from 0.0002 to 0.018 IU/mL). The error bars are the standard deviation of three repetitive measurements. c Fluorescence pictures of the test strips for increasing concentration of tetanus antibody under a 365-nm handheld ultraviolet lamp
Evaluation of the selectivity
For a new fluorescent assay with potential application in real samples, selectivity is an important parameter to evaluate its performance. To investigate the specificity of this assay, boric acid buffer was used as the blank group; 0.018 IU/mL tetanus antibody was used as the test group, and the concentrations of interferences (0.18 IU/mL) were 10-fold of tetanus antibody, which were taken as the negative control group. We can see from Fig. 4, the fluorescence intensity of the test line with 0.018 IU/mL tetanus antibody is remarkably higher than those of other control test strips and blank group. These results indicate that the FNs-Fc-based test strip possesses high selectivity and strong anti-interference for detection of tetanus antibody in complex matrix.
Stability of the assay
Stability is also an important factor for a method which will be used to complex sample. Therefore, to evaluate the stability of the LFTS, test strips were stored in dry environment at 4 °C for 0 day, 1 day, 3 days, 1 week, 1 month, 3 months, and 6 months. Subsequently, the stored strips were tested with 0.018 IU/mL tetanus antibody. As shown in Fig. 5, it can be seen that FNs-Fc can retain their bioactivity, and fluorescence intensity of the test strip has no obvious change even after 6 months’ storage, indicating its great stability and promise in commercial applications in the future.
Application in human serum samples
To further investigate the practicality of the proposed assay in complex biological samples for point-of-care testing (POCT) application, the different serum samples were obtained by the First Affiliated Hospital of Zhengzhou University. We employed the system to detect tetanus antibody in differently diluted positive serum samples. As shown in Table 2, the response of the strips to serum samples is in agreement with the result obtained by using a commercial ELISA kit and has a good consistence with the previously published report [19]. In addition, the accuracy and precision of the FNs-Fc-based lateral flow assay were also evaluated by detecting tetanus antibody spiked negative serum samples. As shown in Table S1 (see ESM), the recoveries are in the range of 96.7–101.4% for measurement of 0.0050, 0.0075, and 0.01 IU/mL tetanus antibody spiked in negative serum samples with a maximum RSD of 2.35%, suggesting that the as-proposed assay holds a great promise in practical applications for tetanus antibody detection.
Conclusions
In this work, a well-performing lateral flow strip platform was reported for highly sensitive detection of tetanus antibody. As promising alternative reporters, FNs possess high brightness and outstanding chemical and colloidal stability, making them a unique and effective fluorescence signal reporter. Under the optimized conditions, this system showed high sensitivity to tetanus antibody with a detection limit of 0.00011 IU/mL and can be completed in 10 min. This assay was validated with different serum samples and spiked human negative serum with satisfactory results. Moreover, it provided one-step detection without extensive purification steps and can be easily read out for nonprofessional operators. In view of these advantages, the FNs-Fc-based test strip platform may open up new opportunities for the early ultra-sensitive detection in fields such as clinical diagnosis, food safety, and environmental monitoring.
References
Vandelaer J, Birmingham M, Gasse F, Kurian M, Shaw C, Garnier S. Tetanus in developing countries: an update on the Maternal and Neonatal Tetanus Elimination Initiative. Vaccine. 2003;21(24):3442–5.
Gall SA, Myers J, Pichichero M. Maternal immunization with tetanus–diphtheria–pertussis vaccine: effect on maternal and neonatal serum antibody levels. Am J Obstet Gyneco. 2011;204(4):334.e1–5.
Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obesity. 2011;36:1072–7.
Jain S, Chattopadhyay S, Jackeray R, Zainul Abid CKV, Kumar M, Singh H. Detection of anti-tetanus toxoid antibody on modified polyacrylonitrile fibers. Talanta. 2010;82(5):1876–83.
King CA, Spellerberg MB, Zhu D, Rice J, Sahota SS, Thompsett AR, et al. DNA vaccines with single-chain Fv fused to fragment C of tetanus toxin induce protective immunity against lymphoma and myeloma. Nat Med. 1998;4:1281–6.
Ramakrishnan G, Pedersen K, Guenette D, Sink J, Haque R, Petri WA, et al. Utility of recombinant fragment C for assessment of anti-tetanus antibodies in plasma. Diagn Micr Infec Dis. 2015;82(1):11–3.
Roper MH, Vandelaer JH, Gasse FL. Maternal and neonatal tetanus. Lancet. 2007;370(9603):1947–59.
Schröder JP, Kuhlmann WD. Detection of tetanus antitoxin using Eu(3+)-labeled anti-human immunoglobulin G monoclonal antibodies in a time-resolved fluorescence immunoassay. J Clin Microbiol. 1991;29(7):1504–7.
Maple PAC, Jones CS, Andrews NJ. Time resolved fluorometric immunoassay, using europium labelled antihuman IgG, for the detection of human tetanus antitoxin in serum. J Clin Patho. 2001;54(10):812–5.
Gupta RK, Maheshwari SC, Singh H. The titration of tetanus antitoxin II: a comparative evaluation of the indirect haemagglutination and toxin neutralization tests. J Biol Stand. 1984;12(2):137–43.
Susković F. Determination of immune antitetanus antibodies using hemagglutination tests. Lijec Vjesn. 1993;115(9–10):273–9.
Peel MM. Measurement of tetanus antitoxin I. Indirect haemagglutination. J Biol Standard. 1980;8(3):177–89.
Herr AE, Throckmorton DJ, Davenport AA, Singh AK. On-chip native gel electrophoresis-based immunoassays for tetanus antibody and toxin. Anal Chem. 2005;77(2):585–90.
Habermann E, Wiegand H. A rapid and simple radioimmunological procedure for measuring low concentrations of tetanus antibodies. N-S Arch Pharmacol. 1973;276(3):321–6.
Golberg A, Yarmush ML, Konry T. Picoliter droplet microfluidic immunosorbent platform for point-of-care diagnostics of tetanus. Microchim Acta. 2013;180(9):855–60.
Liu J, Ji D, Meng H, Zhang L, Wang J, Huang Z, et al. A portable fluorescence biosensor for rapid and sensitive glutathione detection by using quantum dots-based lateral flow test strip. Sensor Actuat B-Chem. 2018;262:486–92.
Chen J, Huang Z, Meng H, Zhang L, Ji D, Liu J, et al. A facile fluorescence lateral flow biosensor for glutathione detection based on quantum dots-MnO2 nanocomposites. Sensor Actuat B-Chem. 2018;260:770–7.
Li Z, Wang Y, Wang J, Tang Z, Pounds JG, Lin Y. Rapid and sensitive detection of protein biomarker using a portable fluorescence biosensor based on quantum dots and a lateral flow test strip. Anal Chem. 2010;82(16):7008–14.
Liu J, Wang J, Li Z, Meng H, Zhang L, Wang H, et al. A lateral flow assay for the determination of human tetanus antibody in whole blood by using gold nanoparticle labeled tetanus antigen. Microchim Acta. 2018;185(2):110.
Wang J, Meng H-M, Chen J, Liu J, Zhang L, Qu L, et al. Quantum dot-based lateral flow test strips for highly sensitive detection of the tetanus antibody. ACS Omega. 2019;4(4):6789–95.
Swierczewska M, Liu G, Lee S, Chen X. High-sensitivity nanosensors for biomarker detection. Chem Soc Rev. 2012;41(7):2641–55.
Zhang J, Lv X, Feng W, Li X, Li K, Deng Y. Aptamer-based fluorometric lateral flow assay for creatine kinase MB. Microchim Acta. 2018;185(8):364.
Ren M, Xu H, Huang X, Kuang M, Xiong Y, Xu H, et al. Immunochromatographic assay for ultrasensitive detection of aflatoxin B1 in maize by highly luminescent quantum dot beads. ACS Appl Mater Interfaces. 2014;6(16):14215–22.
Hu J, Jiang Y-Z, Wu L-L, Wu Z, Bi Y, Wong G, et al. Dual-signal readout nanospheres for rapid point-of-care detection of Ebola virus glycoprotein. Anal Chem. 2017;89(24):13105–11.
Hu J, Zhang Z-L, Wen C-Y, Tang M, Wu L-L, Liu C, et al. Sensitive and quantitative detection of C-reaction protein based on immunofluorescent nanospheres coupled with lateral flow test strip. Anal Chem. 2016;88(12):6577–84.
Ouyang S, Zhang Z, He T, Li P, Zhang Q, Chen X, et al. An on-site, ultra-sensitive, quantitative sensing method for the determination of total aflatoxin in peanut and rice based on quantum dot nanobeads strip. Toxins. 2017;9(4):137.
Qazi S, Schlicksup CJ, Rittichier J, VanNieuwenhze MS, Zlotnick A. An assembly-activating site in the hepatitis B virus capsid protein can also trigger disassembly. ACS Chem Biol. 2018;13(8):2114–20.
Li X, Li W, Yang Q, Gong X, Guo W, Dong C, et al. Rapid and quantitative detection of prostate specific antigen with a quantum dot nanobeads-based immunochromatography test strip. ACS Appl Mater Interfaces. 2014;6(9):6406–14.
Kenrick KG, Wallace RC, Ismay SL. An improved assay for human tetanus anti-toxin and its use in the accession of human plasma for the production of high-titre tetanus immunoglobulin. Vox Sang. 1990;58(1):35–9.
Ochoa R, Martínez JC, Fajardo EM, Alvarez E, Estrada E, García AM, et al. Validación deun ELISA para la cuantificación de antitoxina tetánica en suero humano. Vaccimonitor. 2000;9(4):31–7.
Aggerbecka H, Nørgaard-Pedersenb B, Herona I. Simultaneous quantitation of diphtheria and tetanus antibodies by double antigen, time-resolved fluorescence immunoassay. J Immunol Methods. 1996;190(2):171–83.
Raeisi S, Molaeirad A, Sadri M, Nejad HR. Detection of anti-tetanus toxoid monoclonal antibody by using modified polycarbonate surface. Plasmonics. 2018;13(5):1555–67.
Funding
This work was supported by National Natural Science Foundation of China (21974125, 21605038, and 21877027), China Postdoctoral Science Foundation (2019T120623 and 2016M602245), and the Key Scientific Research Project in Universities of Henan Province (19A150048 and 16A150013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The serum samples were obtained by the First Affiliated Hospital of Zhengzhou University, approved by Life-Science Ethics Review Committee of Zhengzhou University.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 760 kb)
Rights and permissions
About this article
Cite this article
Chen, J., Meng, HM., An, Y. et al. A fluorescent nanosphere-based immunochromatography test strip for ultrasensitive and point-of-care detection of tetanus antibody in human serum. Anal Bioanal Chem 412, 1151–1158 (2020). https://doi.org/10.1007/s00216-019-02343-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02343-7