Abstract
The authors describe a method that can significantly improve the performance of impedimetric detection of bacteria. A multifunctional microfluidic chip was designed consisting of interdigitated microelectrodes and a micro-mixing zone with a Tesla structure. This maximizes the coating of bacterial surfaces with nanoparticles and results in improved impedimetric detection. The method was applied to the detection of Escherichia coli O157:H7 (E. coli). Silver enhancement was accomplished by coating E.coli with the cationic polymer diallyldimethylammonium chloride (PDDA) to form positively charged E. coli/PDDA complexes. Then, gold nanoparticles (AuNPs) were added, and the resulting E. coli/PDDA/AuNPs complexes were collected at interdigitated electrodes via positive dielectrophoresis (pDEP). A silver adduct was then formed on the E. coli/PDDA/AuNP complexes by using silver enhancement solutions and by using the AuNPs as catalysts. The combination of pDEP based capture and of using silver adducts reduces impedance by increasing the conductivity of the solution and the double layer capacitance around the microelectrodes. Impedance decreases linearly in the 2 × 103–2 × 105 cfu·mL−1 E. coli concentration range, with a 500 cfu·mL−1 detection limit. Egg shell wash samples and tap water spiked with E. coli were successfully used for validation, and this demonstrates the practical application of this method.

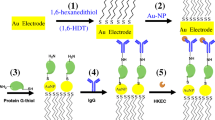
Schematic representation of the AuNP@Ag enhancement method integrated with multifunctional microfluidic chip platform for impedimetric quantitation of bacteria. The method significantly improves the performance of impedimetric detection of bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microfluidic chip based platforms have been broadly applied for portable, fast and sensitive detection of bacteria in a wide range of applications from clinical diagnostics to monitoring of food-borne pathogens with broad prospects [1]. Various detection techniques have been integrated into the microfluidic chip based pathogen detection platforms to improve the sensitivity and reproducibility [2, 3]. Among them, electrochemical impedance has its own advantages such as high-efficiency and ease of integration [4, 5].
Enriching bacteria is an effective strategy to improve the impedance detection sensitivity on microfluidic chips [6]. Integrated dieelectrophoresis (DEP) technology on microfluidic chips can enrich bacteria effectively and non-invasively [7, 8]. Yang [9] improved the sensitivity of bacteria detection by combining DEP and antibody immune-capture. Other reports showed that DEP can enrich bacteria and achieve real-time detection on microfluidic chip [10].
Interdigitated microelectrodes on microfluidic chips have a larger detection area with the advantage of sensitive response. As such, they have been widely applied in bacterial detection. Suehiro et al. developed a bacteria detection method by combining DEP separation and impedance test using interdigitated microelectrodes [11]. Yang and colleagues [12] proved that the impedance value of bacteria sample decreased with increase ofbacterial concentration in suspensions at 1 kHz using interdigitated microelectrodes on a microfluidic chip, and the detection limit of impedance method for bacteria was 3.45 × 106 cfu·mL−1. At present, several researches have focused on impedance detection of bacteria by combining DEP and microfluidic chip technology [13, 14], but these were restricted by detection sensitivity and limition. It has important significance to improve the performance of DEP-impedance detection on a microfluidic chip. Metal nanoparticles have unique properties that have been well characterized with light [15], electricity [16] and magnetism [17]. Due to their superior conductivity, metal nanoparticles have gained tremendous attention in the detection of biological samples. Su [18] protein detection on an electro-microchip intergrated with silver enhancement and immunoassay, which greatly improved the detection sensitivity. A series of novel immune detection methods for DNA samples were developed by Yeh and colleagues [19,20,21] using a silver enhancement reaction to magnify the detection signals. Moreover, Lin et al. [22] established an electrochemical DNA biosensor based on graphene and a silver enhancement strategy, which improved the analytical sensitivity and performance.
These studies have proven the feasibility of silver enhancement reaction in enhancing sensitivity for impedance detection of protein and DNA. However, the microchip used in previous silver enhancement studies only had basic EIS detection zones. In order to enhance the detection automation and to fully take advantage of the microfluidic chip, the integration of functional zones with unique design structure on the microchip is crucial for the improvement of detection efficiency [23, 24]. A Tesla structure has been previously described as a passive microfluidic mixer that can provide excellent mixing capabilities for biochemical samples at low flow rates, based on the “Coanda effect” [25]. More importantly, the Tesla structures are simple, in-plane structures that can be easily fabricated within microfluidic chip devices.
Pathogenic microorganisms in food and water have attracted wide attention in public health [26]. A rapid and sensitive method for bacterial detection is of urgent need to help prevent future outbreaks [27]. Our goal was to design a microchip that would integrate a Tesla mixing zone and a detection zone with interdigital microelectrodes, and to improve the sensitivity of dielectrophoretic impedance detection of bacteria by using silver enhancement. By using E.coli as a model, successive outer membrane coating processes including a cationic PDDA with AuNPs, followed by silver ion reduction catalyzed on the AuNPs surface were applied in this study. The flow rate and duration of exposure of the silver solutions were optimized for amplifing impedance signal. The potentials for application of the method was further demonstrated by detecting E.coli in egg shell wash and tap water samples.
Experimental
Instruments and reagents
Dielectrophoresis stimulation signals were provided by an AC signal generator (33220A, Agilent Technologies Inc. USA) (https://www.agilent.com). Electrochemical workstation (VersaSTAT3, Princeton, USA) (https://www.princetoninstruments.com) and was used to measure the EIS response signal. Sample solutions were injected into the microchannels by using a syringe pump (55,226, Harvard Apparatus, USA) (https://www.harvardapparatus.com). Microscopic images were obtained with an inverted microscope (IX71, Olympus, Japan) (https://www.olympus-global.com). Scanning electron microscope (SEM) micrographs was acquired by using a JSM-7800F (Jeol) field emission scanning electron microscope operated at an acceleration voltage of 5 kV (http://www.jeol.co.jp/en).
PDDA was purchased from Tianjin Sheehan Biochemical Technology Co. (Tianjin, China) (http://www.heowns.com). Hydrogen tetrachloroaurate(III) hydrate (HAuCl4·nH2O, n = 4) was purchased from Sinopharm chemical reagent Co. (Shanghai, China) (http://www.sinoreagent.com), and trisodium citrate was purchased from Chongqing Chuandong Chemical Group (Chongqing China) (http://www.cdhgjt.com). Silver enhancement solutions A and B were purchased from Sigma-Aldrich (Shanghai, China) (https://www.sigmaaldrich.com).
E. coli used in this study were provided by College of Laboratory Medicine, Chongqing Medical University (Chongqing, China). Strains were transferred into the Luria-Bertani broth and incubated at 37 °C for 12 h before use. The bacterial cells were centrifuged for 3 min at 8000 rpm and washed three times with the sterilized deionized water. Finally, bacteria were resuspended in deionized water. The total number of bacteria was determined using the plate count method, and different concentrations of E. coli were obtained by gradient dilution.
Design and fabrication of the microfluidic chip
The multifunctional microfluidic chip presented in Fig. 1 consists of two parts: (1) A PDMS cover with a Tesla mixing zone and a detection zone channel (Fig. 1a), and (2) A glass base plate integrated with gold interdigital microelectrodes (Fig. 1b). Interdigitated microelectrodes contained 10 pairs of interdigital microelectrodes, and each electrode was 30 μm wide with a spacing of 30 μm between each other. The specific structure and dimensions of the PDMS cover are shown in Fig. 1c. The preparation of microfluidic chip is described in the Electronic Supplementary Material.
Schematic of the multifunctional microfluidic chip. PDMS layer with injection ports “a” and “b” leading to a Tesla mixing zone and a detection zone (a) and glass base plate with the interdigitated microelectrodes (b). Structure and dimensions of an individual mixing cell of the Tesla mixing zone (c). Schematic of the interdigitated microelectrodes (d)
Impedance detection of E.coli/PDDA/AuNP@Ag complexes
Target E.coli strain (1 mL) was mixed with 1 mL 1% PDDA at room temperature and kept under continuous gentle shaking for 20 min. Then, the mixture was centrifuged for 3 min at 8000 rpm to pellet the E.coli/PDDA complex and remove the excess PDDA in the solution. The pelleted E.coli/PDDA complex was resuspended in 1 mL deionized water (DI water, 4 μS cm−1). E.coli/PDDA complex and AuNPs solutions (The preparation process of AuNPs are provided in the Electronic Supplementary Materials) were injected simultaneously into micro channels from the two inlets “a” and “b”, at a flow rate of 2 μL min−1 for 10 min. Both of them flowed through the mixing zone to form E.coli/PDDA /AuNPs complexes, which went into the detection zone and were enriched by pDEP on the edges of interdigitated microelectrodes. pDEP enrichment was carried out in the sine AC excitation signal with a frequency of 1.5 MHz and an amplitude of 6 Vpp. Then DI water was injected at a flow rate of 4 μL min−1 for 2 min to remove excess AuNPs which were not bound to the E.coli/PDDA complexes. Subsequently, silver enhancement solutions A and B were simultaneously injected through “a” and “b” inlets into the microchannels, and then the unreacted silver solutions were washed out by using DI water (4 μL min−1, 2 min). Finally, the Bode spectra of EIS signal was detected by using electrochemical workstation, with a disturbance voltage set at 100 mV and a frequency range of 1 KHz - 1 MHz.
E.coli detection in synthetic samples
Eggshell (5 g) was ground using a mortar into a powder and was soaked with 5 mL deionized water for 12 h. Soak solutions and tap water were filtered through a 0.22 μm membrane separately before the experiment started. After filter sterilization, an aliquot from each sample was subjected to culturing conditions to confirm that they were not contaminated with bacteria. For synthetic sample preparation, a certain amount of E.coli was mixed with the soak solution and tap water, respectively. Then, the synthetic samples were detected by the established method.
Results and discussion
Characterization of the microfluidic chip
In order to realize rapid and sensitive detection of bacteria, the integration of pDEP enrichment, EIS test, and silver enhancement was made possible on a microfluidic chip. This particular platform was attractive because compared with traditional electrochemical impedance methods it had the added advantages of ease operation, cost reduction through reagent use savings and ease of integration and detection automation.
The Tesla structure of the mixing zone was crucial to maximize the E.coli/PDDA/AuNPs complex formation. This special passive structure was selected because it was not only produce transverse dispersion with outstanding mixing efficiency under appropriate velocity and shear stress, but also reduces dead volume and avoids nonspecific adsorption. Adequate mixing provided by the mixing zone with Tesla structure played important roles in improving the detection efficiency and sensitivity for the method. First, well-mixed E.coli/PDDA and AuNPs were allowed to have more opportunities to interact and form E.coli/PDDA/AuNPs complexes. The formation of E.coli/PDDA/AuNPs complexes was the first and most important step to conduct silver enhancement, due to the silver ions can only be reduced by AuNP in this study. Second, the full mixing between silver enhancement solutions A (silver salt) and B (initiator) was critical to obtaining steady reduction effects.
Bacteria behave like dielectric particles in an external electric field, and pDEP was used to capture and enrich bacteria on the surface of the microelectrodes of the microfluidic chip. The contact area between the bacterial suspension and capture zone was the main factor influencing capture rate. In order to increase detection efficiency and sensitivity, the detection zone and interdigitated microelectrodes were combined in a single rectangular shaped layout which increased radial length and effective working area (Fig. 2).
Photographs of PDMS cover showing ports “a” and “b”, Tesla mixing zone and detection zone (a) and glass base plate of the microchip with interdigitated microelectrodes (b). Micrograph of an individual mixing cell of the Tesla mixing zone (c). Micrograph of interdigitated microelectrodes (d). Simulation diagram of interdigitated microelectrodes with nonuniformed electric field distribution (e)
The microstructure was characterized using an inverted light microscope. The results showed that 10 pairs of interdigitated microelectrodes were correctly formed. The electrode width and spacing was 33 μm and 29 μm, respectively. We would assume that equally spaced microelectrodes of the same width would display equal electric field distributions between the microelectrodes. In order to verify that the electric field distribution satisfy pDEP using our design, CoventorWare software was used by finite element analysis. As shown in Fig. 2, the results of the simulation of the high electric field region (represented in red), and the low electric field region (represented in blue), demonstrated that the higher electric field intensity was 8.3 × 10−2 V2 μm−2 distributed at the edge of microelectrode, and lower electric field region in the centerline position of the in between electrodes was 2.0 × 10−2 V2 μm−2. Interdigitated microelectrodes in the detection zone of microchip formed nonuniformed electric fields, meeting the design requirements.
Principle of AuNP@Ag enhancement for signal amplification
To further improve EIS sensitivity for bacteria detection, a silver enhancement reaction and pDEP enrichment integrated with microchip were applied in this study. On the one hand, when the concentration of bacteria captured along the microelectrode increased, there are more silver adducts accumulated at the edge of microelectrode which equals to enlarge the contact area between microelectrode and the bacterial suspension, and increase the double layer capacitance (Cdl) (The equivalent circuit is presented in electronic supplementary materials). On the other hand, the conductivity of bacteria suspension increased because of the ionic metabolites released by E.coli [12]. Therefore, higher concentrations of bacteria present a lower impedance value, and the silver adducts resulted in an impedance decrease and significantly improved the sensitivity of bacterial detection using EIS.
A schematic of the process is shown in Fig. 3. E.coli’s surface (strain O157:H7) is spontaneously negatively charged in neutral solution, due to the ionized carboxylate and phosphoryl substituents on outer cell envelope macromolecules [28, 29]. There is a large number of quaternary ammonium cationic in PDDA solution, which is a type of cationic polymer compounds. E.coli/PDDA complexes were formed through static adsorption between the negatively charged E. coli and the positively charged PDDA. Similarly, subsequent coating by AuNPs was performed using the same electrostatic attraction with negatively charged AuNPs resulting in the formation of E.coli/PDDA/AuNPs complexes.
Schematic of the AuNP@Ag enhancement method for E.coli impedance detection on the multifunctional microfluidic chip. The E.coli were coated by PDDA firstly and then were injected into the mixing zone of the micochip at the same time with AuNPs from inlets “a” and “b” respectively. In the mixing zone, E.coli/PDDA/AuNPs complexes were formed because of the electrostatic attraction between the positively charged E. coli/PDDA and the negatively charged AuNPs. After entering detection zone, the E.coli/PDDA/AuNPs complexes were captured and enriched on the edge of microelectrodes followed by silver ions reduction on the surface of AuNPs
After exiting the mixing zone, complexes moved into the detection zone where the appropriate alternating current field was applied. E.coli/PDDA/AuNPs complexes were captured around the microelectrodes by positive dielectrophoretic force. The AuNPs which were not adsorbed on the surface of E.coli/PDDA complexes were expelled out of the detection zone by washing with DI water. Then, the silver enhancement solutions A and B were injected into the microchip simultaneously. Silver ions were reduced to metallic silver, which were precipitated on the surface of the AuNPs of E.coli/PDDA/AuNP complexes in detection zone. The SEM characterization of bacterial nanoparticle complexes are provided in the Electronic Supplementary Materials.
Enrichment of E. coli/PDDA/AuNPs @Ag complexes on impedance microchip
Achieving pDEP enrichment in detection zone of the impedance microchip is of critical importance in this study. Excitation signal applied on the interdigitated microelectrodes was supplied by an AC signal generator with a frequency of 1.5 MHz and amplitude of 6 Vpp. The characterization results of microscopy show that, under these conditions, the complex E.coli/PDDA/AuNPs was enriched on the electrode edges by pDEP (Fig. 4a), and after the reduction of silver ions, the E.coli/PDDA/AuNP@Ag complexes are still captured on the edge of microelectrodes (Fig. 4b). Due to the catalytic activity of AuNPs, silver ions in the silver enhancement solution were reduced and precipitated only at the site of AuNPs [30]. Because of the photon absorbing ability of metallic silver [19, 25], the E.coli/PDDA/AuNP@Ag complexes had darker color than E.coli/PDDA/AuNPs around the microelectrodes.
Optimization of method
The flow velocity and injection duration of silver enhancement solutions were optimized. Respective data and Figures are given in the Electronic Supporting Material. The following experimental conditions were found to give best results: (a) A flow rate of 4 μL min−1; (b) Injection duration of 6 min.
Quantitative analysis
The impedance response signals of different concentrations of E.coli were obtained under the optimized conditions. A standard curve was established in the concentration range from 2 × 103–2 × 105 cfu·mL−1 using the eq. y = -2137lgC + 13,091, with an R2 = 0.9799 (Fig. 5), and detection time was less than 1 h. The detection limit was as low as 5 × 102 cfu·mL−1, four orders of magnitude better than that without silver enhancement [12]. For reasons due to superior electronic transfer ability of the silver precipitate which equaled to the enlarged surface area that increased the Cdl around each microelectrode, the impedance of the E.coli O157:H7 decreased and the signals were enhanced significantly.
Detection of E.coli in synthetic samples
In order to investigate the reliability and practicality of the described method, different concentrations of E.coli artificially were added to filter-sterilized eggshell wash and tap water for detection by the impedance method and verified by conventional plate count method. The results are shown in Table 1. The results of the described impedance method are consistent with the plate count method, and the recoveries of the spiked samples were from 87.69% to 110.86% and the RSD were from 6.3% to 9.0%, demonstrating that the impedance method has the potential for practical applications with reliability. Compared with the plate count method, impedance detection greatly reduced the detection times from 48 h to less than an hour.
Conclusion
In this study, silver enhancement reaction has been proven to be an effective method to improve the performance of a microfluidic chip for impedimetric bacteria detection. A novel impedance microchip integrated a Tesla mixing zone and interdigital microelectrodes was developed. E.coli/PDDA/AuNP@Ag complexes that was captured on the edge of microelectrodes by positive dielectrophoresis increased the conductivity of the solution and the double layer capacitance around the microelectrodes. Under optimized conditions, the detection limit was down to 500 cfu·mL−1 within 1 h. The successful detection of E.coli in solutions of eggshell wash and tap water validates the potential of practical application of this method. In future studies, the selectivity of this method will be constantly improved and refined to enhance its capability.
References
Fabai WCD (2016) Nanofabricated structures and microfluidic devices for bacteria: from techniques to biology. Chem Soc Rev 45:268–280. https://doi.org/10.1039/C5CS00514K
Song Y, Zhang H, Chon CH, Chen S, Pan X, Li D (2010) Counting bacteria on a microfluidic chip. Anal Chim Acta 681(1–2):82–86. https://doi.org/10.1016/j.aca.2010.09.035
Fronczek CF, You DJ, Yoon JY (2013) Single-pipetting microfluidic assay device for rapid detection of Salmonella from poultry package. Biosens Bioelectron 40(1):342–349. https://doi.org/10.1016/j.bios.2012.07.076
Varshney M, Li Y, Srinivasan B, Tung S (2007) A label-free, microfluidics and interdigitated array microelectrode-based impedance biosensor in combination with nanoparticles immunoseparation for detection of Escherichia coli O157:H7 in food samples. Sensors Actuators B Chem 128(1):99–107. https://doi.org/10.1016/j.snb.2007.03.045
Varshney M, Li Y (2007) Interdigitated array microelectrode based impedance biosensor coupled with magnetic nanoparticle-antibody conjugates for detection of Escherichia coli O157:H7 in food samples. Biosens Bioelectron 22(11):2408–2414. https://doi.org/10.1016/j.bios.2006.08.030
Donato SS, Chu V, Prazeres DM, Conde JP (2013) Metabolic viability of Escherichia coli trapped by dielectrophoresis in microfluidics. Electrophoresis 34(4):575–582. https://doi.org/10.1002/elps.201200292
Yang L (2012) A Review of Multifunctions of Dielectrophoresis in Biosensors and Biochips for Bacteria Detection. Anal Lett 45(2–3):187–201. https://doi.org/10.1080/00032719.2011.633182
Yang L, Banada PP, Chatni MR, Seop Lim K, Bhunia AK, Ladisch M, Bashir R (2006) A multifunctional micro-fluidic system for dielectrophoretic concentration coupled with immuno-capture of low numbers of Listeria monocytogenes. Lab Chip 6(7):896–905. https://doi.org/10.1039/b607061m
Yang L (2009) Dielectrophoresis assisted immuno-capture and detection of foodborne pathogenic bacteria in biochips. Talanta 80(2):551–558. https://doi.org/10.1016/j.talanta.2009.07.024
He X, Hu C, Guo Q, Wang K, Li Y, Shangguan J (2013) Rapid and ultrasensitive Salmonella Typhimurium quantification using positive dielectrophoresis driven on-line enrichment and fluorescent nanoparticleslabel. Biosens Bioelectron 42:460–466. https://doi.org/10.1016/j.bios.2012.11.020
Suehiro J, Shutou M, Hatano T, Hara M (2003) High sensitive detection of biological cells using dielectrophoretic impedance measurement method combined with electropermeabilization. Sensors Actuators B Chem 96(1–2):144–151. https://doi.org/10.1016/s0925-4005(03)00517-3
Yang L (2008) Electrical impedance spectroscopy for detection of bacterial cells in suspensions using interdigitated microelectrodes. Talanta 74(5):1621–1629. https://doi.org/10.1016/j.talanta.2007.10.018
Suehiro J, Ohtsubo A, Hatano T, Hara M (2006) Selective detection of bacteria by a dielectrophoretic impedance measurement method using an antibody-immobilized electrode chip. Sensors Actuators B Chem 119(1):319–326. https://doi.org/10.1016/j.snb.2005.12.027
Kim S, Yu G, Kim T, Shin K, Yoon J (2012) Rapid bacterial detection with an interdigitated array electrode by electrochemical impedance spectroscopy. Electrochim Acta 82:126–131. https://doi.org/10.1016/j.electacta.2012.05.131
Wang R, Ni Y, Xu Y, Jiang Y, Dong C, Chuan N (2015) Immuno-capture and in situ detection of Salmonella typhimurium on a novel microfluidic chip. Anal Chim Acta 853:710–717. https://doi.org/10.1016/j.aca.2014.10.042
Chen ZP, Peng ZF, Luo Y, Qu B, Jiang JH, Zhang XB, Shen GL, Yu RQ (2007) Successively amplified electrochemical immunoassay based on biocatalytic deposition of silver nanoparticles and silver enhancement. Biosens Bioelectron 23(4):485–491. https://doi.org/10.1016/j.bios.2007.06.005
Chen X, Zhang L (2017) A review on micromixers actuated with magnetic nanomaterials. Microchim Acta 184(10):3639–3649. https://doi.org/10.1007/s00604-017-2462-2
K-L S, Huang H-H, Chang TC, Lin H-P, Lin Y-C, Chen W-T (2008) An immunoassay using an electro-microchip, nanogold probe and silver enhancement. Microfluid Nanofluid 6(1):93–98. https://doi.org/10.1007/s10404-008-0299-z
Yeh CH, Chang YH, Chang TC, Lin HP, Lin YC (2010) Electro-microchip DNA-biosensor for bacteria detection. Analyst 135(10):2717–2722. https://doi.org/10.1039/c0an00186d
Yeh C-H, Chen W-T, Lin H-P, Chang T-C, Lin Y-C (2009) Development of an immunoassay based on impedance measurements utilizing an antibody–nanosilver probe, silver enhancement, and electro-microchip. Sensors Actuators B Chem 139(2):387–393. https://doi.org/10.1016/j.snb.2009.03.029
Yeh CH, Huang HH, Chang TC, Lin HP, Lin YC (2009) Using an electro-microchip, a nanogold probe, and silver enhancement in an immunoassay. Biosens Bioelectron 24(6):1661–1666. https://doi.org/10.1016/j.bios.2008.08.039
Lin L, Liu Y, Tang L, Li J (2011) Electrochemical DNA sensor by the assembly of graphene and DNA-conjugated gold nanoparticles with silver enhancement strategy. Analyst 136(22):4732–4737. https://doi.org/10.1039/c1an15610a
Sabhachandani P, Sarkar S, Zucchi PC, Whitfield BA, Kirby JE, Hirsch EB, Konry T (2017) Integrated microfluidic platform for rapid antimicrobial susceptibility testing and bacterial growth analysis using bead-based biosensor via fluorescence imaging. Microchim Acta 184(12):4619–4628. https://doi.org/10.1007/s00604-017-2492-9
Jang H, Lee P, Kim S, Kim SM, Jeon T-J (2017) An antibacterial microfluidic system with fish gill structure for the detection of Staphylococcus via enzymatic reaction on a chromatic polydiacetylene material caused by lysostaphin. Microchim Acta 184(11):4563–4569. https://doi.org/10.1007/s00604-017-2517-4
Hong CC, Choi JW, Ahn CH (2004) A novel in-plane passive microfluidic mixer with modified Tesla structures. Lab Chip 4(2):109–113. https://doi.org/10.1039/b305892a
Ravindranath SP, Wang Y, Irudayaraj J (2011) SERS driven cross-platform based multiplex pathogen detection. Sensors Actuators B Chem 152:183–190. https://doi.org/10.1016/j.snb.2010.12.005
Cho Il-Hoon, Radadia AD, Farrokhzad K, Ximenes E, Euiwon B, Singh AK, Oliver H, Ladisch M, Bhunia A, Applegate B, Mauer L, Bashir R, Irudayaraj J (2014) Nano/Micro and Spectroscopic Approaches to Food Pathogen Detection. Annu Rev Anal Chem 7:65–88. https://doi.org/10.1146/annurev-anchem-071213-020249
Dickson JS, Koohmarare M (1989) Cell Surface Charge Characteristics and Their Relationship to Bacterial Attachment to Meat Surfaces. Appl Environ Microbiol 55(4):5
Wilson WW, Wade MM, Holman SC, Champlin FR (2001) Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J Microbiol Methods 43(3):153–164. https://doi.org/10.1016/s0167-7012(00)00224-4
Lackie PM (1996) Immunogold silver staining for light microscopy. Histochem Cell Biol 106:9–17
Acknowledgements
We thank Dr. Zhongwu Zhou and PabloVega for their critical reading of the manuscript. The work was financially supported by National Natural Science Foundation of China (No 21375156); National High Technology Research and Development Program of China (Ministry of Science and Technology 863 Plan) (No 2015AA021104); Frontier Research Key Projects of Chongqing Science and Technology Committee, [cstc2015jcyjBX0010]; Scientific and Technical Innovation Projects for People’s Livelihood of Chongqing Science and Technology Committee, [cstc2015shms,zx00014]; Benefit Projects for People’s Livelihood by Science and Technology, Chongqing Science and Technology Committee 【cstc2015jcsf8001】,2015.07-2017.07; Fundamental Research Funds for the Central Universities (Fund for Brain Science), (No.10611CDJXZ238826) Partial support from the Center for Food Safety Engineering, Agricultural Research Service, under Agreement No. 1935-42000-049-00D at Purdue University is appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 817 kb)
Rights and permissions
About this article
Cite this article
Wang, R., Xu, Y., Sors, T. et al. Impedimetric detection of bacteria by using a microfluidic chip and silver nanoparticle based signal enhancement. Microchim Acta 185, 184 (2018). https://doi.org/10.1007/s00604-017-2645-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2645-x