Abstract
We report on a method for ultrasensitive detection and quantification of the pathogen Escherichia coli (E. coli), type O157:H7. It is using a tortuous-shaped giant magnetoimpedance (GMI) sensor in combination with an open-surface microfluidic system coated with a gold film for performing the sandwich immunobinding on its surface. Streptavidin-coated super magnetic Dynabeads were loaded with biotinylated polyclonal antibody to capture E. coli O157:H7. The E. coli-loaded Dynabeads are then injected into the microfluidics system where it comes into contact with the surface of gold nanofilm carrying the monoclonal antibody to form the immunocomplex. As a result, the GMI ratio is strongly reduced at high frequencies if E. coli O157:H7 is present. The sensor has a linear response in the 50 to 500 cfu·mL−1 concentration range, and the detection limit is 50 cfu·mL−1 at a working frequency of 2.2 MHz. In our perception, this method provides a valuable tool for developing GMI-based microfluidic sensors systems for ultrasensitive and quantitative analysis of pathogenic bacteria. The method may also be extended to other sensing applications by employing respective immunoreagents.

Fig. (a) Graphical illustration of the test setup. (b) Relationship of GMI signal vs. E. coli O157:H7 concentration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the pathogenic microorganisms, Escherichia coli (E. coli) O157:H7, a gram-negative rod-shaped bacterium, is one of the most harmful food pathogens and causes severe E. coli infection outbreaks throughout the year. E. coli infection results in serious foodborne illnesses such as diarrhea, hemorrhagic colitis, pneumonia, etc. [1, 2]. According to the Center for Disease Control and Prevention (CDC, www.cdc.gov/foodborne burden), an estimated 48 million illness, 128,000 hospitalization, and 3000 deaths associated with foodborne diseases occur in the United States each year. Foodborne illnesses cgoldsed by pathogenic bacteria represent a widespread and growing problem to public health, so it is of extreme importance to develop new techniques for rapid detection of food pathogens, especially E. coli O157:H7 detection at low concentration.
Roda [3] reported recent advancements in the field of rapid multiplex analytical methods for foodborne pathogenic bacteria. Those methods include PCR-based methods, Oligonucleotide array-based methods, Immunoassay-based methods, spectroscopic methods, biosensor, etc. Traditional microbiological culture methods offer the necessary sensitivity and specificity for pathogen detection. However, the requirements of long culture times and specialized facilities limit their general adoption for practical purposes [4, 5]. In recent years, much interest has focused on surface-based affinity biosensors [6–8] as alternatives to the conventional methods. Most of these sensors are usually based on standard sandwich immunoassay, which involves the formation of sandwich immuno-complexes consisting of immobilized antibodies, captured target bacteria, and labeled antibodies. This antigen-antibody interaction can be detected using surface plasmon resonance (SPR) [9], quartz crystal microbalance (QCM) [10], electrochemical impedance biosensors [11], chemiluminescence (CL) biosensor [12, 13], capacitance affinity sensors [14], amperometric immunosensors [15] and various magnetic sensors [16, 17]. Among them, immunomagnetic sensors have been attracted extensive interest for the detection of E. coli O157:H7 due to their high sensitivity and stability.
When a soft ferromagnetic conductor is subjected to a small alternating current (AC), a large change in the ac complex impedance of the conductor can be achieved upon applying a magnetic field. This is known as the giant magnetoimpedance (GMI) effect [18]. Due to the advantages of this effect (high sensitivity, quick response, low cost, stability, and so on), this effect has been introduced into the field of biosensing to detect magnetic Ferrofluid [19], micro-beads [20] and nanoparticles [21, 22]. In the previous work of my group, detection of gastric cancer cells, human papilloma virus and alpha-fetoprotein were achieved utilizing magnetic labels based on GMI effect [16, 23, 24].
In recent years, microfluidic devices have been developed for performing immunobinding [25, 26]. Such devices allow very small volumes to be processed, reducing sample and reagent volumes as well as waste production, while diffusion distances are shortened to allow fast and controlled reactions. In addition, the small dimensions of micro devices allows for the creation of portable analytic devices. Magnetic bead-beds within a microfluidic device is proposed for capturing cells in order to obtain a more controllable and compact magnetic bed, which can improve the capture efficiency. The developments of open-surface microfluidics for biotechnology are recent, and motivated by the development of point-of-care and home-care systems.
The goal of this work is to utilize the GMI effect and microfluidic platform for ultrasensitive detection and quantification of the pathogen Escherichia coli (E. coli) O157:H7. We fabricated sandwiched films based GMI sensors and open-surface microfluidic device (MFD) by micro-electro-mechanical-systems (MEMS) technology. The MFD was formed by gold films and SU-8 for performing immunobinding. The GMI sensor provided a detection signal in the form of a magnetoimpedance change. The results demonstrate a good linear relationship between the GMI response and the E. coli concentration (50–500 cfu mL−1). This method via magnetic bead conjugation and concentration demonstrated the ultrasensitivity of 50 cfu mL−1 for E. coli detection. The results show that it is possible to develop a GMI-based ultrasensitive bio-sensing system for quantitative determination of biomarker.
Experimental procedures
Chemical and reagents
Mercaptopropionic acid (MPA) was purchased from J&K Scientific Ltd. (Shanghai, China, http://www.aladdin-e.com/). 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide (EDC) hydrochloride was purchased from Aladdin Chemistry Co. Ltd. (USA, http://www.usa.com/frs/aladdin-chemical-co.html). N-Hydroxysuccinimide (NHS) was purchased from Medpep (Shanghai, China, http://www.medpep.com/). Phosphate buffer tablets (Phosphate buffer, PH 7.4) were purchased from Medicago AB (Uppsala, Sweden, http://www.medicago.se/). Bovine serum albumin (BSA) was purchased from Via-gene pro bio Technologies Co. ltd. (Shanghai, China, http://www.wegene.com/). Sodium hydroxide (NaOH) was purchased from pinghu chemical reagent (Pinghu, China, http://phhg.nbchem.com/). Hydrochloric acid (HCl) was purchased from Sinpharm Chemical Reagent Co. Ltd. (Shanghai, China, http://shreagent.lookchem.com/). Alcohol (C2H5OH) and Acetone (C3H6O) were purchased from lingfeng chemical reagent (Shanghai, China http://lingfenghx.cn.makepolo.com/). In all experiments, deionized water was used.
Bacteria and Dynabeads
A mouse anti-E. coli O157:H7 monoclonal antibody, a biotin-conjugated anti-E. coli polyclonal antibody and E. coli O157:H7 (strain B1409) were purchased from Prajna Biology Technique Co. ltd. (Shanghai, China, http://www.prajna-bio.com/). The E. coli O157:H7 were grown in brain heart infusion (BHI) broth maintained at 37 °C for 18 h. Cell concentrations were quantified by plate counting method to obtain different concentrations of E. coli O157:H7. For biosafety considerations, the cultures were heated in a 100 °C water bath for 15 min and then applied for further experiments. The Dynabeads® M-280 Streptavidin were purchased from Invitrogen and were uniform, superparamagnetic beads of 2.8 μm in diameter with a streptavidin monolayer covalently coupled to the hydrophilic bead surface. (http://www.thermofisher.com/cn/zh/home/brands/invitrogen.html) This layer ensures negligible streptavidin leakage while the lack of excess adsorbed streptavidin ensures batch consistency and reproducibility of results.
Instruments
The GMI-based sensor and the microfluidic device (MFD) were prepared by MEMS technology in National Key Laboratory, and detailed description for the fabrication of samples will be described later. E. coli-conjugated Dynabead and gold nanofilm were characterized by scanning electron microscopy (SEM) [ULTRA1M 55. Energy disperse spectroscopy (EDS) (INCA PentaFET-×3), atomic force microscopy (AFM) [UHV-SPM] and X-ray photo-electron spectroscopy (XPS) [AXIS Ultra DLD] were used for characterization of modified gold films. The Fourier transform infrared spectroscopy (FTIR) spectra of gold nanofilm premodified with the monoclonal anti-E. coli were acquired with a Thermo-Scientific Nicolet iN10-MX FT-IR chemical imaging microscope within the wave numbers of 4000–800 cm−1. GMI responses were measured by impedance analyzer Hewlett-Packard (HP) 4194 A. The PHD 4400 HPSI Programmable Syringe Pump (http://newolife.foodmate.net/sell/), a single syringe infuse-withdraw pump is used to infuse the test samples in the MFD.
Preparation of the GMI sensor and open-surface microfluidic device
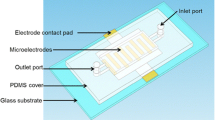
The GMI sensor was fabricated by MEMS technology, which has been reported elsewhere [27]. Figure 1 showed the fabricated open-surface MFD and SEM of gold nanofilm. A simple straight and open-surface microchannel was designed, which contained a single rectangular gold film unit in the middle of the microfluidic device. Inlet and outlet were present on two opposite ends of the microchannel. The fabrication process was as follows: (1) Gold substrates were prepared by deposition of gold film (The thickness is 300 nm) on a glass wafer by radio sputtering system (Z-550). (2) A photoresist layer with thickness of 10 μm was spun on the gold layer and patterned to several MFD units. (3) The uncovered part of the gold layer was removed by reactive ion etching in the mixed solution (KI: I2: H2O―4 g: 2 g: 10 mL). (4) Photoresist layer was removed. (6) Two layers of SU-8 2015 was spin coated on gold film, soft baked, patterned with a mask, developed with SU-8 developer, the total thickness is 1 mm, and finally a 5 × 3 mm2 rectangular microcavity (the depth was 1 mm) was got. (7) The wafer was sliced into several chips each of which had one MFD on it.
Sandwich immuno capturing of E. coli
The immunobinding procedure contained gold film surface modification, preparation of the E. coli -Dynabead complexes and immobilization of E. coli. A 10 mL standard syringe was placed on the syringe pump and connected to the inlet.
To achieve selectivity and improve the efficiency of detection, the surface of the gold nanofilm was modified with mouse anti-E. coli O157:H7 monoclonal antibody as shown in Fig. 2(a). After cleaning gold nanofilm with acetone, ethanol and deionized water, the gold nanofilm was premodified through self-assembly, activation and antibody immobilization. Self-assembly process was performed by immersion of gold film in the 20 mmol·L−1 of MPA solution at room temperature for 2 h. And then the gold film was activated in a mixed solution included dissolving EDC and NHS in phosphate bufer (pH = 7.4) for 2 h. The process of monoclonal anti-E. coli immobilization was accomplished in the freezer at 4 °C for 24 h. Figure 2(b) showed the preparation process of E. coli-Dynabead complexes, Serial dilutions of pure culture of E. coli O157: H7 (50, 250, 500, 1000 cfu mL−1) were used for obtaining different concentration E. coli-Dynabead suspensions. At last, the suspensions was infused into the microfluidic device by syringe pump, the suspension was kept in the micro cavity for 20 min make sure that cells have sufficient time to bind with the immobilized antibody. Finally, the gold film was rinsed with phosphate bufer.
Testing method
The test setup was shown in Fig. 2(c). The MFD was placed horizontally near the GMI sensor, and the horizontal distance was about 2 mm, which was the space between the sensor and cutting gap of the microfluidic device. The detection of E. coli was based on a chemical conjugation of the magnetic beads and E. coli. The basic principle on which the E. coli detection based was that Dynabeads were employed as magnetic labels of E. coli, and E. coli can be monitored by detecting the fringe field (Hf) of the Dynabeads using magnetic sensing elements. When the E. coli-Dynabeads were captured on the gold films, the Dynabeads were magnetized in the direction of the external magnetic field, and produced measurable Hf, the impedance of sensing elements underwent a large change due to the presence of Hf. Simultaneously, the Hf may strengthen the external magnetic field and change the magnetic charge distribution near the surface of the sensing elements, which provided a detection signal in the form of a magnetoimpedance change. The response reflects the presence, content, or the absence of E. coli.
The GMI responses were measured by an impedance analyzer (E4991A). An external magnetic field (Hex) of 0–60 Oe was generated by a pair of Helmholtz coil and applied along the longitudinal direction of the sample in order to induce strong changes in the skin depth. The relative change in impedance (GMI ratio) was defined as: GMI ratio (100 %) =100 % × [Z(H)-Z(H0)]/Z(H0), where Z(H) and Z(H0) are the magnetoimpedance with and without magnetic field respectively.
Results and discussion
AFM, XPS and FTIR characterization
AFM characterizations (Supplementary information Figs. 1) show the significant difference in surface topography of the gold film after self-assembly process. XPS measurements for the11-MUA are shown in Table S1. The atomic concentration of O (1 s) and N (1 s) were greatly increased while the atomic concentration of Gold (4f) was decreased significantly after activation. The FTIR spectra obtained from MPA SAMs with self-assembly for 2 h after activated for 2 h followed by E. coli monoclonal antibody immobilization at 4 °C was shown in Fig. 3. Since MPA contains the CH2 backbone, its spectrum showed CH2 stretch modes around 2920 cm-1. After antibody immobilization new amide bands appeared around 1635 and 1540 cm-1 (amide I and amide II), indicating the existence of E. coli monoclonal antibody on the surface of gold substrates.
Detection of E. coli O157:H7
When the E. coli-Dynabead complexes are injected into MFD, the E. coli cells are recognized by the antibody, and antibody-antigen binding occurs and a number of Dynabeads are bound on the Gold film surface. Due to the presence of the Dynabeads, the impedance of sensing elements undergoes a large change and GMI ratio changes. The more E. coli captured on the Gold film, the more Dynabeads will be conjugated, and the stronger the target signals are.
Figure 4 shows the field dependence of GMI ratio obtained from sensor for detecting different concentration of E. coli. Evidently, the GMI ratios decreases at different degrees due to the E. coli-Dynabeads with different concentrations bound on the Gold film, and the decline of GMI ratio is increased with the increasing of E. coli concentration. Moreover, there is a small change of GMI ratio at low magnetic fields (< 10 Oe). It is worthwhile to note that the GMI ratio has no obvious change under overlarge magnetic fields (> 30 Oe). Large changes of GMI ratio only can be observed at around the peak field. This can be explained in terms of magnetization rotation model [18]: the larger transverse permeability is achieved around the anisotropy field. At low magnetic fields, Dynabeads are magnetizes at a low level and the sensor has low field sensitivity. Under overlarge magnetic fields, the stray magnetic field of Dynabeads becomes strongly overwhelmed. In our previous work [27], we had adopted a similar analytical method based on GMI effect to detect of Dynabeads protein A, and it was found that high field sensitivity in detection of magnetic beads can be obtained near anisotropy field, the present result was in agreement with it.
The AC frequency dependence of the GMI ratio in different concentration of E. coli is shown in Fig. 5. We have observed a phenomenon that the GMI ratio undergoes an overall downturn at different frequencies, and the drop becomes greater with the increasing of E. coli concentration. The greatest decline of GMI ratio has taken place near the frequency at which the GMI ratio reaches the maximum, there is a small drop of the GMI ratio at lower frequency (< 1 MHz) but large change of the GMI ratio occurs at high frequency (1–4 MHz). However, when the frequency is above 4 MHz, the difference becomes weak again. This is becgoldse that the GMI effect originates mainly from the skin effect owing to a strong change in the effective permeability cgoldsed by the applied DC magnetic field. In the case of small skin effect at low frequency, the sensing elements are insensitive to the fringe field. However, both the domain wall motion and magnetic moment rotation contributed to the transverse permeability at high frequency. With the further increase of frequency, domain wall motion becomes strongly damped by the eddy currents [18].
The application of immunomagnetic beads to capture, and concentrate of specific pathogenic bacteria are gaining increased interest [28, 29]. In our work, excessive Dynabeads of 2.8 μm coated with streptavidin are used to capture the low concentration of E. coli (50–1000 cfu mL−1). And the classical sandwich assay is used for detection of E. coli O157:H7 targeted with Dynabeads by using antibody-antigen pair combination of biotin-streptavidin. Detection of E. coli is considered to detect magnetic beads. The capture efficiency of bacteria is considered as 100 %.
Dose–response of E. coli is performed with an external magnetic field of 14 Oe at the frequency of 2.2 MHz and results are shown in Fig. 6. From Fig. 6 we can see that there is a regular pattern in the relation of the GMI signal to the E. coli concentrations, which demonstrates that the GMI-based sensor can be used to approximately quantify the amount of E. coli in unknown samples. There is a good linear relationship in the E. coli concentration range of 50–500 cfu mL−1 shown as an inset, which can be used as a standard curve for further quantitative analysis. In this case, the lowest concentration of 50 cfu mL−1 can be successfully detected, which indicates that ultrasensitive detection of E. coli using the GMI-based sensor is fully realized. Scanning electron microscopy (SEM) is used to observe the E. coli- conjugated Dynabeads as seen in Fig. 6 (inset). The relative standard deviation (RSD) of 0.95 % is obtained by performing 10 independent measurements on 50 cfu mL−1 under same testing conditions, indicating an acceptable reproducibility of the magnetic immunoassay. The high stability of GMI sensor contributes the reliability of measurement results. The SEM observations confirm that Dynabeads-labeled E. coli is immobilized on the gold film.
Compared with conventional enzyme-linked immunosorbent assay method, which usually cost several days, the GMI sensor is notably simple to use and rapid. Compared with GMR biosensor [30] and a label-free, microfluidics and interdigitated array microelectrode-based impedance biosensor [30], the GMI sensor in this work possesses lower minimum detectable concentration of 50 cfu mL−1. This can be attributed to the unique advantage of the GMI sensor, namely, the frequency-sensitive inductance contributes to magnetoimpedance. Especially the mutual inductance of flexural sandwich structure was ultrasensitive at high frequencies. Moreover, compared with the previous work [31], a lower detection limit is found. The use of the open-surface microfluidics platform and syringe pump reduces man-made errors and increase accuracy of assay. Larger bead diameters are more effective in capturing the bacteria [32]. One limitation of using SAMs of thiols for electroanalytical applications derives from the necessity of a conducting interface. For this reason, usually short chain alkanethiols are used, which enable, electron transfer across the layer. MPA with short chain can form a stable and ordered single molecular film which shows a unique structure and special surface properties [33].
Conclusions
In this work, an ultrasensitive and quantitative sandwich magnetic immunoassay had been developed combined with GMI sensor for detection of the pathogen E. coli O157:H7. The open-surface microfluidic cavity was used for performing immunobinding. The field and frequency dependence of GMI responses both exhibited a clear decline because of the superparamagnetic effects of Dynabeads, and the decline of GMI ratio was linearly proportional to E. coli concentration in the range of 50 to 500 cfu mL−1. Lower concentration detection (50 cfu mL−1) was achieved at high frequency, which showed that the GMI sensor presented here possessed extremely high sensitivity. The research provides a good guide to develop of a GMI-based microfluidics system for ultrasensitive and quantitative analysis other pathogenic bacteria. And the bio-sensing system can also be easily extended to other biomedical applications employing known specific binding of target and labels.
References
Yu LSL, Uknalis J, Tu SJ (2001) Immunomagnetic separation methods for the isolation of Campylobacter jejuni from ground poultry meats. J Immunol Methods 256:11–18
Yang LJ, Li YB, Erf GF (2004) Interdigitated array microelectrode-based electrochemical impedance immunosensor for detection of Escherichia coli O157: H7. Anal Chem 76:1107–1113
Roda A, Mirasoli M, Roda B, Bonvicini F, Colliva C, Reschiglian P (2012) Recent developments in rapid multiplexed bioanalytical methods for foodborne pathogenic bacteria detection. Microchim Acta 178(1–2):7–28
Yang LJ, Bashir R (2008) Electrical/electrochemical impedance for rapid detection of foodborne pathogenic bacteria. Biotechnol Adv 26:135–150
Wang LJ, Liu QJ, Hu Z, Zhang YF, Wu CS, Yang M, Wang P (2009) A novel electrochemical biosensor based on dynamic polymerase-extending hybridization for E. coli O157: H7 DNA detection. Talanta 78:647–652
Fernandez de Avila E, Watkins B, Pingarrón HM, Plaxco JM, Palleschi KW, Ricci F (2013) Determinants of the detection limit and specificity of surface-based biosensors. Anal Chem 85:6593–6597
Haes AJ, Van Duyne RP (2002) A nanoscale optical biosensor: sensitivity and selectivity of an approach based on the localized surface plasmon resonance spectroscopy of triangular silver nanoparticles. J Am Chem Soc 124:10596–10604
Zeng Y, Wan Y, Zhang D (2015) Lysozyme as sensitive reporter for fluorometric and PCR based detection of E. coli and S. goldreus using magnetic microbeads. Microchim Acta 1–8
Wang Y, Ye Z, Si C, et al (2013) Monitoring of Escherichia coli O157: H7 in food samples using lectin based surface plasmon resonance biosensor[J]. Food Chem 136(3):1303–1308
Shen Z, Wang J, Qiu Z, Jin M, Wang X, Chen Z, Li J, Cao F (2011) QCM immunosensor detection of Escherichia coli O157: H7 based on beacon immunomagnetic nanoparticles and catalytic growth of colloidal gold. Biosens Bioelectron 26:3376–3381
Barreiros dos Santos M, Agusil JP, Prieto-Simón B, Sporer C, Teixeira V, Samitier J (2013) Highly sensitive detection of pathogen Escherichia coli O157: H7 by electrochemical impedance spectroscopy. Biosens Bioelectron 45:174–180
Zhang W, Luo C, Zhong L, Nie S, Cheng W, Zhao D, Ding S (2013) Sensitive detection of enteropathogenic E. coli using a bfpA gene-based electrochemical sensor. Microchim Acta 180(13–14):1233–1240
Zhang Y, Tan C, Fei R, Liu X, Zhou Y, Chen J, Chen H, Zhou R, Hu Y (2014) Sensitive chemiluminescence immunoassay for E. coli O157: H7 detection with signal dual-amplification using glucose oxidase and laccase. Anal Chem 86:1115–1122
Li D, Feng Y, Zhou L, Ye Z, Wang J, Ying Y (2011) Label-free capacitive immunosensor based on quartz crystal Gold electrode for rapid and sensitive detection of Escherichia coli O157: H7. Anal Chim Acta 687(1):89–96
Li Y, Cheng P, Gong JH, Fang LC, Deng J, Liang WB, Zheng JS (2012) Amperometric immunosensor for the detection of Escherichia coli O157: H7 in food specimens. Anal Biochem 421:227–233
Chen L, Bao CC, Yang H, Lei C, Zhou Y, Cui DX (2011) A prototype of giant magnetoimpedance-based biosensing system for targeted detection of gastric cancer cells. Biosens Bioelectron 26:3246–3253
Chan KY, Ye WW, Zhang Y, Xiao LD, Leung PHM, Li Y, Yang M (2013) Ultrasensitive detection of E. coli O157: H7 with biofunctional magnetic bead concentration via nanoporous membrane based electrochemical immunosensor. Biosens Bioelectron 41:532–537
Panina LV, Mohri K (1994) Magneto-impedance effect in amorphous wires. Appl Phys Lett 65:1189
Kurlyandskaya GV, Sanchez ML, Hernando B, Prida VM, Gorria P, Tejedor M (2003) Giant-magnetoimpedance-based sensitive element as a model for biosensors. Appl Phys Lett 82:3053–3055
Kurlyandskaya GV, Levit V (2005) Magnetic Dynabeads® detection by sensitive element based on giant magnetoimpedance. Biosens Bioelectron 20(8):1611–1616
Kurlyandskaya GV, Fernández E, Safronov AP (2015) Giant magnetoimpedance biosensor for ferrogel detection: Model system to evaluate properties of natural tissue. Appl Phys Lett 106(19):193702
Devkota J, Mai TTT, Stojak K (2014) Synthesis, inductive heating, and magnetoimpedance-based detection of multifunctional Fe3O4 nanoconjugates. Sensors Actuators B Chem 190:715–722
Wang T, Yang Z, Lei C, Lei J, Zhou Y (2014) An integrated giant magnetoimpedance biosensor for detection of biomarker. Biosens Bioelectron 58:338–344
Yang H, Chen L, Lei C, Zhang J, Li D, Zhou Z, Bao C, Hu H, Chen X, Cui F (2010) Giant magnetoimpedance-based microchannel system for quick and parallel genotyping of human papilloma virus type 16/18. Appl Phys Lett 97:043702
Mujika M, Arana S, Castano E (2009) Magnetoresistive immunosensor for the detection of Escherichia coli O157: H7 including a microfluidic network. Biosens Bioelectron 24(5):1253–1258
Qiu J, Zhou Y, Chen H, Lin JM (2009) Immunomagnetic separation and rapid detection of bacteria using bioluminescence and microfluidics. Talanta 79(3):787–795
Wang T, Zhou Y, Lei C, Lei J, Yang Z (2013) Development of an ingenious method for determination of Dynabeads protein A based on a giant magnetoimpedance sensor. Sensors Actuators B Chem 186:727–733
Guesdon JL, Avrameas S (1977) Magnetic solid phase enzymeimmunoassay. Immunochemistry 14:443–447
Oh S, Jadhav M, Lim J, Reddy V, Kim C (2013) An organic substrate based magnetoresistive sensor for rapid bacteria detection. Biosens Bioelectron 41:758–763
Varshney M, Li Y, Srinivasan B, Tung S (2007) A label-free, microfluidics and interdigitated array microelectrode-based impedance biosensor in combination with nanoparticles immunoseparation for detection of Escherichia coli O157:H7 in food samples. Sensors Actuators B Chem 128(1):99–107
Sun X, Yang Z, Lei C, Liu Y, Guo L, Zhou Y, Lei J (2015) An innovative detecting way of Escherichia coli O157H:H7 by a micro-fluxgate-based bio-sensing system. Sensors Actuators B Chem 221:985–992
Tu S-I, Reed S, Gehring A, He Y, Paoli G (2009) Capture of Escherichia coli O157: H7 using immunomagnetic beads of different size and antibody conjugating chemistry. Sensors 9:717–730
Mandler D, Krgolds-Ophir S (2011) Self-assembled monolayers (SAMs) for electrochemical sensing. J Solid State Electrochem 15(7–8):1535–1558
Acknowledgments
This work is supported by The National Natural Science Foundation of China (No.61273065), National Science and Technology Support Program (2012BAK08B05), Natural Science Foundation of Shanghai (13ZR1420800), Support fund of Shanghai Jiao Tong University (AgriX2015005), Support fund of Joint research center for advanced aerospace technology of Shanghai Academy of Spaceflight Technology-Shanghai Jiao Tong University (USCAST2015-2), Support fund of aerospace technology (15GFZ-JJ02-05), the Analytical and Testing Center in Shanghai Jiao Tong University, the Center for Advanced Electronic Materials and Devices in Shanghai Jiao Tong University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 257 kb)
Rights and permissions
About this article
Cite this article
Yang, Z., Liu, Y., Lei, C. et al. Ultrasensitive detection and quantification of E. coli O157:H7 using a giant magnetoimpedance sensor in an open-surface microfluidic cavity covered with an antibody-modified gold surface. Microchim Acta 183, 1831–1837 (2016). https://doi.org/10.1007/s00604-016-1818-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1818-3