Abstract
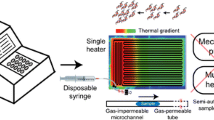
The authors describe a microreactor for performing a multiplexed polymerase chain reaction (PCR) which is operated with minimal accessories such as a single heater for gene amplification and a hand-held syringe for sample actuation. It was fabricated by wrapping a polytetrafluoroethylene (PTFE) tube around a rectangular poly(dimethylsiloxane) (PDMS) block having a predetermined thickness. The resulting portable microreactor was applied to PCR using a single heater because the rectangular PDMS block spatially segregated the upper and lower arrays of the PTFE tube. This warrants the adjustment of distinct temperatures inside the upper and lower tubes. A hand-held plastic syringe was connected to the inlet of the PTFE tube and used as a portable pump to achieve a homogeneous transport of a sample liquid inside the microreactor. The use of gas-impermeable PTFE prevents the formation of bubbles during thermal cycling. The microreactor was successfully applied to the amplification of typical DNA fragments of three foodborne pathogens in less than 30 min. In our perception, this method paves the way to the construction of a truly portable PCR chip that is applicable to rapid clinical diagnosis and the detection of foodborne pathogens.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Micro total analysis systems are becoming increasingly important as portable devices for various biological and chemical applications. In early days, microdevices were fabricated using hard materials such as silicon, and glass [1, 2]. Although the mechanical durability and chemical resistance inside microchannels made from the said materials were high, the fabrication requiring photolithographic process was costly. As an alternative, microdevices have been built using a soft and transparent elastomer, poly(dimethylsiloxane) (PDMS), because of replica molding, ease of bonding, and low fabrication cost [3]. PDMS is a multifunctional material with numerous potential applications, some of which are already realized in the versatile fields of research. Nevertheless, PDMS has limited solvent resistance, and its high porosity is a disadvantage when the device must be heated, as this will result in sample loss inside a microchannel where the surface effect predominates over volume [4, 5]. Thermoplastics are gaining a lot of attention because of their hardness, transparency, low price, ease of molding, and potential for mass production [6, 7]. Despite the numerous advantages, however, microdevices made of thermoplastics deform easily when heated above the glass transition temperature (T g), and except for a few thermoplastics such as polycarbonate [8, 9] and poly(methyl methacrylate) [10, 11], most of them have a low T g, and therefore, they are inappropriate for conducting polymerase chain reactions (PCRs) that require temperatures as high as 95 °C.
For microdevice fabrication, new materials were explored to overcome the shortcomings of the abovementioned conventional materials. Of the emerging materials, fluoropolymers are gaining popularity owing to their low gas permeability, surface inertness, and high chemical resistance to organic solvents [12,13,14]. Of the various forms of fluoropolymer, the use of polytetrafluoroethylene (PTFE) capillary tube has been so far applied for continuous-flow PCR. In 1994, the first use of a single Teflon capillary tube as a high speed PCR reactor achieved amplification in less than 20 min [15]. However, three successive heaters were used for the reaction, and the sample was introduced into the reactor using a pump. Wang et al. used a single PTFE capillary tube placed on three copper heating blocks as an oscillatory-flow PCR reactor in which repeated back and forth movements of a sample plug within a short distance enabled genetic amplification [16]. Li et al. used a long PTFE capillary tube for performing a continuous-flow PCR employing cylindrical copper heating blocks [17]. The abovementioned fluoropolymer-based continuous-flow microdevices exhibit a big advantage in simplifying the fabrication process while maximizing the device performance, making continuous-flow PCR a potential tool for multiplex analysis besides other common configuration like microarray-based amplification [18, 19] or digital PCR [20]. However, the use of multiple heater for amplification and pump systems for sample delivery reported in the current continuous-flow PCR systems still remain challenging in realizing a truly portable microdevice. This challenge, however, can be resolved by the construction of a 3D microreactor with minimal accessories required for performing PCR as presented in our study.
In this study, we fabricated a tube-type 3D Teflon microreactor employing a PTFE tube. PTFE, the commercial name of which is Teflon, is a type of thermoplastic with relatively high T g of approximately 115 °C [13, 21]. Owing to its high thermal stability [22, 23] and relatively low gas permeability [24], PTFE can be used satisfactorily for performing a flow-through PCR during the denaturation process, which requires a temperature as high as 95 °C while suppressing bubbles [25], one of the critical issues when performing an on-chip PCR. Most of all, capillary PTFE tubes are readily available commercially and the issue of device sealing is resolved because the tube itself is adopted as a complete unit for constructing the device. Therefore, there is no assembly process during which tiny pores can form and act as a nucleation site for bubble generation. For these reasons, PTFE tube was selected to fabricate the microreactor non-photolithographically by wrapping the tube around a PDMS mold having a particular thickness. Here, we aim to downsize the overall system as well as the PCR chip and operational accessories, which hinder system miniaturization. In our previous studies, we have successfully demonstrated a flow-through PCR employing a single heater [26, 27] as an attempt to minimize the number of heaters required for the reaction. We have also demonstrated replacing the bulky mechanical syringe pump with a hand-held plastic syringe to achieve a self-powered sample delivery [28,29,30]. Here, we perform a flow-through PCR using a single heater for genetic amplification and a hand-held plastic syringe for sample delivery inside a PTFE tube over 2 m long. We varied and systematically tested several operational parameters to examine the controllability of the flow rate, and we evaluated the feasibility and practical applicability of the introduced system by amplifying gene fragments as effective markers for diagnosing multiple foodborne pathogens. In addition, multiplex PCRs were performed for detecting three most familiar foodborne pathogens [31], namely, Salmonella spp., Staphylococcus aureus (S. aureus), and Escherichia coli (E. coli) O157:H7 using the 3D spiral PTFE microdevice.
Principle
As shown in Fig. 1(a), a portable syringe is connected into a PTFE tube at the inlet, and a silicone tube is inserted into the outlet of the thermoplastic microdevice. The tube at the outlet of the microdevice is made completely air-tight using a clip. V 1 represents the total volume of air contained inside the syringe as well as the posterior end of the sample plug, that is, inside the PTFE tubes in both inlet and microchannel behind the sample plug. V 2 represents the volume of air contained in the anterior end of the sample plug, that is, inside the microchannel in front of the sample plug and the silicone tube inserted in the outlet. P 1 and P 2 represent the air pressures in the posterior and anterior ends of the sample plug, respectively. When the air captured inside the syringe and the fluidic conduit (referred to as microchannel and silicone tube) is compressed with a piston, the pressure inside the closed fluidic conduit becomes higher than the atmospheric pressure. Once the sample plug, originally positioned at the corner of the syringe (shown in Fig. 1(a)), has moved into the tip of the syringe and entered into the fluidic conduit, the sample plug completely prevents air exchange between the anterior and posterior ends of the sample plug, physically segregating the two ends. At this moment, the pressure exerted by the air on both ends of the sample plug becomes equal.
Because air diffuses out through the anterior end of the sample plug, that is, through the gas-permeable silicone tube outlet, this results in a decrease of P 2. However, as no air diffuses out through the plastic syringe at the posterior end of the sample plug, this results in a constant P 1. Owing to this, the sample moves toward the outlet in the initial stage. Considering the ideal gas law, PV = nRT, where n is the number of moles of gas, T is the Kelvin temperature, and R is the gas constant, P 1 and P 2 can be rewritten as n 1 RT/V 1 and n 2 RT/V 2, respectively. Therefore, at the same temperature, the pressure drop is mainly dependent on dn and dV.
Assuming that the sample plug is located in the middle of the microchannel, air diffuses out at the anterior end of the sample plug because of the permeability of air through the outlet silicone tube. However, there is no air diffusing out at the posterior end of the sample plug by using PTFE tube at the inlet. Concerning the effect of dn, n 1 > n 2 owing to the large volume of air inside the syringe; however, because the PTFE tube was used in the inlet and silicone tube in the outlet, air diffusion becomes negligible in the posterior end of the sample plug, resulting in dn 1 < dn 2. Concerning the effect of dV, as the sample plug moves forward, dV is added to V 1 while dV is subtracted from V 2. However, because V 1 > V 2 owing to the relatively large volume of air contained inside the syringe, the effect of dV on V 1 is negligible; thus, we can assume that V 1 is maintained almost constant, whereas the effect of dV on V 2 is significant. Because the effects of both dn 1 and dV 1 are negligible, we can assume that P 1 is almost constant. However, P 2 is significantly affected by both dn 2 and dV 2. Nevertheless, as the gas continuously diffuses out and the sample plug progresses forward, both n 2 and V 2 decreases, resulting in seemingly constant P 2. Because both P 1 and P 2 are maintained constant, the pressure gradient driving the sample plug toward the outlet is also constant throughout the sample flow. The magnitude of the pressure gradient, which determines the speed of the sample flow, is solely dependent on the length of the gas-permeable silicone tube in the outlet, when the wall thickness of the outlet silicone tubes as well as the length of the inlet silicone tube are fixed, because the length of the outlet silicone tube determines dn 2, which determines dV 2, and eventually P 2.
Materials and methods
Chemicals and materials
Polytetrafluorethylene (PTFE) tube (ID = 0.30 mm, OD = 0.76 mm) was purchased from Cole-Parmer ( www.coleparmer.com ) while poly(dimethylsiloxane) (PDMS) prepolymer (Sylgard 184) and a curing agent were purchased from Dow Corning (www.dowcorning.com). The Taq DNA polymerase, PCR buffer, and dNTPs were purchased from Promega (www.promega.com ). Moreover, 100 bp DNA ladder, agarose powder, bovine serum albumin (BSA, V fraction), loading star, human genomic DNA, and premade primers were purchased from Takara (www.takara-bio.com), BioShop (www.bioshopcanada.com), Sigma (www.sigmaaldrich.com), DyneBio (www.dynebio.co.kr), Roche (www.roche.com), and Bioneer (www.bioneer.co.kr), respectively.
Microdevice design and fabrication
To fabricate the microcreactor, the PTFE tube is rotated around a 0.8 cm thick rectangular PDMS block (Fig. 2(a)). The PDMS block was prepared by mixing the PDMS prepolymer and a curing agent in a 10:1 (w/w) ratio and by curing at 80 °C for 30 min. After curing, the PDMS block was cut into 3 × 5 × 0.8 cm in its width, length, and height, respectively, as shown in Fig. 2(a). The PDMS block functions as a physical support for constructing the microreactor. The height of the microreactor was freely adjusted based on the annealing temperature of the target to be amplified, because the temperature gradient is formed in the vertical direction, and the upper layer of the microreactor represents the annealing temperature of the target, while the lower layer represents the denaturation temperature. The total length of the PTFE tube was 250 cm when 30 rotations were made, which corresponded to 30 thermal cycles as shown in Fig. 2(a-1). Each thermal cycle required an 8 cm long tube. After wrapping the tube around the PDMS block, a cyanoacrylate-based instant adhesive (Loctite 401) was used to maintain the tube in a fixed position by reacting for 15 min at room temperature as shown in Fig. 2(a-2). In this way, the rectangular-shaped microreactor was fabricated, and the assembly of the PTFE tube and PDMS block inserted inside the wrapping tube was used as a microreactor for performing the flow-through PCR employing a single heater. Owing to the 3D configuration of the microreactor, two distinct temperature regimes were generated at the top and bottom of the rectangular structure, and the lab-made rubber heater was placed at the bottom of the microreactor to achieve a two-temperature PCR using a single heater. Figure 2(a-3) shows the connection between the PTFE tube and silicone tube (ID = 0.64 mm and OD = 1.19 mm) at the outlet to achieve a semi-automated liquid flow and to obtain a stable flow rate inside the microreactor.
Various methods for foodborne pathogen detection have been reported as shown in Table S1 (Supplementary information). However, the use of a microreactor system based on flow through PCR for performing multiple amplifications simultaneously was used as shown in Fig. 2(b). In this system, the portable PCR microreactor consists of three components: the microreactor, single lab-made rubber heater, and hand-held syringe, which functions as the driving force for sample delivery inside the PTFE tube.
Temperature measurement
The temperatures were measured using an infrared (IR) camera (FLIR Thermovision A320). In this experiment, annealing and extension were performed at the same temperature [ 30 ]. The lab-made rubber heater was used as a source of heat. Ten spots on the surface of the lab-made rubber heater as well as on the upper arrays of the PTFE tube were arbitrarily selected to determine the denaturation and annealing/extension temperatures, respectively. The temperatures were estimated by analyzing the captured images obtained using an image analyzer (ThermaCAM Quick Plot).
Flow-through PCR inside the microreactor
The purified DNA from three foodborne pathogens, namely, Salmonella spp., S. aureus, and E. coli O157:H7 were amplified using the microreactor; the target amplifications were 113, 207, and 384 bp in size, respectively. The primer sequences for amplifying a 113 bp gene fragment in invA gene from Salmonella spp. were as follows: 5′–AAA ACA TAT GCT GGA CCA ACT GGA AGC–3′ (forward) and 5′–GTT CGC TTA ACA AAC GCT GCA AAA CTT–3′ (reverse). The primer sequences for amplifying a 207 bp gene fragment in nuc gene from S. aureus were as follows: 5′–ACA CCT GAA ACA AAG CAT CC–3′ (forward) and 5′–TAG CCA AGC CTT GAC GAA CT–3′ (reverse). The primer sequences for amplifying a 384 bp gene fragment in eaeA gene from E. coli O157:H7 were as follows: 5′–GAC CCG GCA CAA GCA TAA GC–3′ (forward) and 5′–CCA CCT GCA GCA ACA AGA GG–3′ (reverse). The whole-cell bacteria (107 CFU mL−1) from the culture solution were isolated and used as templates (10 ng μL−1) for amplifying the foodborne pathogens. The 25 μL sample plug contained a mixture of DNA template (1 ng μL−1) and PCR reagents. The PCR reagent contained a green-colored buffer, 0.16 mM dNTPs mixture, 1.5 μg μL−1 BSA, 0.5 μM of forward and reverse primers, and 0.06 U μL−1 Taq DNA polymerase. After the PCR reaction, agarose gel electrophoresis was performed, and the results were detected at 254 nm using a UV illuminator. Positive control experiments were performed using a thermal cycler (Bio-Rad C1000) under the same temperature conditions for all three targets. Denaturation and annealing/extension were performed at 95 °C and 58 °C, respectively, and amplifications were performed for 30 thermal cycles.
Results and discussion
Flow analyses: Effect of initial internal pressure on the speed of liquid flow
Prior to performing the PCR using the microreactor, flow analyses were performed inside the microreactor utilizing a 30 mL disposable plastic syringe. Figure 3(a–d) shows the experimental setup and procedures for the preparation of a hand-held pumping device constructed using the disposable plastic syringe [28, 32]. Figure 3(a) shows the overall components for the semi-automated sample injection. First, the outlet part of the PTFE tube was connected with a 5 cm long silicone tube and was made blunt-ended by clamping. Second, the piston was pulled to the brim of the syringe and a red ink (used as the model for a real sample in this flow analyses) was introduced into the tip of the syringe using a pipette (Fig. 3(b)). Once the ink was securely positioned in the corner of the syringe (Fig. 3(c)), the syringe was connected to the inlet of the PTFE tube, and the piston was pushed to a predetermined graduation of the syringe (Fig. 3(d)). Finally, the piston was fixed at the abovementioned position using a wire as shown in Fig. 3(a).
a–d Setup and procedure for constructing the semi-automated sample delivery inside a microreactor using a disposable syringe. e Graph showing the rates of ink flowing inside the microreactor when initial internal pressures were 1.4 and 1.67 atm. Insets above the graphs show the relative positions of the red ink plug inside the microreactor at a specific cycle number. Pairs of syringes in the insets below the graphs show the initial (V initial ) and final (V final ) graduation values for obtaining initial internal pressures of 1.4 and 1.67 atm. The experiments were repeated three times
Figure 3(e) shows the results of flow analyses performed by varying the initial pressure of the syringe. A commercially available 30 mL disposable plastic syringe was used as a hand-held pumping device. Although the maximum volume of the syringe used in this experiment was 30 mL, it reached a volume of almost 35 mL when the piston was pulled to the brim. For this reason, we considered the maximum volume of the air captured inside the syringe to be 35 mL. After connecting the syringe to the inlet of the microreactor, the piston was pushed to a graduation value of 25 mL. Because the outlet is blunt-ended, the air inside the syringe becomes compressed. For example, if the piston graduation was initially 35 mL and was pushed to 25 mL, then the initial internal pressure of the syringe becomes approximately 1.4 atm. In case the piston was pushed to a graduation of 21 mL, the initial internal pressure becomes approximately 1.67 atm. As shown in Fig. 3(e), the flow of the liquid was maintained stable until the ink came out of the outlet. A smaller initial internal pressure resulted in a slower flow rate and vice versa. For example, when the initial internal pressure was 1.4 atm, it took approximately 91.7 s to complete each cycle, and the overall reaction took approximately 45 min, composed of 30 thermal cycles. On the other hand, when the initial internal pressure was 1.67 atm, it took approximately 71.1 s to complete each cycle, resulting in an overall reaction time of 35.5 min. In either case, the flow was highly homogeneous regardless of the initial internal pressure applied. This is probably because of the low gas permeability of the PTFE tube and its tube-type nature, which did not require device assembling. The higher initial internal pressure shortened the overall reaction speed without sacrificing flow rate stability.
Temperature control
Figure 4(a) shows the real image of a single lab-made rubber heater system (4 cm width, 15 cm length, and 10 cm height) consisting of four components (circuit protector, power supply, temperature control, and silicone rubber heater). As shown in Fig. 4(b, c), the IR camera images showing the sides and the top of the microreactor, corresponding to the denaturation and annealing/extension temperature regimes, were measured to be approximately 95.5 ± 1.2 °C (CV = 1.2% (n = 10)) and 58.1 ± 1.2 °C (CV = 2% (n = 10)), respectively. A homogeneous temperature was maintained over 1 h, which was more than sufficient for running one complete reaction (Fig. 4(d)). After the device fabrication, the PDMS block, which was used as a support, was not released so air cannot fill in the space. As the thermal conductivities of PTFE [33, 34] (0.25 W m−1 K−1) and PDMS [35] (0.17 W m−1 K−1) were similar, thermal stability was maintained, while providing structural rigidity to the device. The annealing/extension temperature can be readily controlled by varying the thickness of the PDMS block, making the microreactor a versatile and flexible platform for performing a flow-through PCR for a variety of samples.
Temperature measurement. a Photo of lab-made rubber heater system. b IR camera images showing the side views of both the microreactor and the rubber heater. c IR camera images showing the top and the side views of a microreactor placed on a rubber heater. d IR camera images showing time-dependent temperature changes of the microreactor measured at 0, 5, 30, and 60 min after the heating
On-chip flow-through PCR
Figure 5(a, b) show the photos of a microreactor and a microreactor system equipped with a single lab-made rubber heater and a hand-held plastic syringe. Figure 5(c–e) show the results of the flow-through PCR for detecting three widely known foodborne pathogens: Salmonella spp., S. aureus, and E. coli O157:H7, using the microreactor. Because the annealing temperatures for all three pathogens were within close proximity, the same annealing temperature of 58 °C was applied. An initial internal pressure of 1.4 atm was applied. Figure 5(c) shows the results of 113 bp gene fragments amplified from Salmonella spp. using a thermocycler (lanes 1 and 2) and the microreactor (lane 3). The intensity of the target amplicons obtained using the microreactor was almost identical with that obtained from the thermocycler. Figure 5(d) shows the results of 207 bp gene fragments amplified from S. aureus using a thermocycler (lane 1) and the microreactor (lanes 2 and 3). The average intensity of the target obtained using the microreactor was approximately 87% of that obtained using a thermocycler. Figure 5(e) shows the results of 384 bp gene fragments amplified from E. coli O157:H7 using a thermocycler (lanes 1 and 2) and microreactor (lane 3). The intensity of the amplicons obtained using the microreactor was approximately 80% of that obtained using a thermocycler. The results show that the microreactor offers wide possibilities in the multiple detection of foodborne pathogens in much simpler and rapid manner as compared with traditional methods.
Multiplex PCR on chip
Figure 6 shows the results of the multiplex PCR performed using the microreactor; either two or three sets of primer pairs were used in a single reaction. As shown in Fig. 6, two or three targets were successfully amplified simultaneously with high selectivity and sensitivity, after optimization of reaction conditions by increasing the concentrations of dNTPs and Taq polymerase. In case of multiplex PCR, target bands amplified using the microreactor generally displayed weaker intensity compared to those amplified using the thermocycler. For two-target amplifications, there was a common trend that relatively shorter targets displayed a stronger band, as shown in Fig. 6(a–c). These phenomena can be explained based on the fact that shorter targets can be synthesized more rapidly owing to shorter length. It took less than 30 min for amplifying two targets in one PCR reaction when the initial internal pressure was controlled at 1.67 atm. As demonstrated in Fig. 6(d), three target genes from Salmonella spp., S. aureus, and E. coli O157:H7 were also successfully amplified using the microreactor. When the initial internal pressure was 1.4 atm, the total reaction took approximately 45 min. Obviously, the multiplex PCR is much more complex and difficult to achieve compared with a single-target amplification that is demonstrated by the appearance of a smear (Fig. 6(d)); however, three distinct target amplicons were clearly distinguishable. The method can be used for constructing a portable device that can be directly applied on site with high sensitivity at reduced manufacturing cost for the simultaneous detection of multiple foodborne pathogens with fast reaction.
Results of multiplex PCR realized using the microreactor. Amplifications of (a) Salmonella spp. and E. coli O157:H7 (113 and 384 bp), (b) Salmonella spp. and S. aureus (113 and 207 bp), (c) S. aureus and E. coli O157:H7 (207 and 384 bp), and (d) Salmonella spp., S. aureus, and E. coli O157:H7 (113, 207, and 384 bp). For reaction using the microreactor, initial internal pressure of 1.67 atm was used for all multiplex PCRs
Conclusion
In this study, we introduced a miniaturized PCR device employing a 3D Teflon microreactor equipped with minimum accessories for the rapid identification of multiple foodborne pathogens. The design took advantage of the PTFE tube in terms of material and shape to create a seamless microreactor that aided in the suppression of bubble formation under heated condition and achieving a semi-automated and stable sample transport inside a microchannel over 2 m long. The 3D configuration of the Teflon microreactor enabled the two-temperature PCR to employ only a single heater. The portable PCR microreactor is simple in design and easy to fabricate without demanding highly professional skills, while giving sufficiently reliable results, opening a new perspective in the rapid identification of foodborne pathogens, which are major public health concerns worldwide.
References
Taylor TB, Harvey SE, Albin M, Lebak L, Ning Y, Mowat I, Schuerlein T, Principe E (1998) Process control for optimal PCR performance in glass microstructures. Biomed Microdevices 1:65–70
Effenhauser CS, Bruin GJM, Paulus A, Ehrat M (1997) Integrated capillary electrophoresis on flexible silicone microdevices: analysis of DNA restriction fragments and detection of single DNA molecules on microchips. Anal Chem 69:3451–3457
Xia Y, Whitesides GM (1998) Soft lithography. Annu Rev Mater Sci 28:153–184
Lee JN, Park C, Whitesides GM (2003) Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal Chem 75:6544–6554
Dangla R, Gallaire F, Baroud CN (2010) Microchannel deformations due to solvent-induced PDMS swelling. Lab Chip 10:2972–2978
Lee LJ (2006) Polymer nanoengineering for biomedical applications. Ann Biomed Eng 34:75–886
Wang X, Engel J, Liu C (2003) Liquid crystal polymer (LCP) for MEMS: processes and applications. J Micromech Microeng 13:628–633
Ye MY, Yin XF, Fang ZL (2005) DNA separation with low-viscosity sieving matrix on microfabricated polycarbonate microfluidic chips. Anal Bioanal Chem 381:820–827
Ogończyk D, Węgrzyn J, Jankowski P, Dąbrowski B, Garstecki P (2010) Bonding of microfluidic devices fabricated in polycarbonate. Lab Chip 10:1324–1327
Lounsbury JA, Karlsson A, Miranian DC, Cronk SM, Nelson DA, Li J, Haverstick DM, Kinnon D, Saul DJ, Landers JP (2013) From sample to PCR product in under 45 minutes: a polymeric integrated microdevice for clinical and forensic DNA analysis. Lab Chip 13:1384–1393
Becker H, Gartner C (2008) Polymer microfabrication technologies for microfluidic systems. Anal Bioanal Chem 390:89–111
Arcella V, Ghielmi A, Tommasi G (2003) High performance perfluoropolymer films and membranes. Ann N Y Acad Sci 984:226–244
Dolbier WR (2005) Fluorine chemistry at the millennium. J Fluor Chem 126:157–163
Rae PJ, Dattelbaum DM (2004) The properties of poly(tetrafluoroethylene) (PTFE) in compression. Polymer 45:7615–7625
Nakano H, Matsuda K, Yohda M, Nagamune T, Endo I, Yamane T (1994) High speed polymerase chain reaction in constant flow. Biosci Biotechnol Biochem 58:349-352
Wang H, Zhang C, Xing D (2011) Simultaneous detection of Salmonella enterica, Escherichia coli O157:H7 and Listeria monocytogenes using oscillatory-flow multiplex PCR. Microchim Acta 173:503–512
Li Y, Zhang C, Xing D (2011) Integrated microfluidic reverse transcription-polymerase chain reaction for rapid detection of food- or waterborne pathogenic rotavirus. Anal Biochem 415:87–96
Kersting S, Rausch V, Bier FF, Nickisch-Rosensgk M (2014) Multiplex isothermal solid-phase recombinase polymerase amplification for the specific and fast DNA-based detection of three bacterial pathogens. Microchim Acta 181:1715–1723
Santiago-Felipe S, Tortajada-Genaro LA, Puchades R, Maquieira A (2016) Parallel solid-phase isothermal amplification and detection of multiple DNA targets in microliter-sized wells of a digital versatile disc. Microchim Acta 183:1195–1202
Whale AS, Huggett JF, Cowen S, Speirs V, Shaw J, Ellison S, Foy CA, Scott DJ (2012) Comparison of microfluidic digital PCR using conventional quantitative PCR for measuring copy number variation. Nucleic Acids Res 40:e82
Patrick CR (1958) Thermal stability of polytetrafluoroethylene. Nature 181:698
Teng H (2012) Overview of the development of the fluoropolymer industry. Appl Sci 2:496–512
Zheng W, Wang Z, Zhang W, Jiang X (2010) A simple PDMS-based microfluidic channel design that removes bubbles for long-term on-chip culture of mammalian cells. Lab Chip 10:2906–2910
Chen Z, Qian S, Abrams WR, Malamud D, Bau HH (2004) Thermosiphon-based PCR reactor: experiment and modelling. Anal Chem 76:3707–3715
Robb WL (1968) Thin silicone membranes their permeation properties and some applications. Ann N Y Acad Sci 146:119–137
Trinh KTL, Wu W, Lee NY (2014) Planar poly(dimethysiloxane) (PDMS)-glass hybrid microdevice for a flow-through polymerase chain reaction (PCR) employing a single heater assited by an intermediate metal alloy layer for temperature gradient formation. Sens Actuators B 190:177-184
Wu W, Lee NY (2011) Three-dimensional on-chip continuous-flow polymerase chain reaction employing a single heater. Anal Bioanal Chem 400:2053–2060
Wu W, Trinh KTL, Lee NY (2012) Hand-held syringe as a portable plastic pump for on-chip continuous-flow PCR: miniaturization of sample injection device. Analyst 137:983–990
Wu W, Lee NY (2013) Two-layer microdevice for parallel flow-through PCRs employing plastic syringes for semi-automated sample injection and a single heater for amplification: toward process simplification and system miniaturization. Sens Actuators B 181:756-765
Wu W, Trinh KTL, Lee NY (2015) Flow-through polymerase chain reaction inside a seamless 3D helical microreactor fabricated utilizing a silicone tube and paraffin mold. Analyst 140:1416–1420
Wu S, Duan N, Shi Z, Fang CC, Wang Z (2014) Simultaneous aptasensor for multiplex pathogenic bacteria detection based on multicolour upconversion nanoparticles lables. Anal Chem 86:3100–3107
Wu W, Trinh KTL, Zhang Y, Lee NY (2015) Portable plastic syringe as a self-actuated pump for long-distance uniform delivery of liquid inside a microchannel and its application for flow-through polymerase chain reaction on chip. RCS Adv 5:12071-12077
Kim SL, Choi K, Tazebay A, Yu C (2014) Flexible power fabrics made of carbon nanotubes for harvesting thermoelectricity. ACS Nano 8:2377–2386
Khedkar J, Negulescu I, Meletis EI (2002) Sliding wear behavior of PTFE composites. Wear 252:361–369
Shin YS, Cho K, Lim SH, Chung S, Park SJ, Chung C, Han DC, Jang JK (2013) PDMS-based micro PCR chip with parylene coating. J Micromech Microeng 13:768–774
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. NRF-2017R1A2B4008179) and also by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2014R1A1A3051319).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Trinh, K.T.L., Lee, N.Y. A portable microreactor with minimal accessories for polymerase chain reaction: application to the determination of foodborne pathogens. Microchim Acta 184, 4225–4233 (2017). https://doi.org/10.1007/s00604-017-2451-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2451-5