Abstract
The authors describe an optical assay for determination of mercury(II) ion by making use of gold nanorods (AuNRs) which are found to aggregate after addition of 6-mercaptopurine (6-MP) by forming a covalent Au-S bond. Aggregation is accompanied by a color change to blue. However, the 6-MP induced aggregation is inhibited in the presence of Hg(II) results because of the formation of a more stable Hg-S bond. The different degrees of aggregation of the AuNRs is indicated by variations in the absorption spectra and accompanied by a color change from blue to brown. Under the optimal conditions, the ratio of absorbances at 780 and 665 nm varies in the 1–100 nM Hg(II) concentration range. The effect can also be detected with bare eyes. The limit of detection is 0.48 nM. The method is rapid, sensitive, and does not require sophisticated instrumentation. It was successfully applied to the determination of Hg(II) in spiked water samples.

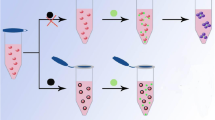
Schematic of colorimetric determination of Hg2+ based on the inhibition of the aggregation of gold nanorods caused by 6-mercaptopurine. This method is sensitive with a 0.48 nM detection limit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The contamination by heavy metals has aroused extensive public concern since the exposure of the Minamata event in the 1950s. Mercury ion (Hg2+), the most stable inorganic form of mercury, is a hazardous environmental contaminant due to its accumulative and high toxic properties [1]. The maximum allowable level of Hg2+ defined by United States Environmental Protection Agency (EPA) is 10 nM in drinking water [2]. The methods for determination of Hg2+ include high performance liquid chromatography (HPLC) [3], inductively coupled plasma mass spectrometry (ICPMS) [4], and atomic absorption spectrometry (AAS) [5]. Although these methods provide adequate qualitative and quantitative determination of Hg2+, the sample preparation is complication and the cost of the equipment is high. An inexpensive, sensitive and rapid analytical method is required and many rapid detection methods for determination of Hg2+ have been reported. These include fluorescent detection methods [6, 7] colorimetric methods [8], electrochemical sensing methods [9, 10] and surface-enhanced Raman spectroscopy methods [11, 12]. The fluorescence quencher [13, 14], oligonucleotide [15], DNA enzyme [16], and colorimetric probe [17,18,19] are used as the probe to determination of Hg2+. Among these probes, the colorimetric probe is paid more attention due to Hg2+ can be determined based on a color change without using special equipment.
Colorimetric sensors of gold nanoparticles (AuNPs) attracted a great deal of interest for their optical properties [20]. Such as large absorption coefficients, and that characters are depended on the size, shape, and local environment of the nanoparticles [21, 22]. When the distance between AuNPs is shorter than the average diameter of the nanoparticles, the color of the AuNPs is changed, which will cause the change of the absorption spectrum of the AuNPs [23]. Recently, several AuNPs based colorimetric probes have been designed for determination of Hg2+ by modifying nanoparticle surfaces with aptamers [24], peptides [25], proteins [26], fluorophores [27], and others [28, 29]. The preparation of these ligands is easy and inexpensive; the use of commercially available ligands is more desirable for production of cost effective and practical colorimetric assays by eliminating synthesis steps [30]. For instance Hg2+ can specifically bind to two thymine bases (T) and mediated the formation of stable DNA duplexes through T–Hg2+–T coordination, various methods have been reported for Hg2+ detection based on this recognition system [31, 32]. For gold nanorods (AuNRs),two absorption bands can be observed, one associated with the transverse mode (~520 nm) and the other with the longitudinal mode (usually >600 nm) [33]. The longitudinal plasmon wavelengths are more sensitive to the changes in the dielectric properties of the surroundings and the sensitivity increases with the aspect ratio of the nanorods [34, 35].
In this work, AuNRs were used as colorimetric probe to determination of Hg2+ in water. 6-mercaptopurine (6-MP) can be strongly adsorbed on the surface of AuNRs by –SH and induce the aggregation of AuNRs. However, in the presence of Hg2+, 6-MP induced aggregation of AuNRs was inhibited and the aggregation degree is related to the concentration of Hg2+. The separation and concentration of the analyte are not required. Hg2+ can be determined with a UV-vis-NIR spectrophotometer and any specialized equipments were not required. This method can be applied to the determination of Hg2+ in environmental samples. The experimental results indicated that this method has some benefits, such as sensitivity, simplicity, speediness and stability.
Experimental
Materials
Tetrachloroauric acid (HAuCl4·4H2O, 99.99%), sodium borohydride (NaBH4, 99%), silver nitrate (AgNO3, 99%), ascorbic acid (AA, 99.7%), cetyltrimethylammonium bromide (CTAB, 99%) and 6-mercaptopurine (6-MP) were purchased from Beijing Ding Guo Biotech. Co. Ltd., China (http://www.bioon.com.cn/show/index.asp?id=123197). The metal salts of analytical reagent grade (>99%) were purchased from Beijing Chemical Reagent Company (Beijing, China, http://www.crc-bj.com/). The stock solution of Hg2+ was prepared by dissolving HgCl2 in ultrapure water with several drops of concentrated hydrochloric acid (HCl). The working solution of Hg2+ was prepared by diluting the stock solution with ultrapure water. Britton-Robinson (BR) buffer was used to control the acidity of the sample solution. Aqua regia solution was used to clean the glassware. Other chemicals used here were of analytical reagent grade and all the solutions used in this study were prepared with ultrapure water.
Equipments
Absorption spectra were recorded on an Australian GBC Cintra 10e UV-vis-NIR spectrophotometer (http://www.quasi-s.com.sg/) in the wavelength range of 400 to 950 nm. The transmission electron microscope (TEM) image was obtained with a Hitachi H 800 transmission electron microscope (http://www.labbase.net/) operated at an accelerating voltage of 200 kV. The sample was prepared by dropping the analytical solutions on the carbon-coated copper grid and dried at room temperature.
Preparation of AuNRs
AuNRs were synthesized by seed-mediated growth method improved by El-Sayed [36, 37] with slight modification. 5.0 mL of 0.2 M CTAB solution and 5.0 mL of 5 × 10−4 M HAuCl4 solution were mixed in a round bottom flask. Then, 0.6 mL of 0.01 M freshly prepared ice-cold NaBH4 solution was added into the flask. The color of the solution changed from dark yellow to brownish yellow immediately after adding NaBH4, which indicated the formation of gold seeds. The mixed solution was vigorously stirred for 2 min and the flask was placed in a water bath at 25 °C. After 2 h, this seed solution was used for the synthesis of AuNRs. Figure 1a shows the TEM image of gold seeds. The gold seeds were of near monodispersion and homogeneous. The shape of gold seed was spherical and the size of gold seed was between 3 to 5 nm.
3.0 mL of 4 mM AgNO3 solution was added into 50.0 mL of 0.2 M CTAB solution under gentle stirring at 25 °C in a flask. After that, 50.0 mL of 1 mM HAuCl4 solution was added into this solution with gently mixed and the resulted solution was referred as growth solution. Then, 0.7 mL of 0.1 M AA was added into the growth solution under gentle stirring. The color of the growth solution changed from dark yellow to colorless. Finally, 240 μL of the gold seed solution was added into the growth solution at 27–30 °C. The gold seed started growing and as the new atoms join the nanocrystal lattice, they were protected by the surfactant monomers from the solution. Silver ions were adsorbed on the surface of gold nanoparticle in the form of AgBr and restricted the growth on the local surface of the gold nanoparticle. The nanorods were not prepared in the absence of silver ions [37]. Excellent tuning of aspect ratios was achieved by changed the content of AgNO3 in the growth solution [38]. The color of the solution gradually changed within 10–20 min. The resulting solution was kept at 27–30 °C for 24 h to ensure the AuNRs reached full growth. The solution was then centrifuged at 14000 rpm for 20 min twice to remove the excess surfactant CTAB. The resulting deposition containing the rods was redisposed in ultrapure water via the ultrasound and AuNRs solution was obtained.
Preparation of water samples
The tap and lake water were collected from the laboratory and Xinyue Lake (Jiaozuo, China), respectively. All the samples collected were spiked with a suitable amount of standard solution of Hg2+. These samples were filtered through a 0.22 μm membrane to remove the suspended particles.
Determination of Hg2+
AuNRs solution was mixed with BR buffer in 5 mL colorimetric tube. 6-MP and the sample solution were added into the colorimetric tube. The obtained solution was diluted to 2.0 mL with ultrapure water and mixed thoroughly with gentle shaken. The resulted solution was incubated for appropriate time at room temperature and the absorption spectrum of the solution was recorded with 1 cm path-length cell.
Results and discussion
Sensing mechanism
Figure 2 show the sensing mechanism of this method. The AuNRs were of monodispersion and the color of the AuNRs appears brownish. After addition of 6-MP into the AuNRs solution in the absence of Hg2+, 6-MP was adsorbed on the surface of AuNRs by the strong covalent interaction between Au and –SH, which destroyed the bilayer structure of AuNRs and caused the aggregation of AuNRs in the solution. The color of the AuNRs solution changed from brownish to blue which can be observed with bare eye. However, in the presence of Hg2+, the color of AuNRs solution changed back to brownish gradually. The absorption spectra of 6-MP-AuNRs remain almost unchanged in the presence of high concentration of Hg2+. The changes in the solution color and UV–vis spectra, induced by the competitive interactions of 6-MP with AuNRs and Hg2+, allowed the colorimetric determination of Hg2+ in aqueous solution by bare eyes or UV–vis–NIR spectroscopy.
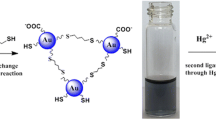
In this system, 6-MP and Hg2+ played the role of aggregation reagent and anti-aggregation reagent, respectively. The aggregation of AuNRs was reversible after adding the 6-MP followed by Hg2+. That is because the covalent bond formed between Hg and sulfur is stronger than that between Au and sulfur. In the presence of Hg2+, 6-MP firstly preferred binding to Hg2+ through Hg–S bond and lost the ability to induce the aggregation of AuNRs. The formation of covalent bond of Au-S and Hg-S can be further confirmed by UV-vis spectra (Fig. 3). As shown in Figs. 3 and 6-MP had two absorption bands located at 227 nm and 323 nm, respectively. After addition of 6-MP into the AuNRs solution, the absorbance at 323 nm was increased and the absorption bands located at 227 nm was disappeared, which indicated the formation of covalent bond of Au-S. After addition of 6-MP into the AuNRs solution in the presence of Hg2+, the absorbance at 323 nm decreased with a blue shift to 297 nm and the absorption bands located at 227 nm was disappeared. The UV-vis spectrum indicated the formation of covalent bond of Hg-S and when in the presence of Hg2+, 6-MP firstly preferred binding to Hg2+.
The TEM images (Fig. 1) of AuNRs provides direct evidence of the sensing mechanism. The AuNRs were synthesized by the seed-mediated growth method. CTAB molecule formed bilayer structure on the surface of the AuNRs. In the bilayer structure, two inner and outer layers of surfactants were present. Presumably the inner layer was bound to the gold surface via the headgroups and connected to the outer layer through hydrophobic interactions, while the headgroups of the outer layer were in the aqueous medium [37]. The prepared AuNRs were of near monodispersion and homogeneous (Fig. 1b). After addition of 6-MP, 6-MP caused the aggregation of AuNRs in the solution and the aggregation had two modes: end-to-end and side-to-side. CTAB molecules had different adsorption ability to different part of the AuNRs. Because CTAB bound to the side was more strongly than to the end of AuNRs, the end of AuNRs had less protection, 6-MP molecules can be easily bound to the ends of AuNRs. After addition of 6-MP, the AuNRs were preferentially assembled in an end-to-end mode to form linear and branched chains (Fig. 1c). However, AuNRs were of near monodispersion in the presence of 6-MP and Hg2+ (Fig. 1d), which supported Hg2+ inhibit the 6-MP induced aggregation of AuNRs.
Figure 4 shows the absorption spectra of this system. As shown in Fig. 4, the absorption spectra of the prepared AuNRs exhibit two well defined absorption bands: the transverse plasmon band located at 514 nm and the longitudinal plasmon band located at 665 nm. After the addition of 6-MP, a new peak located at 780 nm was observed in the absorption spectra which indicated the aggregation of AuNRs. The absorbance at 780 and 665 nm are related to the amounts of aggregated and dispersed AuNRs, respectively. So, the ratio of the absorbance at 780 nm to that at 665 nm (A 780/A 665) was used to express the molar ratio of aggregated to disperse AuNRs. After addition of 6-MP in the presence of Hg2+, the absorbance at 780 nm decreased with a blue shift with the increase of Hg2+ concentration. However, the absorbance at 665 nm increased with the increase of Hg2+ concentration. The changes between the ratios of absorbances in the absence ((A 780/A 665)0) and presence (A 780/A 665) of Hg2+ (ΔA 780/A 665 = (A 780/A 665)0–A 780/A 665) was referred to analytical signal. The real photographs of 6-MP-AuNRs-Hg2+ system were shown in the inset of Fig. 4. The color change of 6-MP-AuNRs induced by 1.0 nM Hg2+ can be viewed with bare eye and the color of AuNRs solution changed back to brownish gradually with the increase of Hg2+ concentration.
Optimization of method
The following parameters were optimized: (a) Sample pH value; (b) 6-MP concentration and (c) incubation time. Respective data and Figures are given in the Electronic Supporting Material. We found the following experimental conditions to give best results: (a) sample pH value of 5.0 (Fig. S1, ESM); (b) 6-MP concentration of 1.5 μM (Fig. S2, ESM) and (c) incubation time of 10 min (Fig. S3, ESM).
The selectivity of the method
To evaluate the selectivity of this method, the change of the absorption ratio in the presence of Al3+, Ba2+, Ca2+, Ce2+, Co2+, Cr3+, Fe3+, Fe2+, Mg2+, Mn2+, and Ni2+ (all at such as 6 μM), Ag+, Cd2+, Cu2+, and Pb2+ (all at such as 3 μM), was tested under the same experimental conditions when Hg2+ concentration was 0.03 μM. The inset of Fig. 5 shows the photographic images of 6-MP-AuNRs solution in the presence of various metal ions and Hg2+. The 6-MP-AuNRs solution remained aggregated with a blue color except for Hg2+. The color of the 6-MP-AuNRs kept brownish after addition of Hg2+ due to Hg2+ inhibited the aggregation of 6-MP-AuNRs. Figure 5 shows the A 780/A 665 value of 6-MP-AuNRs system in the absence and presence of various metal ions. As shown in Fig. 5, the A 780/A 665 values were similar to that of the blank in the presence of other metal ions, an obvious decrease of A 780/A 665 value only found in the presence of Hg2+ relative to that of the blank, which indicating this method had good selectivity for Hg2+ detection. Hg2+ exhibits much stronger thiophilic tendency than other metal ions. Though the concentration of Hg2+ in real water samples was lower or nearly equal to the concentration of other metal ions, Hg2+ can be determined by this method.
Photographs (a) and A 780 /A 665 (b) of 6-MP-AuNRs systems in the absence (blank) and presence of various metal ions. Concentration of Al3+, Ba2+, Ca2+, Ce2+, Co2+, Cr3+, Fe3+, Fe2+, Mg2+, Mn2+, and Ni2+, 6 μM; Concentration of Ag+, Cd2+, Cu2+, and Pb2+, 3 μM; Concentration of Hg2+, 0.03 μM. The colors of all systems except for that in the presence of Hg2+ are blue, the color of the system in the presence of Hg2+ is brownish
The sensitivity of the method
The capability of this method for quantitatively detecting Hg2+ was evaluated by the obvious changes in color and absorption spectrum of 6-MP-AuNRs towards Hg2+. Under the optimum conditions, the calibration curve for determination of Hg2+ was constructed. It can be seen from Fig. 6 that the ΔA 780/A 665 increases linearly with the increase of Hg2+ concentration and there is a direct correlation between ΔA 780/A 665 and the concentration of Hg2+ ranging from 1.0 to 100.0 nM. The regression equation is ΔA 780/A 665 = 0.04298 + 0.0099 × 10 9 C. The corresponding correlation coefficient is 0.997, which indicates that there is good linear relationship between the ΔA 780/A 665 and Hg2+ concentration. The limit of detection (LOD), which corresponds to signal-to-noise ratio 3, is 0.48 nM. The sensitivity and linear range of other methods for determination of Hg2+ are listed in Table 1 for comparison. It can be seen that our colorimetric Hg2+ sensor showed a superior sensitivity and broader linear range. Compared to AuNPs, the sensitivity of AuNRs was higher.
Analysis of samples
In order to examine the applicability and accuracy of the method, two water samples were collected and analyzed. The water samples were spiked with Hg2+ at two concentration levels and the experimental results are listed in Table 2. The recovery values were satisfied (96.7–102.4%) and the RSDs are lower than 3.6%. The results agreed reasonably well with those obtained by ICP-MS (Agilent 7500) measurements, which demonstrates that the method is suitable for Hg2+ detection in environmental samples.
Conclusions
In summary, a simple system for colorimetric determination of Hg2+ at room temperature based on the inhibition of the aggregation of AuNRs caused by 6-MP was demonstrated. Hg2+ can be detected based on the change of the absorbance ratios. This method had a wide linear range of 1 to 100 nM and with a 0.48 nM detection limit. The applicability of this probe has been successfully proven in real samples with good recovery percentages. This method also shows excellent selectivity and the experimental procedure is quite simple. The samples can be analyzed without previous separation and concentration. This method can be applied for the determination of Hg2+ in environmental samples. This detection scheme can also be used to other ions if respective aptamers are available. Therefore, the method holds a great potential for environmental monitoring various metal ions.
References
Stoichev T, Amouroux D, Martin-Doimeadios RCR, Monperrus M, Donard OFX, Tsalev DL (2006) Fluorescence spectroscopy of biological tissues-a review. Appl Spectrosc Rev 41:591–619
EPA US (2005) Regulatory impact analysis of the clean air mercury rule: EPA-452/ R-05-003
Krishna MVB, Castro J, Brewer TM, Marcus RK (2007) Online mercury speciation through liquid chromatography with particle beam/electron ionization mass spectrometry detection. J Anal At Spectrom 22:283–291
Leermakers M, Baeyens W, Quevauviller P, Horvat M (2005) Mercury in environmental samples: speciation, artifacts and validation. TrAC Trend Anal Chem 24:383–393
Hsu IH, Hsu TC, Sun YC (2011) Gold-nanoparticle-based graphite furnace atomic absorption spectrometry amplification and magnetic separation method for sensitive detection of mercuric ions. Biosens Bioelectron 26:4605–4609
Wang RZ, Zhou DL, Huang H, Zhang M, Feng JJ, Wang AJ (2013) Water-soluble homo-oligonucleotide stabilized fluorescent silver nanoclusters as fluorescent probes for mercury ion. Microchim Acta 180:1287–1293
Wang ZX, Guo YX, Ding SN (2015) Fluorometric determination of cadmium(II) and mercury(II). Microchim Acta 182:2223–2231
Chen YJ, Yao L, Deng Y, Pan DD, Ogabiela E, Cao JX, Adeloju SB, Chen W (2015) Rapid and ultrasensitive colorimetric detection of mercury (II) by chemically initiated aggregation of gold nanoparticles. Microchim Acta 182:2147–2154
Wang G, He X, Wang B, Zhang X, Wang L (2012) Electrochemical amplified detection of Hg2+ based on the supersandwich DNA structure. Analyst 137:2036–2038
Zhu Z, Su Y, Li J, Li D, Zhang J, Song S, Zhao Y, Li G, Fan C (2009) Highly sensitive electrochemical sensor for mercury (II) ions by using a mercury-specific oligonucleotide probe and gold nanoparticle-based amplification. Anal Chem 81:7660–7666
Senapati T, Senapati D, Kumar Singh A, Fan Z, Kanchanapally R, Chandra Ray P (2011) Chandra ray, highly selective SERS probe for hg (II) detection using tryptophan-protected popcorn shaped gold nanoparticles. Chem Commun 47:10326–10328
Wang GQ, Chen LX (2009) Aptameric SERS sensor for Hg2+ analysis using silver nanoparticles. Chin Chem Lett 20:1475–1477
Cheng X, Li S, Zhong A, Qin J, Li Z (2011) New fluorescent probes for mercury(II) with simple structure. Sensors Actuators B Chem 157:57–63
Wang B, Zhuo S, Chen L, Zhang Y (2014) Fluorescent graphene quantum dot nanoprobes for the sensitive and selective detection of mercury ions. Spectrochim Acta Part A 131:384–387
Zuo X, Wu H, Toh J, Li SFY (2010) Mechanism of mercury detection based on interaction of single-strand DNA and hybridized DNA with gold nanoparticles. Talanta 82:1642–1646
Li T, Li BL, Wang EK, Dong SJ (2009) G-quadruplex-based DNAzyme for sensitive mercury detection with the naked eye. Chem Commun:3551–3553
Zarlaida F, Adlim M (2017) Gold and silver nanoparticles and indicator dyes as active agents. Microchim Acta 184:45–58
Li X, Su X, Shi Z, Cheng X, Liu S, Zhao Q (2014) Highly selective and reversible colorimetric detection of mercury ions by a hydrophilic cycloruthenated complex in water. Sensors Actuators B Chem 201:343–350
Sun MZ, Wu FY, Wu YM, Liu WM (2008) A ditopic colorimetric sensor for fluoride ion based on thiourea mercury complex. Spectrochim Acta Part A 71:814–817
Zarlaida F, Adlim M (2017) Gold and silver nanoparticles and indicator dyes as active agents in colorimetric spot and strip tests for mercury(II) ions: a review. Microchim Acta 184:45–58
Sepulveda B, Angelome PC, Lechuga LM, Liz-Marzan LM (2009) LSPR-based nanobiosensors. Nano Today 4:244–251
Saha K, Agasti SS, Kim C, Li X, Rotello VM (2012) Gold nanoparticles in chemical and biological sensing. Chem Rev 112:2739–2779
Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ (1996) A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382:607–609
Wang Y, Yang F, Yang X (2010) Colorimetric detection of mercury (II) ion using unmodified silver nanoparticles and mercury-specific oligonucleotides. ACS Appl Mater Interfaces 2:339–342
Slocik JM, Zabinski JSJ, Phillips DM, Naik RR (2008) Colorimetric response of peptide-functionalized gold nanoparticles to metal ions. Small 4:548–551
Xie J, Zheng Y, Ying JY (2010) Highly selective and ultrasensitive detection of Hg2+ based on fluorescence quenching of au nanoclusters by Hg2+–au+ interactions. Chem Commun 961−963
Liu D, Wang S, Swierczewska M, Huang X, Bhirde AA, Sun J, Wang Z, Yang M, Jiang X, Chen X (2012) Highly robust, recyclable displacement assay for mercuric ions in aqueous solutions and living cells. ACS Nano 6:10999–11008
Liu D, Qu W, Chen W, Zhang W, Wang Z, Jiang X (2010) Colorimetric detection of mercury (II) in aqueous media by quaternary ammonium group-capped gold nanoparticles at room temperature. Anal Chem 82:9606–9610
Huang CC, Yang Z, Lee KH, Chang HT (2007) Synthesis of highly fluorescent gold nanoparticles for sensing mercury (II). Angew Chem Int Ed 46:6824–6828
Chen L, Xiu FL, Lu W, Chen L (2013) Sensitive and selective colorimetric sensing of Hg2+ based on the morphology transition of silver Nanoprisms. ACS Appl Mater Interfaces 5:284–290
Miyake Y, Togashi H, Tashiro M, Yamaguchi H, Oda S, Kudo M, Tanaka Y, Kondo Y, Sawa R, Fujimoto T, Machinami T, Ono A (2006) MercuryII-mediated formation of thymine−HgII−Thymine Base pairs in DNA duplexes. J Am Chem Soc 128:2172–2173
Lee JS, Han MS, Mirkin CA (2007) Colorimetric detection of mercuric ion (Hg2+) in aqueous media using DNA-functionalized gold nanoparticles. Angew Chem 119:4171–4174
Perez-Juste J, Pastoriza-Santos I, Liz-Marz’an LM, Mulvaney P (2005) Gold nanorods: synthesis, characterization and applications. Coord Chem Rev 249:1870–1901
Lee KS, El-Sayed MA (2006) Gold and silver nanoparticles in sensing and imaging: sensitivity of Plasmon response to size, shape, and metal composition. J Phys Chem B 110:19220–19225
Link S, El-Sayed MA (2005) Simulation of the optical absorption spectra of gold Nanorods as a function of their aspect ratio and the effect of the medium dielectric constant. J Phys Chem B 109:10531–10532
Nikoobakht B, El-Sayed MA (2003) Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem Mater 15:1957–1962
Nikoobakht B, El-Sayed MA (2001) Evidence for bilayer assembly of cationic surfactants on the surface of gold nanorods. Langmuir 17:6368–6374
Jana NR, Gearheart L, Murphy CJ (2001) Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Adv Mater 13:1389–1393
Chen ZB, Zhang CM, Tan Y, Zhou TH, Ma H, Wan CQ, Lin YQ, Li K (2015) Chitosan-functionalized gold nanoparticles for colorimetric detection of mercury ions based on chelation-induced aggregation. Microchim Acta 182:611–616
Shamsipur M, Safavi A, Mohammadpour Z, Ahmadi R (2016) Highly selective aggregation assay for visual detection of mercury ion based on competitive binding of sulfur-doped carbon nanodots to gold nanoparticles and mercury ions. Microchim Acta 183:2327–2335
Ma Y, Jiang L, Mei Y, Song R, Tian D, Huang H (2013) Colorimetric sensing strategy for mercury(II) and melamine utilizing cysteamine modified gold nanoparticles. Analyst 138:5338–5343
Hu T, Yan X, Na W, Su X (2016) Aptamer-based aggregation assay for mercury(II) using gold nanoparticles and fluorescent CdTe quantum dots. Microchim Acta 183:2131–2137
Ding XJ, Qu LB, Yang R, Zhou YC, Li JJ (2015) A highly selective and simple fluorescent sensor for mercury (II) ion detection based on cysteamine-capped CdTe quantum dots synthesized by the reflux method. Luminescence 30:465–471
Zhu SS, Zhuo Y, Miao H, Zhong D, Yang XM (2015) Detection of mercury(II) by DNA templated gold nanoclusters based on forming thymidine-Hg2+-thymidine duplexes. Luminescence 30:631–636
Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 61505049, No.21404033 and No.21401046), the Doctoral Fund of Henan Polytechnic University (No. B2013-044 and B2013-013), and the Research Projects of Science and Technology of the Education Department Henan Province (No.14B430026).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 92 kb)
Rights and permissions
About this article
Cite this article
Bi, N., Hu, M., Xu, J. et al. Colorimetric determination of mercury(II) based on the inhibition of the aggregation of gold nanorods coated with 6-mercaptopurine. Microchim Acta 184, 3961–3967 (2017). https://doi.org/10.1007/s00604-017-2427-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2427-5