Abstract
The authors describe an ultrasonic-assisted headspace method for solid phase micro-extraction (UA-HS-SPME) of 7 polychlorinated biphenyls (PCBs) with codes PCB28, PCB52, PCB101, PCB118, PCB138, PCB153 and PCB180. The coating is based on a poly-dopamine metal-organic framework [PDA-MIL-53(Fe)] on a stainless steel wire. The coating can be prepared and evenly deposited on the stainless fiber by dipping the PDA fiber into a solution of MIL-53(Fe). The assay is also environmentally friendly because water is used as the solvent. The effects of extraction time, addition of salts, pH value and power of ultrasonic power were optimized. The coating is found to possess a high selectivity and adsorption capacity for PCBs compared to commercial SPME fibers such as the divinylbenzene/carboxen/polydimethylsiloxane fibers. Following desorption, the PCBs were quantified by GC-MS. The detection limits are between 50 and 90 pg⋅g−1 of PCBs in soil. The fibers can be easily prepared, and the batch-to-batch reproducibility (RDS) is <10% (for n = 6). The fibers are inexpensive, re-usable and can be easily manipulated, and particularly well suited for screening polychlorinated biphenyls in soil.

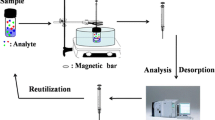
Schematic of the preparation of an extraction fiber using stainless steel wire as substrate, PDA as adhesive, and MIL-53(Fe) as the adsorbent. It was applied to the extraction of PCBs from soil. The fiber is durable and inexpensive.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The toxicity of polychlorinated biphenyls (PCBs) residues makes the public extremely concern, because they may contribute to carcinogenicity or tumorigenicity even at ultratrace level [1]. Thus, it becomes more important to build an ultrasensitive detection assay for trace PCBs in environmental samples. Headspace SPME (HS-SPME) [2–4], it is a green pre-treatment method which overcomes many shortcomings such as time consuming, consuming large amounts of analytes and organic solvents [5, 6]. Moreover, one of the key techniques for SPME is to fabricate coatings with high selectivity and adsorption capacity. Nowadays there are many commercial SPME fibers in market, but the fibers are extremely fragile and expensive. And their absorption selectivity for PCBs is normally poor [7]. Thus, in order to selectively detect trace level of PCBs in soil, we need to develop a novel selective coating for fiber with high adsorption capacity.
Metal organic frameworks (MOFs) possess high porosity and large specific area [8, 9] which can show excellent adsorption properties towards some organic pollutants [10, 11]. There are many MOFs being used as adsorbent to adsorb some organic pollutants for detection, such as MIL-88B [10], MOF-199 [12], ZIF-8 [13], MIL-53(Al) [14], ZIF-90 [15]. Our research group found that iron-based MOFs can selectively and effectively enrich PCBs [7]. Our group chose MOF-5(Fe) as coating of stir bar to extract PCBs, the fibers showed higher capacity towards the targets. However, the sir bar cannot be directly injected into sample inlet of GC-MS and needed some organic solvent to elute the PCBs. In order to employ iron-based MOFs as SPME fiber coating for direct injection, we select MIL-53(Fe) which has good thermal stability as the coating material of SPME fiber.
Nevertheless, it is crucial to employ proper method to stably modify MIL-53(Fe) onto the fiber’s surface. Epoxy resins are usually employed as adhesive agent to immobilize MOF on fiber [16–18]. However, the modification process is not easy especially pasting it evenly onto fiber. Moreover, some of them can’t resist high temperature which is needed to desorb PCBs on SPME fiber [19]. Polydopamine is a good biological adhesive to many kinds of substrates, e.g. metal, silica, metal-oxide [20–22]. Moreover, it can be easily prepared just by immersing the substrate in dopamine solution under alkali conditions. Compared with other literatures with epoxy resin as adhesive agent, PDA is a kind of environment-friendly material which can evenly self-aggregate to the stainless steel wire and has certain adsorption for PCBs. As it is well known, most of the conventional SPME fiber’s substrate is silica which is fragile and liable to be destroyed. Stainless steel wire is a good candidate to form stable fiber substrate. Based on it, we hope to use stainless fiber as substrate then PDA to adhere MIL-53(Fe) as coating in order to prepare a firm home-made SPME fiber with high and specific absorption capacity for PCBs.
PCBs are a kind of semi-volatile organic compounds [23]. In some SPME methods [24, 25], water is employed as medium to disperse samples. There are three possible reasons: Firstly, water is a green solution while PCBs are hydrophobic. They can easily be expelled from water to headspace assisted by some driving force. Secondly, the deionized water can prevent some water-soluble impurities with lower boiling point from entering the headspace. Thirdly, deionized water can easily generate a ‘cavitation effect’ by ultrasonic wave to increase the extraction effectiveness. However, in some previous reports [2–4], it is generally needed to heat the samples to increase PCBs’ volatilization amount in the headspace for SPME, but the effectiveness is not obvious because most of the boiling points of PCBs are more than 100 °C of water. Ultrasonic wave has been investigated and proved that it can assist extraction effectiveness of organic pollutants from samples [24, 25]. Ultrasonic wave can accelerate samples evenly dispersed in the solvent and promote PCBs strip from the soil by cavitation effect. A novel ultrasonic-assisted headspace solid phase micro-extraction (UA-HS-SPME) method was applied for the extraction of volatile organics [24–26]. So it may be also applied to assist driving semi-volatile PCBs from soil-water mixtures to headspace of SPME vials. Therefore, in the whole experiment (except the synthesis of MOF), we just use water as dispersive solvent and ultra-sonic wave as driving force to push PCBs in the soil into headspace for SPME enrichment. Thus a green pretreatment method can be fabricated.
Above all, in this work, we fabricated a novel MIL-53(Fe) films on stainless steel fibers with PDA. The home-made PDA- MIL-53(Fe) fiber was applied for extracting PCBs from soil samples in headspace with good efficiency via ultrasonic-assisted headspace solid phase micro-extraction (UA-HS-SPME) method. The preparation and extraction procedures are shown in Scheme 1. Moreover, some parameters were optimized such as extraction time, addition of salts, pH and ultrasonic power. The method was successfully employed for PCBs’ selective extraction in natural soil samples.
Experimental
Materials and reagents
Seven standards of PCB congeners (PCBs: PCB28, PCB52, PCB101, PCB118, PCB138, PCB153, PCB180) in isooctane solution (10 mg L−1) were obtained from Accu Standard (https://www.accustandard.com/, USA). Isooctane (HPLC grade), hydrochloric acid, andiron (III) chloride hexahydrate (FeCl3 · 6H2O, 99%), terephthalic acid, N, N-dimethylformamide (DMF, 99%), NaCl, NaOH, dopamine, 4-difluorobiphenyl (internal standard) were purchased from Sigma–Aldrich (http://www.sigmaaldrich.com, USA). Commercial SPME fiber coated with DVB/CAR/PDMS was purchased from Supelco (https://www.supelco.com/, USA). Deionized water was used in whole experiment.
Fabrication of the SPME fiber with PDA- MIL-53(Fe)
Preparation of metal-organic framework MIL-53(Fe)
MIL-53(Fe) was prepared using the method reported in the literature [27]. Briefly, 810 mg of FeCl3·6H2O and 498 mg of terephthalic acid were mixed with 15 mL of DMF solution at room temperature. After stirring for 10 min, the mixture was transferred into a 20 mL Teflon-lined bomb and heated at 150 °C for 6 h in muffle furnace. After cooling it to room temperature, the yellow products were collected by centrifugation with 6000 rpm for 5 min and washed with distilled water (5 × 10 mL). Then, the products were suspended in 600 mL deionized water with mixing overnight. Next, the yellow powder was collected and dried at 60 °C for 24 h in a vacuum drying oven.
Fabrication of the PDA- MIL-53(Fe) fiber
Stainless steel wire fiber (about 10 cm in length and the diameter of 0.22 mm) was applied as substrate to prepare SPME coating. In order to obtain a rough surface to increase loading area to MOFs, one end of the wire (about 1.5 cm in length) was etched with aqua regia for 10 min. After then, it was washed by distilled water and dried in air. Then, the PDA coating was synthesized onto the etched stainless steel wire, 20 mg dopamine (DA) were mixed with 15 mL 100 mM Tris-HCl (pH = 8.5), and the etched stainless steel wire was immersed into the solution for 12 h. Next, the PDA fiber was washed with phosphate buffer and solidified at 160 °C in oven for 0.5 h. At the same time, 10 mg MIL-53(Fe) was equably mixed with 20 μL distilled water. Finally, we dipped the PDA fiber into the MIL-53(Fe) solution repeatedly for five times (5 mins for each time) and solidified at 160 °C for 0.5 h in the GC injector at each time.
GC–MS analysis
PCB separations were operated on a GCMS-QP2010E (Shimadzu, Japan) system fitted with RXi-5MS capillary column (30 m × 0.25 mm, 0.25 μm film thickness). Helium (purity 99,999%) was used as the carrier gas with 0.96 mL⋅min−1 flow rate. The oven temperature program was set as follows: 70 °C, held for 2 min, ramped 20 °C⋅min−1 to 230 °C, held for 2 min, ramped 5 °C⋅min−1 to 250 °C, held for 2 min, ramped from 250 °C to 280 °C at a rate of 20 °C⋅min−1, held for 2 min. The instrumental conditions were: split less mode; solvent delay was 5 min; ion source temperature was 200 °C; the injector temperature was 270 °C; the interface temperature was 280 °C. The quantitative and qualitative ions were shown in Table S1 in supplementary document.
UA-HS-SPME process and quantification of PCBs in soil samples
Standard addition method was employed for quantification of PCBs in soils. Firstly, the samples which were collected from the central garden of Ningbo University were dried at room temperature, and crushed in a mortar to make powder about 200 meshes by filters. Three soil samples of 10 g were weighted and put in three 40 mL head space bottles respectively. Then, various concentrations of PCB solutions (0, 0.05, 0.10, 1.00, 1.50 ng⋅g−1) were added to the bottles respectively. 10 μL of 1 μg⋅mL−1 difluorobiphenyl was added as internal standard. The bottles were shocked to make the samples mix evenly and were added 10 mL of deionized water. Then, three PDA- MIL-53(Fe) fibers were inserted to head space of the three bottles respectively. Next, the bottles were placed into an ultrasonic bath with the power of 90 W for 1 h. Then, the fiber was take out from the head space bottles and placed into a bottle with KOH for 5 min and directly inserted into the GC-MS. The content of PCBs in soil was quantified by the calibration line of standard addition curve. The area ratio of target and difluorobiphenyl (Ax/Ai) was employed as Y axis, and △Cs (added standard substance) as X axis.
Results and discussion
Choice of materials
Based on our previous work, we found that iron-based MOFs can selectively and effectively enrich PCBs in soil and fish samples. And many MIL MOFs had good stability and high temperature resistance (It can also resist high temperature during desorption process in the injection port of GC-MS). MIL-53(Fe) is a universal and widely used MIL material containing iron central ions, so it is selected as a candidate adsorbent for PCBs. However, the MIL-53(Fe) is difficult to be coated onto stainless steel wire by dipping. Therefore, an adhesive was required for its immobilization on fibers. Polydopamine is a good biological adhesive which can self-aggregate on the surface of the stainless steel wire to form an even film. Moreover, PDA can adsorb PCBs with certain capacity, and possess higher temperature resistance than some other widely-used adhesive such as epoxy resin for coating development. Even if PANI, a universal coating in SPME fibers, also exhibits good adsorption capacity for organic volatile pollutants, it can’t be employed as adhesive to connect the MOF coating to the SPME fiber. Above all, we employed PDA as an adhesive of MOF coating and co-existed adsorption agent for PCBs as well.
Characterization of the PDA- MIL-53(Fe) fiber
By comparing the XRD pattern of the MIL-53(Fe) on the fiber and the simulated one referenced from literature [27], the MIL-53(Fe) coating was characterized. The XRD pattern of MIL-53(Fe) which was scraped from the home-mage fiber is shown in Fig. S1A and the peaks positions are very consistent with that of the simulation one, showing that the MIL-53(Fe) coating is synthesized successfully. The TG curve, it can be seen that the MIL-53(Fe) is stable up to 300 °C (Fig. S1B). Moreover, we also prepared PDA coating and scratched it for TG experiment, it can be seen PDA can also maintain stable at 280 °C (Fig. S1B). The results show that the PDA and MIL-53(Fe) has high thermal stability which is beneficial to the thermal desorption of the adsorbed PCBs from the SPME fiber for GC separation. And the morphology of the PDA and MIL-53(Fe) show that the PDA and MIL-53(Fe) is evenly coated the surface (Fig. S1C and Fig. S1D). The SEM image of Fig. 1 shows that the MIL-53(Fe) as a homogenous and rough coating appeared on the surface of the fiber by the PDA. MIL-53 is monodisperse in needle shape with diameter of 500 nm. The micro porous structure of MIL-53(Fe) allows the MOF to have a larger surface area.
Comparison of UA-HS-SPME and WB-HS-SPME
According to literature [28, 29], water-bathed headspace solid-phase micro-extraction (WB-HS-SPME) was a common pretreatment method for PCBs. Here, UA-HS-SPME was employed to replace WB-HS-SPME to extract PCBs in soil. The extraction efficiencies of UA-HS-SPME and WB-HS-SPME (60 °C) are shown in Fig. 2. The results demonstrated that UA-HS-SPME has better adsorption capacity than WB-HS-SPME (increasing 5%–66% for different PCB species). The reason may be due to that ultrasonic cavitation effect promotes the volatilization of PCBs. Moreover, we found ultrasonic wave can also increase the temperature of water. All these can accelerate PCBs’ volatilization rate in headspace of SPME vials and increase their extraction capacity.
Comparison of the MIL-53(Fe) coated fiber with commercial DVB/CAR/PDMS fiber
To show the extraction performance of MIL-53(Fe) for the UA-HS-SPME of PCBs, the MIL-53(Fe) coated fiber was compared with commercial DVB/CAR/PDMS fiber (we found it has the largest adsorption capacity to PCBs comparing with commercial PDMS, PDMS/DVB and PA SPME coating) (Fig. 3). The extraction capacity of MIL-53(Fe) coated fiber for PCBs is higher than the commercial DVB/CAR/PDMS fiber. Especially the adsorption amount increased obviously for PCBs with more chlorine substituent groups, e.g. PCB118, 153, 180 (the number of Cl group >4). The multiples of MIL-53 coating comparing to commercial one is larger than 4. Among them, the adsorption of PDA for PCBs is weak, and MIL-53 plays a major role in this fiber (Fig. S1). This maybe because MIL-53 has bare iron ion which can enhance the absorbing ability to PCBs by the interaction between iron ion and chlorine groups of PCBs, thus the adsorption capacity increase with chlorine group’s numbers. The result shows the great potential of MIL-53(Fe) for the effective enrichment of PCBs.
Optimization of extraction conditions
In order to obtain the best extraction efficiency of UA-HS-SPME of PCBs on MIL-53(Fe) coating, the following parameters were optimized: (a) Extraction time; (b) Addition of salt; (c) Sample pH value; (d) Ultrasonic power. Respective data and Figures are given in Fig. S2. We found the following experimental conditions to give best results: (a) 60 min was chosen for extraction time; (b) no salt; (c) a sample pH value of 7.0; (d) an ultrasonic power of 90 W.
The figure shows that the extraction efficiencies for PCBs presented an exponential growth after 50 min (Fig. S2A). However, ultrasonic instrument cannot work long hours (less than 60 min) otherwise the instrument will be damaged. Consequently, 60 min is chosen for the following work. For sample pH value, the extraction efficiency reach to the maximum when the pH value of was 7.0 (Fig. S2B). This may be due to that the MIL-53 has the highest stability at neutral conditions. It can be seen that the extraction efficiency for PCBs decrease with the addition of salt from Fig. S2C. Hence, salt addition for the extraction is not recommended in this work. Ultrasonic power can accelerate cavitation effect and molecular mass transfer rate. The best extraction efficiency is observed with the power of 90 W from Fig. S2D.
Selectivity of the MIL-53(Fe) coated fiber
Our group has found Fe-MOF have some selectivity towards chlorinated organic compounds because the iron central atom has high affinity to Cl group [7]. In order to test the selectivity of MIL-53(Fe) coating to PCB, the extraction ability of MIL-53(Fe) coated fiber for PCBs was compared with the extraction ability for some of PAHs (without Cl groups). Three PCBs (PCB28,PCB52 and PCB101: 10 ng⋅g−1)) and three PAHs without Cl groups while fused rings like PCBs (naphthalene, acenaphthene and acenaphthylene: 10 ng g−1) which has almost same volatile points with the above 3 PCBs respectively, were employed as models for comparison because they have the same peak time. Comparing the curve a to b in Fig. 4, it can be seen that MIL-53 coating almost doesn’t have affinity to the three PAHs comparing with commercial fiber (CAR/PDMS/DVB). While when comparing curve c to d, it can be seen that MIL-53 coating has higher adsorption capacity than the commercial fiber. Fig. 4 can prove that the MIL-53(Fe) coated fiber has a selective adsorption for PCBs. And this selectivity maybe due to the central Fe ions has chelation to Cl groups in PCBs, while not only because π-π stacking effects.
Chromatographs of PCBs and PAHs (Sample volume 10 g, spiked at 10 ng·g−1); a the MIL-53(Fe) coated fiber for three PAHs (naphthalene, acenaphthene and acenaphthylene); b the commercial SPME fiber for three PAHs (naphthalene, acenaphthene and acenaphthylene); c the MIL-53(Fe) coated fiber for three PCBs (PCB28,PCB52 and PCB101); d the DVB/CAR/PDMS fiber for three PCBs (PCB28,PCB52 and PCB101)
Reproducibility, stability of the MIL-53(Fe) fiber
The reproducibility of this fiber was evaluated with the response of 10 ng·g−1 PCBs for measurement repeated six times and the relative standard deviation (RSD) were less than 10% (Table S2). The tiny RSD intimated that the strategy has well reproducibility and the results come from it is reliable. And the precisions about inter-day and intra-day of this method were measured and the results are shown in Table 2. It can be seen that the two relative precisions are less than 7.5%. The recovery of this method was measured by the three levels of standard PCBs samples (0.1 ng⋅g−1, 1.0 ng⋅g−1, 10 ng⋅g−1) with six replicates. From the results (Table S3), the recoveries are from 92.4% to 97.1%. The values suggest that the reproducibility of the fiber is satisfactory.
The stability of the prepared fiber was examined after extraction for 50 times. We found the adsorption capacity maintain nearly 85% of its initial value. It indicates that the fiber has good stability.
Real soil sample analysis by standard addition method
To validate the practical performance of MIL-53(Fe) coated UA-HS-SPME in real samples with complex matrix, some natural soil was selected as analytes. The analytical results are shown in Table 1. It can be found three PCBs (PCB28: 3.552 ng⋅g−1; PCB52: 0.204 ng⋅g−1; PCB101: 0.058 ng⋅g−1) in soil in this work.
It can be seen from Table 2, the extracted PCBs present favorable linearity (0.3–100 μg⋅L−1) with correlation coefficients of more than 0.992. And the low detection limits for the seven PCBs can reach to between 0.05 and 0.09 ng⋅g−1 using gas chromatography-mass spectrometry (GC-MS) for measurement. Some methods have been compared with the UA-HS-SPME method concerning the analysis of PCBs in soil samples. The results are summarized in Table 3. Compared with other methods, the LODs of PCBs by this method are relatively lower, and higher than those reported by Pérez RA et al. However, the UA-HS-SPME method is faster and greener (not use any organic solvent) compared to mSPE. The results show that the PDA-MIL-53(Fe) has good absorption effectiveness to indicator PCBs and this method can apply to the determination of trace level of PCBs in soil samples.
Conclusion
In this paper, we fabricated a novel PDA-MIL-53(Fe) SPME coating for selective and sensitive headspace extraction of seven indictor PCBs in soil samples being assisted by ultrasonic wave. It is easy for preparation just using PDA as binder and stable which can be reused for at least 50 times. It shows better adsorption capacity and selectivity for PCBs than some commercial fibers due to the MIL-53(Fe)‘s specific adsorption to PCBs. Ultrasonic wave can obviously enhance the extraction effectiveness than just only heating the samples. Distilled water was employed to assist ultrasonic wave extraction which made the SPME assay environment-friendly and easy for manipulation. Based on the novel fiber, an ultrasonic-assisted headspace on fiber solid phase micro-extraction (UA-HS-SPME) method was successfully employed for extraction of PCBs in natural soils. The method can be used for other semi volatile pollutants in complex soil samples. And the coating of fiber can be replaced by other adsorption materials to detect other pollutants. But the method also has some defects, for example, we performed the experiments with the assistance of ultrasonic wave which can shorten the working life of the fiber. We hope to improve the working life and reusability of the MOF based fiber in the next work.
References
Safe SH (1994) Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol 24:87–149
Vichi S, Castellote AI, Pizzale L, Conte LS, Buxaderas S, Lopez-Tamames E (2003) Analysis of virgin olive oil volatile compounds by headspace solid-phase microextraction coupled to gas chromatography with mass spectrometric and flame ionization detection. J Chromatogr A 983:19–33
Mills GA, Walker V (2000) Headspace solid-phase microextraction procedures for gas chromatographic analysis of biological fluids and materials. J Chromatogr A 902:267–287
Siebert TE, Smyth HE, Capone DL, Neuwöhner C, Pardon KH, Skouroumounis GK, Pollnitz AP (2005) Stable isotope dilution analysis of wine fermentation products by HS-SPME-GC-MS. Anal Bioanal Chem 381:937–947
Ouyang G, Pawliszyn J (2006) SPME in environmental analysis. Anal Bioanal Chem 386:1059–1073
Hu X, Hu Y, Li G (2007) Development of novel molecularly imprinted solid-phase microextraction fiber and its application for the determination of triazines in complicated samples coupled with high-performance liquid chromatography. J Chromatogr A 1147:1–9
Lin S, Gan N, Qiao L, Zhang J, Cao Y, Chen Y (2015) Magnetic metal-organic frameworks coated stir bar sorptive extraction coupled with GC–MS for determination of polychlorinated biphenyls in fish samples. Talanta 144:1139–1145
Li H, Eddaoudi M, O'Keeffe M, Yaghi OM (1999) Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 402:276–279
Alvaro M, Carbonell E, Ferrer B, Llabrés i Xamena FX, Garcia H (2007) Semiconductor behavior of a metal-organic framework (MOF). Chem-Eur J 13:5106–5112
Wu YY, Yang CX, Yan XP (2014) Fabrication of metal–organic framework MIL-88B films on stainless steel fibers for solid-phase microextraction of polychlorinated biphenyls. J Chromatogr A 1334:1–8
Gu ZY, Jiang JQ, Yan XP (2011) Fabrication of isoreticular metal–organic framework coated capillary columns for high-resolution gas chromatographic separation of persistent organic pollutants. Anal Chem 83:5093–5100
Cui XY, Gu ZY, Jiang DQ, Li Y, Wang HF, Yan XP (2009) In situ hydrothermal growth of metal− organic framework 199 films on stainless steel fibers for solid-phase microextraction of gaseous benzene homologues. Anal Chem 81:9771–9777
Yang XQ, Yang CX, Yan XP (2013) Zeolite imidazolate framework-8 as sorbent for on-line solid-phase extraction coupled with high-performance liquid chromatography for the determination of tetracyclines in water and milk samples. J Chromatogr A 1304:28–33
Chen XF, Zang H, Wang X, Cheng JG, Zhao RS, Cheng CG, Lu XQ (2012) Metal–organic framework MIL-53 (al) as a solid-phase microextraction adsorbent for the determination of 16 polycyclic aromatic hydrocarbons in water samples by gas chromatography–tandem mass spectrometry. Analyst 137:5411–5419
Yu LQ, Yan XP (2013) Covalent bonding of zeolitic imidazolate framework-90 to functionalized silica fibers for solid-phase microextraction. Chem Commun 49:2142–2144
Wang XM, Du XZ, Rao HH, Lu XQ (2010) Determination of polycyclic aromatic hydrocarbons in water by a novel mesoporous-coated stainless steel wire microextraction combined with HPLC. J Sep Sci 33:3239–3244
Chen XF, Zang H, Wang X, Cheng JG, Zhao RS, Cheng CG, Lu XQ (2012) Metal–organic framework MIL-53 (al) as a solid-phase microextraction adsorbent for the determination of 16 polycyclic aromatic hydrocarbons in water samples by gas chromatography–tandem mass spectrometry. Analyst 137:5411–5419
Shang HB, Yang CX, Yan XP (2014) Metal–organic framework UiO-66 coated stainless steel fiber for solid-phase microextraction of phenols in water samples. J Chromatogr A 1357:165–171
Liu H, Ran F, Tao C, Zhao M, Jia Y, Guo Y (2016) A highly thermal stable solid phase microextraction fiber prepared by an inorganic binder. Anal Chim Acta 918:35–42
Brubaker CE, Messersmith PB (2012) The present and future of biologically inspired adhesive interfaces and materials. Langmuir 28:2200–2205
Zhu LP, Jiang JH, Zhu BK, Xu YY (2011) Immobilization of bovine serum albumin onto porous polyethylene membranes using strongly attached polydopamine as a spacer. Colloids Surf B 86:111–118
Yu M, Hwang J, Deming TJ (1999) Role of L-3, 4-dihydroxyphenylalanine in mussel adhesive proteins. J Am Chem Soc 121:5825–5826
Cousins IT, Gevao B, Jones KC (1999) Measuring and modelling the vertical distribution of semi-volatile organic compounds in soils. I: PCB and PAH soil core data Chemosphere 39:2507–2518
Ghiasvand AR, Nasseri M, Farsizaeh S, Meshkatalsadat MH, Sadeghi-Sarabi R, Shadabi S, Borzoei M (2011) Chemical characterization of cultivated Tagetes minuta L. by use of ultrasound-assisted head space SPME and GC–MS. Chromatographia 73:1031–1035
Rahimi A, Hashemi P, Talei GR, Borzuei M, Ghiasvand AR (2014) Comparative analyses of the volatile components of Citrus aurantium L. flowers using ultrasonic-assisted headspace SPME and Hydrodistillation combined with GC-MS and evaluation of their antimicrobial activities. Anal Bioanal Chem Res 1:83–91
Zulj MM, Maslov L, Tomaz I, Jeromel A (2015) Determination of 2-aminoacetophenone in white wines using ultrasound assisted SPME coupled with GC-MS. J Anal Chem 70:814–818
Ai L, Li L, Zhang C, Fu J, Jiang J (2013) MIL-53 (Fe): a metal–organic framework with intrinsic peroxidase-like catalytic activity for colorimetric Biosensing. Chem–Eur J 19:15105–15108
Llompart M, Li K, Fingas M (1998) Solid-phase microextraction and headspace solid-phase microextraction for the determination of polychlorinated biphenyls in water samples. Anal Chem 70:2510–2515
Shu YY, Wang SS, Tardif M, Huang Y (2003) Analysis of polychlorinated biphenyls in aqueous samples by microwave-assisted headspace solid-phase microextraction. J Chromatogr A 1008:1–12
Hassine SB, Ameur WB, Gandoura N, Driss MR (2012) Determination of chlorinated pesticides, polychlorinated biphenyls, and polybrominated diphenyl ethers in human milk from Bizerte (Tunisia) in 2010. Chemosphere 89:369–377
Pauwels A, Wells DA, Covaci A, Schepens PJC (1999) Improved sample preparation method for selected persistent organochlorine pollutants in human serum using solid-phase disk extraction with gas chromatographic analysis. J Chromatogr B Biomed Appl 723:117–125
Poli D, Caglieri A, Goldoni M, Castoldi AF, Coccini T, Roda E, Mutti A (2009) Single step determination of PCB 126 and 153 in rat tissues by using solid phase microextraction/gas chromatography–mass spectrometry: comparison with solid phase extraction and liquid/liquid extraction. J Chromatogr B 877:773–783
Pérez RA, Albero B, Tadeo JL, Sánchez-Brunete C (2016) Oleate functionalized magnetic nanoparticles as sorbent for the analysis of polychlorinated biphenyls in juices. Microchim Acta 183:157–165
Diao C, Li C, Yang X, Sun A, Liu R (2016) Magnetic matrix solid phase dispersion assisted dispersive liquid liquid microextraction of ultra trace polychlorinated biphenyls in water prior to GC-ECD determination. Microchim Acta 183:1261–1268
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 51403110), the Natural Science Foundation of Zhejiang (LY17C200007, LY15B050002, LY16B050003, 2017C37023), the Natural Science Foundation of Ningbo (2016A610084), and the K.C. Wong Magna Fund in Ningbo University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic Supplementary Material
ESM 1
(DOCX 0.97 mb)
Rights and permissions
About this article
Cite this article
Lv, F., Gan, N., Huang, J. et al. A poly-dopamine based metal-organic framework coating of the type PDA-MIL-53(Fe) for ultrasound-assisted solid-phase microextraction of polychlorinated biphenyls prior to their determination by GC-MS. Microchim Acta 184, 2561–2568 (2017). https://doi.org/10.1007/s00604-017-2208-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2208-1