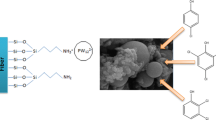
Abstract
We report on a nanostructured polysiloxane as a coating for solid-phase microextraction (SPME) of chlorobenzenes from water, wastewater, sludge and sediment samples. Methyltriethoxysilane was used to prepare the nanostructured fibrous polysiloxane coatings, which were deposited onto a stainless steel wire. Headspace SPME, followed by GC with electron capture detection was applied for separation and quantitation. The effects of stirring rate, salt concentration, equilibrium and extraction time, extraction temperature, desorption time and temperature were optimized. The extraction efficiency of the analytes using the new fiber was 5–10 and 10–30 times better than those obtained by using the commercial PDMS fiber and nonfibrous polysiloxane fiber, respectively. The relative standard deviations for intra- and inter-day precision for a single fiber were below 6 %. The fiber to fiber reproducibility was in the range of 3.3–9.7 % (for n = 3). The detection limits were between 0.15 and 75 ng L−1. The relative recoveries for water, wastewater, sludge, and sediment samples were in the range from 90 to 99 %.

Polysiloxane nanofibers coated onto a stainless steel wire were prepared and used as a SPME coating for extraction of chlorobenzenes from water, sediment and soil samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid-phase microextraction (SPME) was developed by Pawliszyn and co-workers in 1989 to overcome some difficulties in other sample preparation and pre-concentration techniques such as liquid-liquid extraction and solid-phase extraction [1, 2]. SPME is a solvent free, simple, sensitive, portable and easy to automate technique in which the sample extraction and pre-concentration are performed in a single step [3, 4]. Since its development, the technique has been widely used for the determination of different compounds in the environmental, nutritive, pharmaceutical, toxicological and forensic fields [5–8].
The principle of SPME is based on exposing the fiber, which is coated with an extraction phase, to the headspace above the sample solution or directly into the solution of interest. After extraction, the fiber containing analytes, is directly transferred to the GC injection port or LC desorption chamber for thermal or solvent desorption, respectively [3, 4]. Since the fiber coating serves an important function in the extraction efficiency of SPME, much attention has been focused on making new fibers in recent years. Different sorbents can be coated on the SPME fibers. Polydimethylsiloxane (PDMS), polyacrylate (PA), polydimethylsiloxane/divinylbenzene (PDMS/DVB) and carbowax/divinylbenzene (CW/DVB) are some commercial fibers that have been successfully applied in many fields. Despite wide applications, the commercial fibers have some drawbacks such as low operating temperature (generally, in the range of 240–280 °C), the few choices of the available fibers, fragility, instability in organic solvents and the high cost. Therefore, during the last decade, most efforts have been devoted to making new coatings for SPME [9–11].
The sol-gel technology is an attractive, universal and inexpensive method in the preparation of SPME coatings. It is a convenient technique used to obtain both inorganic and hybrid organic-inorganic polymeric networks. Thermal stability, chemical bonding between the sorbent and the fused silica surface, porous structure and a high surface area that ensures better extraction and simplicity in preparation are the advantages of the sol-gel technique. However, its SPME application is limited to the coating of fragile silica fibers. Recently, some researches have been focused on the preparation of sol-gel coating on other supports, like metals instead of the fused silica, to improve the mechanical resistance of the fiber and overcome its fragility [12, 13].
Nanostructured materials have distinct advantages in separation science. As novel stationary phases and dynamic coatings, they greatly enhance resolution, selectivity and efficiency of a separation process [14]. As SPME coatings, they have good thermal and chemical stability, and display a large surface area-to-volume ratio, thus promoting the extraction efficiency of the coating. So far, different nanomaterial coatings such as carbon nanotubes, graphene, fullerenes and nanofibrous polymers (e.g., polyurethane, polycarbonate, polyamide and polyvinylchloride) have been successfully applied as SPME coatings [15]. Polysiloxane materials are the most favorite and frequently used SPME coatings due to their suitable and notable chemical and thermal properties. Despite the obvious advantages of polysiloxane SPME fibers, there is no report on the use of nanostructured polysiloxane as SPME coatings [15].
In this study, polysiloxane nanofibers were synthesized using liquid phase method at room temperature. They were used as SPME coating on a stainless steel wire for the first time. The coating was prepared using methyltriethoxysilane in toluene in the presence of HCl as the catalyst. The extraction capability of the coating was investigated using headspace SPME for the extraction of four chlorobenzene compounds (1,4-dichlorobenzene, 1,2-dichlorobenzene, 1,2,4-trichlorobenzene and 1,2,3,4-tetrachlorobenzene). The effects of the main parameters on the extraction efficiency, such as salt concentration, stirring rate, equilibrium time, extraction time and temperature, desorption time, and temperature, were studied. The analytical characteristics of the method were obtained under the optimized conditions and the extraction efficiency of the new fiber was compared with the commercial PDMS and a nonfibrous polysiloxane fiber. Finally, the possible application of the method in real sample analysis was investigated.
Experimental
Material
Chlorobenzene compounds (1,4-dichlorobenzene, 1,4-DCB; 1,2-dichlorobenzene, 1,2-DCB; 1,2,4-trichlorobenzene, 1,2,4-TCB; and 1,2,3,4-tetrachlorobenzene, 1,2,3,4-TeCB) were purchased from Merck (Darmstadt, Germany, www.merck-chemicals.com). An individual stock standard solution of chlorobenzene compounds was prepared in methanol at the concentration of 1,000 mg L−1. The standard mixture solution at the concentration of 2.5–1,200 mg L−1 (1,4-DCB, 1,200 mg L−1; 1,2-DCB, 600 mg L−1; 1,2,4-TCB, 25 mg L−1; and 1,2,3,4-TeCB, 2.5 mg L−1) was prepared by diluting the stock standard solution in methanol. Working standard solutions of the analytes were prepared daily by successively diluting the secondary standard solution using pure water.
Extra pure toluene was purchased from Merck. To prepare extra dry toluene, it was dried by P2O5 and then distilled. Methyltriethoxysilane was obtained from Sigma & Aldrich (St. Louis, USA, www.sigmaaldrich.com). Other reagents were also purchased from Merck. Pure water was prepared by OES (Overseas Equipment & Services) water purification system (OK, USA). The stainless steel wire (0.25 mm i.d.) was used as the substrate for preparing SPME fibers.
Instrumentation
A homemade SPME holder was assembled and used for the extraction of chlorobenzenes with the fibers constructed in the present work. For the extraction of compounds with commercial fiber (30 μm PDMS), the SPME device was purchased from Supelco (Bellefonte, PA, USA, www.sigmaaldrich.com).
Chromatographic analysis was performed by a SP-3420 gas chromatograph equipped with a split/splitless injector and an electron capture detector (BFRL, Beijing, China, www.bfrl.com.cn). The injector was equipped with a low-volume insert designed for the analysis by SPME (Restek, Bellefonte, PA, USA, www.restek.com). Nitrogen (99.999 %) was used as carrier and make up gas at the flow rates of 2 and 30 mL min−1, respectively. Separation was carried out with a BP5 fused silica capillary column, 30 m × 0.25 mm, with a 0.25 μm stationary phase thickness (SGE, Australia, www.sge.com). The column was initially held at 50 °C for 2 min and then temperature was raised to 250 °C at a rate of 20 °C min−1 (5 min hold). The injector and detector temperatures were set at 240 and 280 °C, respectively. The morphology and surface characteristics of the ploysiloxane nanofibers coating were investigated using scanning electron microscopy (Philips XL400 SEM, The Netherlands, www.panalytical.com).
Preparation of polysiloxane nanofibers coating
Stainless steel wires were cut into 4 cm pieces. The surface of the wires was roughened with a sand paper and then washed with methanol. The wires were placed in 2 M NaOH solution for 4 h to form silanol groups on the surface of the stainless steel and then washed with deionized water [16]. The substrates were then placed in 0.1 M HCl for 15 min to neutralize the excess NaOH. After 15 min, the wires were rinsed with water and dried under a nitrogen stream. The activated wires were instantaneously used for the fabrication of the polysiloxane nanofibers coating by liquid phase method [17]. The wires were placed in a homemade Teflon chamber containing 15 mL dry toluene. The toluene was purged with nitrogen gas for 5 min. Then, 2 μL HCl (catalyst), 2 μL water and 25 μL methyltriethoxysilane were added to the chamber and the mixture was stirred at 450 rpm for 20 h. A length of 1 cm from the end part of the stainless steel wire was coated. The fibers were then removed from the chamber, rinsed with methanol and dried with nitrogen gas flow. To end-cap the residual silanol groups, the fibers were placed in the headspace of a vial containing chlorotrimethylsilane for 5 min, and then washed with methanol. Finally, the fibers were first conditioned at 150 °C for 30 min and then 280 °C for 2 h under a nitrogen flow in the GC injection port.
Preparation of nonfibrous polysiloxane fiber
The fiber was synthesized by the sol-gel technique according to a previously reported method [18]. Seven hundred sixty nine microlitre methyltriethoxysilane, 216 μL pure water, 40 μL hydrochloric acid (0.1 M) and 975 μL methanol were mixed. The sol-gel solution was stirred at room temperature for 4 h. The stainless steel wire was activated with NaOH and HCl solution (according to the method explained in the above section), and then immersed in the sol-gel solution for 20 min. After that, the fiber was end-capped by placing it into the headspace of chlorotrimethylsilane solution for 5 min. The fiber was then washed with methanol and conditioned at 150 °C for 30 min and then 280 °C for 2 h under a nitrogen flow in a GC injection port.
Headspace solid-phase microextraction procedure
A 5 mL standard aqueous solution of chlorobenzenes containing 1 g sodium sulfate was introduced in a 10 mL glass vial equipped with a screw cap and a silicon septum. The glass vial was placed in a water bath (35 °C) on a magnetic stirrer (MR 3000D, Heidolph, Germany, www.heidolph.com) and stirred for 5 min at 1,300 rpm. After 5 min (equilibrium time), the SPME syringe needle was inserted through the silicon septum and the fiber was exposed to the headspace of sample solution for 10 min. Then, the fiber was retracted into the needle and introduced into the GC injection port. The compounds were desorbed in the GC injector at 240 °C in splitless mode for 4 min.
Real samples
River water and sediment were collected from Zayandeh-rood river (Isfahan, Iran). Wastewater samples were collected from different wastewater treatment plants of Isfahan Mobarakeh Steel Company. Sample 1 and 2 were collected from the local primary treatment plants of the manufacturing units. The compositions of these samples were a mixture of different chemical compounds such as surfactants and cleaning agents used in the steel making industry. Sample 3, which was collected from the final effluent of the wastewater treatment plant of Isfahan Mobarakeh Steel Company, was a mixture of industrial and domestic wastewater. The activated sludge of wastewater treatment plant was also taken from the wastewater treatment plant of Isfahan Mobarakeh Steel Company.
The river water and wastewater samples were filtered with a 0.45-μm nylon membrane filter (Millipore, Bedford, MA, USA). The sediment and sludge samples were dried at room temperature. For each analysis, 1 g of the dried sediment or sludge (unspiked and spiked with the appropriate amounts of chlorobenzenes) was introduced into the glass vial, and 5 mL water was added to the sample before extraction [19].
Results and discussion
Selection of SPME coatings
Successful application of SPME technique depends primarily on the selection of a suitable fiber for a particular analysis. The chemical nature of analyte determines the type of polymer used for the extraction. Selection of coating is based on the polarity and volatility of the target compound. The well-known rule of thumb, “like prefers like”, applies well for the SPME coatings. Among the commercially available extracting phases, PDMS is the most studied and characterized fiber. PDMS is a non-polar coating suitable for the extraction of non-polar compounds such as substituted benzenes [20]. Therefore, chlorobenzenes were selected as the model compounds in this work to evaluate the extraction capability of the polysiloxane nanofibers coating. The extraction efficiency of the present fiber was also compared with the commercial PDMS and a nonfibrous polysiloxane coating.
SEM characterization of polysiloxane nanofibers coating
Scanning electron microscopy images of the coated and uncoated stainless steel wire are shown in Fig. 1. Based on the SEM images, the thickness of about 13 μm was observed for the coating. The SEM images of the coating with different magnifications are shown in Fig. 2. The fabrication of polysiloxane nanofibers on the surface of stainless steel wire was obviously confirmed. The nanofibrous structure of the coating increased the surface area and thus, enhanced the extraction capability of the fiber.
Headspace solid-phase microextraction
Experimental parameters including salt concentration, stirring rate, equilibrium time, extraction time, extraction temperature, desorption time and desorption temperature can affect the efficiency of SPME procedure. Therefore, the influence of these factors on the extraction efficiency of the fiber was investigated, and four chlorobenzenes were used as model compounds. All experiments were performed three replicates.
Ionic strength
Addition of salt to the sample solution can have two different effects. Increasing salt concentration in the solution can enhance the extraction efficiency due to the salting out effect and also, in some cases, decrease the efficiency of extraction by reducing the mass transfer rate [21]. To investigate the effect of salt on the extraction of chlorobenzenes from the sample solution to the fiber, the amounts of sodium sulfate in the range of 0–0.4 g mL−1 were studied. The addition of sodium sulfate increased the extraction of the analytes up to 0.2 g mL−1 (Fig. S1, Electronic Supplementary Material, ESM). More concentration of sodium sulfate reduced the extraction efficiency. So, an amount of 0.2 g mL−1 was chosen for the subsequent experiments.
Stirring rate
In headspace SPME, agitation of the sample solution can increase convection, thereby enhancing the mass transfer of analytes in the sample solution. Therefore, a new sample solution surface is regenerated and analyte mass transfer from the sample solution to the headspace is thus increased [22]. It can be concluded that increasing the stirring rate could reduce the time required to reach the equilibrium. In this study, the effect of stirring rates between 500 and 1,300 rpm on the extraction efficiency was investigated. By increasing the stirring rate, the peak areas of the analytes were enhanced (Fig S2, ESM). Therefore, the stirring rate of 1,300 rpm was used for all extractions.
Extraction temperature
The influence of sample temperature on the extraction capability was studied by changing the temperature in the range of 25–55 °C. The extraction efficiency was increased when temperature was changed from 25 to 35 °C and after that, by increasing temperature, the extraction efficiency was decreased. Higher temperature enhanced the diffusion of the analytes towards the fiber, and therefore, reduced the time required for reaching the equilibrium [23]. On the other hand, partition coefficient of the analytes between sample solution and the fiber could be diminished at higher temperatures, thus reducing the extraction yield. According to the results (Fig. S3, ESM), the temperature of 35 °C was selected for further experiments.
Equilibrium and extraction time
In HS-SPME, the sample solution is usually stirred for a period of time before extraction to allow the analytes to reach equilibrium between sample solution and headspace above the solution. The effect of different equilibrium times from 1 to 15 min on the extraction efficiency was investigated. Based on the results (Fig. S4, ESM), a 5-min time was enough to achieve equilibrium.
In microextraction techniques, the extraction of analytes from the solution to the fiber is an equilibrium process in which enough time should be given to analytes to reach the equilibrium [24]. Meanwhile, the thickness of fiber coating is also an important factor that affects the extraction time. By using thick films, the time for extraction and desorption is increased. To study the effect of time on the extraction yield, the sample solution was extracted in different times between 5 and 30 min. Based on the results (Fig. S5, ESM), the extraction time of 10 min provided good extraction efficiency and extraction times more than 10 min had no significant effect on the extraction.
Desorption time and temperature
After the extraction of the analytes by the fiber, they had to be thermally desorbed into the GC. To ensure rapid transfer of the analytes from the SPME fiber to the GC column and avoid the carry-over of the analytes during the extraction process, desorption time and desorption temperature had to be optimized. To study desorption time, the fiber was introduced in the GC injection port (260 °C) and different times (2, 3, 4 and 5 min) were investigated. The data (Fig. S6, ESM) showed that the desorption time of 4 min was enough to desorb the analytes. By checking the desorption temperatures in the range of 220–280 °C using desorption time of 4 min, it was found that a desorption temperature of 240 °C was sufficient to desorb the analytes from the fiber (Fig. S7, ESM). No carry-over on second desorption was found for the fiber with a 4 min desorption time at 240 °C.
Method validation
To evaluate the HS-SPME method with the novel fiber coating, the analytical parameters such as linear dynamic range, detection limit and precision were determined under the optimized conditions (0.2 g mL−1 sodium sulfate concentration, stirring rate of 1,300 rpm, 5 min equilibrium time, 10 min extraction time, extraction temperature of 35 °C, and 4 min desorption time at 240 °C). The results of the analytical figures of merit for the method are shown in Table 1. As can be seen, the method showed good linearity in the range of 0.0005–24 μg L−1 with determination coefficients (r 2) greater than 0.9970. Based on peak-to-peak noise (S/N = 3), the limits of detections were found to be in the range of 0.15–75 ng L−1. The intra-day RSDs of chlorobenzenes for a single fiber were in the range of 2.1–4.2 % for three replicate extractions of spiked water samples (1,4-DCB, 2,400 ng L−1; 1,2-DCB, 1,200 ng L−1; 1,2,4-TCB, 50 ng L−1; and 1,2,3,4-TeCB, 5 ng L−1). The inter-day relative standard deviations for a single fiber were between 2.8 and 5.7 % as calculated by extracting the chlorobenzene compounds (three times) each day over a period of three working days. The fiber-to-fiber reproducibility was studied using three different fibers prepared under the same conditions. The RSDs ranged from 3.3 to 9.7 %.
Fiber durability
In this study, polysiloxane nanofibers were coated on a stainless steel wire to overcome the drawbacks of silica fibers. The stainless steel as a support was flexible, sturdy and unbreakable. The polysiloxane nanofibers SPME coating could be used for approximately 100 times without any significant reduction in the extraction efficiency. After 100 extraction-desorption cycles, the decrease in efficiency was only 6 % (±0.5), 4 % (±0.3), 6 % (±0.6) and 5 % (±0.6) for 1,4-DCB, 1,2-DCB, 1,2,4-TCB, and 1,2,3,4-TeCB, respectively. Polysiloxane coating had good thermal stability and could tolerate high temperatures. In addition, the fiber had good mechanical stability.
Comparison of the polysiloxane nanofibers coating with other fibers
The extraction of chlorobenzenes using PDMS commercial fiber has previously been reported [25, 26]. To compare the extraction efficiency of the present fiber with PDMS fiber, the analytes were extracted with both fibers. The commercial PDMS fiber was used under a previously reported condition [26] (20 % NaCl, extraction time of 30 min at room temperature, stirring rate of 1,500 rpm, and 10 min desorption time at 200 °C). In addition, a nonfibrous polysiloxane fiber was fabricated using the conventional sol-gel technique [18]. As shown in Fig. 3, the extraction efficiency of chlorobenzenes with polysiloxane nanofibers was much better than that of other fibers. The new fiber showed 5–10 and 10–30 times better extraction efficiency compared to the commercial PDMS fiber and the nonfibrous polysiloxane fiber, respectively. Since the chemical composition of the studied fibers was approximately the same, the high extraction efficiency of the polysiloxane nanofibers coating could be due to the nanostructure of the fiber. The nanofibrous material enhanced the surface area of the fiber and provided fast mass transfer rate.
Effect of SPME fiber type on the extraction efficiency. Extraction conditions for polysiloxane nanofiber and sol-gel fiber: concentration of the analytes: 5–2,400 ng L−1; sample volume: 5.0 mL; extraction time: 10 min; salt concentration: 0.2 g mL−1; stirring rate 1,300 rpm; desorption time: 4 min; extraction temperature: 35 °C; desorption temperature: 240 °C. Conditions for PDMS commercial fiber: concentration of the analytes: 5–2,400 ng L−1; sample volume: 5.0 mL; salt concentration: 0.2 g mL−1; stirring rate 1,500 rpm; extraction time: 30 min at room temperature; desorption time: 10 min; desorption temperature: 200 °C
In comparison to other SPME methods used for the determination of chlorobenzenes (Table 2), the present method showed better precision and low detection limit in a shorter extraction time. In addition, the method required a lower desorption temperature. This could be due to the lower thickness of the present fiber.
Real sample analysis
To evaluate the possibility and reliability of the HS-SPME method in the determination of chlorobenzene compounds in real samples, different samples such as water, wastewater, activated sludge and river sediment were analyzed. The samples were spiked with different amounts of the chlorobenzenes, and the analytes were determined with multiple standard addition procedure.
The samples were spiked with the chlorobenzenes at concentration levels between 0.001 and 2.4 μg L−1 for water and wastewater, and 0.001–2.4 ng g−1 for sediment and sludge samples (three spiked levels). The results (Table S1, ESM) showed that the activated sludge and sample 3 were either free of the analytes or had concentrations below the method detection limit. The determination coefficients of calibration curves for the chlorobenzene compounds were in the range of 0.9940–0.9990, showing good linearity for real sample analysis. The relative recoveries (the ratio of the concentration found in real sample to the concentration found in pure water, spiked with the same amounts of analytes) for water and wastewater samples were obtained to be between 91 and 98 % for the samples spiked at concentration levels of 0.002–0.96 μg L−1. The relative standard deviations were 1.1–5.9 % (n = 3), and the results were accurate with the relative error varying from 2.7 to 8.5 %. The relative recoveries for sediment and sludge samples spiked at the concentration of 0.002–0.96 ng g−1 were 90–99 %. The RSDs were in the range of 2.1–7.7 % for three replicate extractions. The relative error was in the range of 1.0–9.1 % for the concentrations examined. The results confirmed the capability of the present method in analyzing different real samples with different matrices. The chromatograms of spiked and non-spiked wastewater sample (sample 1) are shown in Fig. 4.
Conclusions
In this study, based on polysiloxane nanofibers, a novel SPME coating was fabricated on the stainless steel wire. The coating was simply prepared in one step at room temperature. The fiber was used for the extraction of four chlorobenzenes in water, wastewater, sludge and sediment samples. The nanofibrous structure of the coating enhanced the surface area and caused a remarkable increase in the extraction efficiency at a short extraction time. In the meantime, the relatively low thickness of the fiber allowed the use of less desorption temperature and thus, a high fiber life time (over 100 times usability) was obtained. Compared to the polysiloxane fiber fabricated by the conventional sol-gel technology and also, the commercial PDMS fiber, the fiber prepared by the present technique had higher extraction efficiency. In comparison to other SPME methods used for the determination of chlorobenzene compounds, the present method also showed lower detection limit with short extraction time and good reproducibility.
References
Theodoridis G, Koster EHM, de Jong GJ (2000) Solid-phase microextraction for the analysis of biological samples. J Chromatogr B 745:49–82. doi:10.1016/S0378-4347(00)00203-6
Belardi RP, Pawliszyn J (1989) The application of chemically modified fused silica fibers in the extraction of organics from water matrix samples and their rapid transfer to capillary columns. Water Pollut Res J Can 24:179–191
Balasubramanian S, Panigrahi S (2011) Solid-phase microextraction (SPME) techniques for quality characterization of food products. Food Bioprocess Technol 4:1–26. doi:10.1007/s11947-009-0299-3
Peñalver A, Pocurull E, Borrull F, Marcé RM (1999) Trends in solid-phase microextraction for determining organic pollutants in environmental samples. Trends Anal Chem 18:557–568. doi:10.1016/S0165-9936(99)00145-4
Budziak D, Martendal E, Carasek E (2009) Application of an NiTi alloy coated with ZrO2 solid-phase microextraction fiber for determination of haloanisoles in red wine samples. Microchim Acta 164:197–202. doi:10.1007/s00604-008-0057-7
Mehdinia A, Khani H, Mozaffari S (2013) Fibers coated with a graphene-polyaniline nanocomposite for the headspace solid-phase microextraction of organochlorine pesticides from seawater samples. Microchim Acta 181:89–95. doi:10.1007/s00604-013-1071-y
Cai L, Koziel JA, Davis J, Lo YC, Xin H (2006) Characterization of volatile organic compounds and odors by in-vivo sampling of beef cattle rumen gas, by solid-phase microextraction, and gas chromatography-mass spectrometry-olfactometry. Anal Bioanal Chem 386:1791–1802. doi:10.1007/s00216-006-0799-1
Yuan H, Mullett WM, Pawliszyn J (2001) Biological sample analysis with immunoaffinity solid-phase microextraction. Analyst 126:1456–1461. doi:10.1039/B101854J
Xu J, Zheng J, Tian J, Zhu F, Zeng F, Su C, Ouyang G (2013) New materials in solid-phase microextraction. Trends Anal Chem 47:68–83. doi:10.1016/j.trac.2013.02.012
Spietelun A, Pilarczyk M, Kloskowski A, Namieśnik J (2010) Current trends in solid-phase microextraction (SPME) fibre coatings. Chem Soc Rev 39:4524–4537. doi:10.1039/C003335A
Aziz-Zanjani MO, Mehdinia A (2014) A review on procedures for the preparation of coatings for solid phase microextraction. Microchim Acta. doi:10.1007/s00604-014-1265-y
Augusto F, Carasek E, Silva RGC, Rivellino SR, Batista AD, Martendal E (2010) New sorbents for extraction and microextraction techniques. J Chromatogr A 1217:2533–2542. doi:10.1016/j.chroma.2009.12.033
Li XJ, Ye CW, Huo XL, Zeng Z (2010) Solid-phase microextraction using a diglycidyloxycalix[4]arene coated fiber combined with gas chromatography: very simple, rapid and sensitive method for the determination of chlorobenzenes in water. Microchim Acta 168:161–167. doi:10.1007/s00604-009-0276-6
Guihen E (2013) Nanoparticles in modern separation science. Trends Anal Chem 46:1–14. doi:10.1016/j.trac.2013.01.011
Mehdinia A, Aziz-Zanjani MO (2013) Recent advances in nanomaterials utilized in fiber coatings for solid-phase microextraction. Trends Anal Chem 42:205–215. doi:10.1016/j.trac.2012.09.013
Saraji M, Farajmand B (2012) Microporous silica with nanolayer structure coated with renewable organic solvent film as a novel extracting phase: a combination of solid- and liquid-phase microextraction. Anal Chim Acta 721:61–67. doi:10.1016/j.aca.2012.01.046
Stojanovic A, Olveira S, Fischer M, Seeger S (2013) Polysiloxane nanotubes. Chem Mater 25:2787–2792. doi:10.1021/cm400851k
Gbatu TP, Sutton KL, Caruso JA (1999) Development of new SPME fibers by sol–gel technology for SPME-HPLC determination of organometals. Anal Chim Acta 402:67–79. doi:10.1016/S0003-2670(99)00532-2
Sarrión MN, Santos FJ, Galceran MT (1998) Strategies for the analysis of chlorobenzenes in soils using solid-phase microextraction coupled with gas chromatography–ion trap mass spectrometry. J Chromatogr A 819:197–209. doi:10.1016/S0021-9673(98)00466-X
Supelco (2004) SPME application guide, 5th edn. Supelco, Bellefonte
Liu W, Zhang L, Chen S, Duan H, Chen X, Wei Z, Chen G (2009) A method by homemade OH/TSO-PMHS fibre solid-phase microextraction coupling with gas chromatography-mass spectrometry for analysis of antiestrogens in biological matrices. Anal Chim Acta 631:47–53. doi:10.1016/j.aca.2008.10.012
Sarafraz-Yazdi A, Ghaemi F, Amiri A (2012) Comparative study of the sol–gel based solid phase microextraction fibers in extraction of naphthalene, fluorene, anthracene and phenanthrene from saffron samples extractants. Microchim Acta 176:317–325. doi:10.1007/s00604-011-0718-9
Asadollahzadeh H, Noroozian E, Maghsoudi S (2010) Solid-phase microextraction of phthalate esters from aqueous media by electrochemically deposited carbon nanotube/polypyrrole composite on a stainless steel fiber. Anal Chim Acta 669:32–38. doi:10.1016/j.aca.2010.04.029
Magdic S, Boyd-Boland A, Jinno K, Pawliszyn JB (1996) Analysis of organophosphorus insecticides from environmental samples using solid-phase microextraction. J Chromatogr A 736:219–228. doi:10.1016/0021-9673(95)01349-0
Santos FJ, Sarrion MN, Galseran MT (1997) Analysis of chlorobenzenes in soils by headspace solid-phase microextraction and gas chromatography-ion trap mass spectrometry. J Chromatogr A 771:181–189. doi:10.1016/S0021-9673(97)00132-5
He Y, Wang Y, Lee HK (2000) Trace analysis of ten chlorinated benzenes in water by headspace solid-phase microextraction. J Chromatogr A 874:149–154. doi:10.1016/S0021-9673(00)00067-4
Li X, Chen J, Du L (2007) Analysis of chloro- and nitrobenzenes in water by a simplepolyaniline-based solid-phase microextraction coupled with gas chromatography. J Chromatogr A 1140:21–28. doi:10.1016/j.chroma.2006.11.044
Zhang G, Li Z, Zang X, Wang C, Wang Z (2014) Solid-phase microextraction with agraphene-composite-coated fiber coupled with GC for the determination of some halogenated aromatic hydrocarbons in water samples. J Sep Sci 37:440–446. doi:10.1002/jssc.201301183
Li X, Zeng Z, Xu Y (2006) A solid-phase microextraction fiber coated with diglycidyloxycalix[4]arene yields very high extraction selectivity and sensitivity during the analysis of chlorobenzenes in soil. Anal Bioanal Chem 384:1428–1437. doi:10.1007/s00216-005-0281-5
Acknowledgments
The authors acknowledge the Research Council of Isfahan University of Technology (IUT) and the Center of Excellence in Sensor and Green Chemistry for their financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 182 kb)
Rights and permissions
About this article
Cite this article
Saraji, M., Mehrafza, N. Polysiloxane coated steel fibers for solid-phase microextraction of chlorobenzenes. Microchim Acta 182, 841–848 (2015). https://doi.org/10.1007/s00604-014-1395-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1395-2