Abstract
A copolymer was prepared from glycidyl methacrylate, ethylene glycol dimethacrylate and oxidized single-walled carbon nanohorns via photo-polymerization and used in spin columns for the extraction of nonsteroidal anti-inflammatory drugs (NSAIDs) from human urine samples. All microextraction procedures (loading, washing and elution) can be performed by centrifugation. The hybrid monolithic polymers were characterized by scanning electron microscopy and nitrogen intrusion porosimetry. Following elution with methanol, the NSAIDs (naproxen, fenbufen, flurbiprofen, and ibuprofen) were quantified by reversed-phase HPLC with UV detection. The detection limits varied between 0.1 and 10 μg·L−1, and the precision (relative standard deviation) ranged from 3.5 to 11.8%. Relative recoveries between 81 and 106% were found when analyzing spiked urine samples.

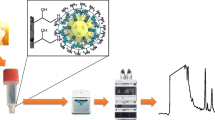
A hybrid monolithic solid based on the copolymerization of methacrylate monomers and single-walled carbon nanohorns was covalently anchored and synthetized into a spin column device via photo-polymerization approach. The spin column was used for the extraction of nonsteroidal anti-inflammatory drugs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the first synthesis of monolithic polymers at the end of the twentieth century [1] organic monoliths derived from methacrylates, acrylamides, and styrenes have been successfully applied as stationary phases in chromatography [2], and as sorbents in sample treatment techniques [3] but to a lesser extent. Among the microextraction formats where monolithic sorbents have been used, spins columns can be cited as one of the most advantageous. Thus, the spin-column format offers a simple operation procedure, allows a high-throughput sample, requires a low eluate volume and does not involve solvent evaporation, all features in rough agreement with the principles of green analytical chemistry. Monolithic silica spin column was first introduced by Namera and Saito in 2008 [4, 5]. The monolithic solid is packed in the bottom of the column unit without using frits, and then solvents are passed through the sorbent phase by centrifugation. This approach has been used to extract target compounds from biological matrices [6, 7]. While there are already several monolithic silica spin columns used to isolate and preconcentrate target analytes, a potential growth area of interest may be the development of polymer-based monoliths. In this context, Güzel et al. have developed an erbium phosphate doped poly(glycidyl methacrylate-co-ethylene glycol dimethacrylate (poly(GMA-co-EDMA)) spin column for selective enrichment of phosphopeptides [8].
To achieve more specific interaction of the analytes with the polymeric networks, nanomaterials including graphene [9], carbon nanotubes [10, 11], and carbon nanohorns [12], have been combined with monolithic material to prepare novel stationary phases or sorbents with enhanced performances.
Single-walled carbon nanohorns (SWNHs) were firstly discovered by Iijima et al. in 1999 [13]. Concretely, SWNHs are cone-shaped carbon structures with a single wall, which have natural tendency to form dahlia-shaped aggregates [14]. Their conical structure provides high porosity and large surface area showing a good absorbent capacity for organic compounds [15]. Up to date, their potential and usefulness has widely demonstrated in microextraction techniques [16–19].
In this study, a hybrid monolithic phase based on a combination of methacrylate monomers and oxidized SWNHs (o-SWNHs) has been synthetized into a spin column device employing a UV-polymerization approach. To ensure covalent attachment of the monolith to the inner wall of the polypropylene device, a surface modification was first carried out with grafted chains of EDMA. Then, the optimization of variables affecting to the more convenient preparation of the hybrid monolith in terms of uniformity, rigidity and robustness was addressed. Moreover, the monolith copolymerized with o-SWNHs was characterized by scanning electron microscopy (SEM) and a porosimetry study was also carried out. The spin column was evaluated for the extraction and preconcentration of four nonsteroidal anti-inflammatory drugs (NSAIDs) in urine samples prior to HPLC-UV analysis.
Experimental section
Reagents and materials
All reagents were of analytical grade or better. Nonsteroidal anti-inflammatory drugs (NSAIDs) (naproxen, fenbufen, flurbiprofen, and ibuprofen) were purchased from Sigma-Aldrich (Madrid, Spain. http://www.sigmaaldrich.com). Standard solutions of each analyte were prepared in methanol (Sigma-Aldrich) at a concentration of 1 g·L−1 and stored at 4 °C. Working standard solutions were prepared on a daily basis by rigorous dilution of the stocks in ultrapure Milli-Q water. Methanol was also used for NSAIDs elution.
Single–walled carbon nanohorns (SWNHs) were purchased from Carbonium S.r.l. (Padua, Italy. http://www.carbonium.it/public/site/index.php). SWNHs form stable dahlia-shaped aggregates with an average diameter of 60–80 nm. Individually, the lengths of these SWNHs are in a range 40–50 nm, and the diameter in the cylindrical structure varies between 4 and 5 nm. Laboratory-oxidized carbon nanohorns were prepared following a procedure previously optimized [18]. In brief, SWNHs were weighed (5 mg) and added to a glass vial, which was further introduced into a microwave oven, being the solid irradiated at 800 W for 10 min.
Acetonitrile, acetic acid (Scharlab, Barcelona, Spain. http://scharlab.com) and ultrapure Milli-Q water were employed as components of the chromatographic mobile phase.
The reagents used for the synthesis of the monolithic phase, glycidyl methacrylate (GMA), ethylene dimethacrylate (EDMA), cyclohexanol, 1-dodecanol, lauroyl peroxide (LPO), ethanol, acetone, 2,2-dimethoxy-2-phenylacetophenone (DMPA), α,α′-azobisisobutyronitrile (AIBN), and benzophenone (BP) were purchased from Sigma-Aldrich. The spin columns were supplied by Sigma-Aldrich (SigmaPrep™ Spin Columns with Break-Away Tip), which have a maximum volume of 700 µL.
Blank urine samples were collected from healthy adult volunteers and stored in polytetrafluoroethylene flasks at −20 °C until analysis. Prior to the microextraction process, urine samples were diluted to 1:1 with phosphate buffer (pH 2.11, 25 mM). Next, each sample filtered through a disposable nylon filter of 0.45 μm of pore size (Análisis Vínicos, Tomelloso, Spain. http://www.analisisvinicos.com). In order to validate the method, samples collected from individuals treated with NSAIDs, were also employed.
Instrumentation
The photo-polymerization was carried out using a UV crosslinker (model CL1000) from UVP Inc. (Upland, CA, USA) equipped with UV lamps (5 × 8 W, 254 nm). SEM photographs of monolithic materials were taken with a scanning electron microscope (S-4100, Hitachi, Ibaraki, Japan) provided by a field emission gun and an EMIP 3.0 image data acquisition system (Rontec, Normanton, UK). Samples for SEM analysis were previously sputter-coated with Au/Pd for 2 min to avoid charging problems. This treatment was necessary since these materials are not electrically conducting. Optical microscope SZH (Olympus) was used to obtain the pictures of the hybrid monolithic solid. An ultracentrifuge (Sigma Laborzentrifugen, model 2–15 Osterode am Harz, Germany) was used for the different extraction steps.
Nitrogen adsorption/desorption experiments were carried out at −196 °C using a Quantachrome®ASiQwinTM-Automated Gas Sorption Data. The specific surface area values were calculated according to the BET (Brunauer-Emmett-Teller) eq. T-plot method was used to determine the micropore surface areas, and the average pore volumes were evaluated from the desorption branches of isotherms based on the BJH (Barrett-Joyner-Halenda) model.
An 1100 Series HPLC chromatograph (Agilent Technologies, Waldbronn, Germany), provided with a quaternary pump, including a thermostatic column compartment and a UV-Vis diode array detector was used. For the optimized procedure, a Kinetex C18 column (Phenomenex, Torrance, US, 2.6 μm, 100 Å, 150 × 4.6 mm) was used. The flow rate was 1 mL·min−1. UV detection wavelengths were set at 220, 230, and 254 nm using 360 nm as reference. Separation was accomplished using two mobile phase solvents: ultrapure Milli-Q water (mobile phase A) and ACN (mobile phase B) both containing 0.1% (v/v) of acetic acid. An isocratic gradient (50% B) in 11 min was performed at 25 °C. Prior to use, all mobile phases were degassed with a D-78224 ultrasonic bath (Elma, Germany).
Modification of spin wall surface and preparation of hybrid monolithic material
To ensure covalent attachment of the monolithic beds to the inner spin column wall for enhanced mechanical stability, the polypropylene wall surface was previously photo-chemically modified with BP and EDMA. For this purpose, the spin column was sequentially washed with ethanol and acetone, and dried under a nitrogen stream. Next, it was filled with 80 μL of a 5% w/v methanolic BP solution and irradiated with UV light at 1 J·cm−2 for 10 min. BP was removed with methanol followed by a drying step with nitrogen. Then, the spin column was filled with a 15% (v/v) methanolic EDMA solution (80 μL) and placed again under UV irradiation for 10 min. Finally, the spin column modified was washed with acetone and dried under a nitrogen stream.
The polymerization mixture is composed of 60 wt% monomers (48 wt% GMA and 12 wt% EDMA), 40 wt% porogens (37 wt% cyclohexanol and 3 wt% 1-dodecanol) and 0.3 wt% of LPO (out of the total weight of monomers) as free-radical initiator. In the next step, o-SWNHs (0.1 wt%) was added to the polymerization mixture. Afterward, the mixture was sonicated for 10 min and purged with nitrogen for an additional 10 min. The spin column previously modified was filled with 70 μL of the polymerization mixture. Polymerization was accomplished by irradiation of the spin column within the UV crosslinker chamber at 1 J·cm−2 for 4 h. After polymerization, the spin column was thoroughly washed with methanol and Milli-Q water to remove the pore-forming solvents and any possible unreacted monomers.
Microextraction procedure
The poly(GMA-co-EDMA-co-o-SWNHs) monolithic material was used for the extraction of NSAIDs from urine samples. The general scheme of the microextraction procedure is depicted in Fig. 1. Prior to extraction of NSAIDs, the hybrid monolith was preconditioned with 0.1 mL of methanol and 0.1 mL of Milli-Q water by centrifugation at 14000 rpm (18,000 g) for 10 min, respectively. Urine samples were diluted to 1:1 with phosphate buffer (pH 2.11) and filtered through a disposable nylon filter (0.45 μm). Then, 0.6 mL of sample, were placed into the pre-conditioned spin column, and it was centrifuged at 18000 g (for 55 min. The monolithic phase was then rinsed with Milli-Q water (0.1 mL) by centrifuging (18,000 g) for 10 min. Finally, the adsorbed analytes were eluted with 50 μL of methanol (5 min, 18,000 g) for further HPLC analysis.
Results and discussion
Surface attachment of monolith to polypropylene spin column
Polypropylene surface modification is required for the successful attachment of the monolithic polymer. This fact prevents both the formation of voids at the monolith-wall interface due to the shrinkage of the monolith during the polymerization process in bulk polymerization as well as the monolith detachment during extraction operation steps. This is especially remarkable in non-treated conical housing materials. Supersonic adhesion is usually used to fix monolithic silica rod into the spin column devices. However, covalent binding of the organic polymer-based monoliths to the spin column inner wall has not yet been reported. In this work, a UV grafting process based on the initial UV immobilization of BP derived free-radical initiator followed by the grafting of a polymer layer (EDMA) with a multiplicity of pendant double bonds from the polypropylene inner surface has been optimized. The influence of the UV irradiation time, at both steps, keeping the irradiation power level fixed, has been studied within the interval 5–60 min. The results showed that 10 min was selected as the time required to reach a covalent attachment of the hybrid monolith to the spin column wall (respective figure is given in the Supporting Information).
Choice of materials
One of the benefits of polymer monolithic materials is their high permeability due to their large through pores. However, these materials exhibit small surface areas due the absence of meso- and nano-porous structures, which can reduce the number of interaction sites required to achieve a sufficient sample loading capacity, an important parameter in sorption-based extraction techniques. Then, the combination of monolithic technology and the specific features of SWNHs is an attractive way of obtaining novel sorbents with enhanced adsorption (retention) performances. In order to demonstrate the improvement in the extraction performance of polymeric monoliths after incorporation SWNHs, monoliths from a relatively polar monomer, glycidyl methacrylate (GMA) were selected.
Incorporation of o-SWNHs to methacrylate monoliths
Taking into account these considerations, our approach was to incorporate SWNHs in the monolith by direct addition (dispersion) of these carbon nanostructures to the polymerization mixture in order to obtain a hybrid monolith with mechanical stability and large extraction efficiency. To reach this goal, initial polymerization conditions were as follows; 20 wt% monomers (15 wt% GMA and 5 wt% EDMA) and 80 wt% (75 wt% cyclohexanol and 5 wt% 1-dodecanol), in the presence of different free-radical initiators. Furthermore, 0.1 wt% of o-SWNHs was well-dispersed in this mixture by sonication for 10 min, and then purged with nitrogen. Once the mixture was filled into the spin column, it was placed under UV irradiation for 4 h.
Thus, several types of free-radical initiators were tested in terms of the polymerization rate as well as homogeneity of the polymer formed. The behavior of AIBN (1 wt%), DMPA (0.2 wt%) and LPO (0.3 wt% out of the total weight of the monomers), which affects the kinetics of the free-radical polymerization as well as the morphology of the resulting polymer [20, 21], was evaluated. When AIBN or DMPA were used as radical initiator, the o-SWNHs were entrapped into the monolithic network remaining some carbon nanoparticles on the pore surface available to interact with the analytes. However, a copolymerization of monomers (GMA and EDMA) with o-SWNHs was produced using an organic peroxide initiator such as LPO. This may be due to the existence of a competition between monomer and o-SWNHs for the initiator radicals [22]. In this regard, the role of initiator is a key point, AIBN and DMPA undergo a reaction mechanism which leads to the production and subsequent propagation of monomer radicals in comparison with the active o-SWNHs surface generated by LPO. Thus, hybrid monoliths initiated with LPO exhibited the most favourable and homogeneous structure for the extraction performance, and therefore LPO was selected as photo-initiator.
The second variable studied, in order to obtain a rigid and stable monolithic polymer with high extraction capacity, was the monomers/porogens ratio within the following proportions: 20/80% (w/w), 40/60% (w/w), 60/40% (w/w), and 80/20% (w/w). The permeability and mechanical stability of the hybrid monolith were controlled by the percentage of porogenic solvents. While monoliths prepared with ratios of 20/80 and 40/60% (w/w) showed a too porous and brittle structure causing their break in the centrifugation step, when the proportion was 80/20% (w/w) the monolithic phase resulted in the smaller pores, hindering the flow of solvents through it. The best morphological characteristics were obtained for a 60/40% (w/w) ratio and therefore it was selected for further experiments.
Next, the amount of o-SWNHs was evaluated in the range from 0.05 to 0.5 wt%. Monolithic polymers formed at high concentrations of nanoparticles showed a more rigid structure avoiding the breakage of the material during the passage of the solvents through it and promoting the reuse of the monolithic solid. By contrast, monoliths were not formed in the absence of o-SWNHs in the polymerization mixture because polymer radicals for methacrylate monomers are not as reactive as the o-SWNHs conical surface due to its reactivity is associated with their areas rich in pentagons and heptagons [23].
When the percentage was 0.5 wt%, the solid resulted in the smaller pores and therefore it led to an increased flow resistance. Thus, an amount of 0.1 wt% of SWNHs was selected as the best compromise for high throughput purposes. Figure 2 shows a picture of this hybrid monolithic polymer into the spin column.
Characterization of hybrid monolithic material
The monoliths were characterized by SEM, elemental analysis, and nitrogen adsorption/desorption measurements. Figure 3 shows the SEM images of the UV polymerized monoliths obtained in presence of 0.1 wt% of SWNHs at different magnification powers. The macroporous structure with different levels of pore sizes is visible which demonstrated large pore (∼3 μm) sizes, and consequently, an adequate permeability (Fig. 3a). A larger magnification provided evidence that the addition of SWNHs in the polymeric matrix led to globules with larger surface roughness compared to those found in the typical globular structure of polymeric monoliths [1, 2, 20, 21].
Data from nitrogen adsorption-desorption isotherms evidenced that the monolithic solid exhibited an isotherm type IV which is typical of solids with a mainly mesoporous structure. Furthermore, t-plots (using Harkins-Jura correlation) from the adsorption branch of the isotherm, showed the absence of microporosity. The specific surface area of the hybrid solid was determined by the BET method and the pore size distribution by the method of Barrett, Joyner, and Halenda (BJH). The values of surface area, pore diameter, and pore volume, obtained are compiled in Table 1. Thus, the hybrid monoliths containing o-SWNHs showed larger values surface areas (ca. 700 m2·g−1) compared to the typical organic polymer-based monolith, where these values did not exceed few tens of m2·g−1 This remarkable increase in surface area will benefit undoubtedly the retention and extraction efficiency, which will be described in details below.
Application to the extraction and preconcentration of NSAIDs from urine samples
The hybrid monolithic sorbent presented in this work was employed for the extraction of the four NSAIDs (naproxen, fenbufen, flurbiprofen, and ibuprofen) from urine samples following the microextraction procedure described previously. The extraction conditions were adapted from previous studies of our research group [24]. Prior to (micro) solid-phase extraction using the poly(GMA-co-EDMA-co-o-SWNHs) spin column, each sample was diluted to 1:1 with phosphate buffer and filtered through a disposable nylon filter. The pH of the sample was adjusted to 2.11 by using dilute phosphate buffer to maximize the interaction of the analyte with the hybrid monolith via hydrophobic and hydrogen bonds interactions.
The method was validated in terms of sensitivity, linearity, and precision. The corresponding calibration graphs were constructed by extracting in duplicate nine working aqueous standards containing the four analytes at different concentrations (0.01–10,000 μg·L−1). For all the analytes, a good linearity (R > 0.99) was observed. The limits of detection (LODs) were calculated by using a signal-to-noise ratio of 3, giving values comprised between 0.1 (naproxen) and 10 (ibuprofen) μg·L−1 (Table 2). The limits of quantification (LOQs), calculated as the concentration providing chromatographic peak areas ten times higher than the background noise, ranged from 0.5 to 20 μg·L−1 (Table 2).
The precision of the method (intra and inter-units), expressed as relative standard deviation (RSD) and also given in Table 2, was calculated from three individual standards prepared at a concentration of 50 μg·L−1 and it was lower than 11.8% for all the analytes. The enrichment factors for all the analytes were calculated by comparison of the slopes of the calibration graphs before and after the extraction process. They were in the range from 10.4 to 13.2 (see Table 3). The absolute extraction recoveries, which refer to the percentage of total analyte that can be extracted efficiently by the sorbent and finally eluted with methanol, were in the interval of 81–106%.
The identification of potential interferences from the matrix on the quantification of the analytes is a relevant issue, especially when analyzing unknown samples. Therefore, the accuracy of the method was evaluated through a recovery study. Different blank urine samples were fortified with the four target analytes (naproxen, fenbufen, flurbiprofen, and ibuprofen) at a concentration of 50 μg·L−1, and they were left to stand for 24 h prior to analysis. Then, the fortified samples were analyzed using the extraction method, and the concentration for each NSAID was calculated by interpolating the peak area obtained in the corresponding calibration graph. The recovery values were calculated dividing the concentration found by the concentration added, and expressed in percentage. Each sample was analyzed by triplicate; the results obtained are listed in Table 3. As it can be seen, in all instances, excellent recovery values (ranged from 81.3 to 105.6%) were obtained.
The method was then applied to the determination of the NSAIDs in urine samples (3 h after drug intake). Urine samples, collected from an individual treated with naproxen (550 mg) were analyzed. The extraction was carried out for standards and spiked samples following the procedure detailed above. The presence of naproxen was definitely confirmed by the comparison of its retention time with the spiked standard of the same analyte. The corresponding value was 1.79 mg·L−1 which is comparable with pharmacokinetic values for the NSAIDs, and is closely related to half-life values reported in literature [25, 26]. Furthermore, the method allows the quantitative determination of the target analytes without interference of endogenous compounds.
Comparison with other nanomaterial-based extraction procedures
Table 4 compares the characteristic features for our method with other nanomaterial-based extraction methods reported in the literature for the determination of NSAIDs in biological samples. Regarding absolute extraction recovery values obtained in this study, these were similar to those found in most reported studies with the exception of those given in reference [30], where the recoveries were quite low. Concerning the LODs, most of these studies have been focused on the determination of naproxen, in this sense, our LOD value was similar [31] or better [28] using magnetic SPE. Besides, our method provided the widest linearity range of all the methods reported. Regarding to the preparation of sorbent few simple steps are required, and moreover our protocol simplifies the handling of more samples simultaneously and speeds the preconcentration process of NSAIDs. In particular, this method allowed a sample throughput of 10 samples· h−1, whereas a rate of 1–2 samples· h−1 may be achieved with other nanomaterials-based protocols [27, 29].
Conclusions
A monolithic spin column, based on copolymerization between methacrylate monomers and o-SWNHs, was prepared via in situ photo-polymerization. The potential of this new sorbent was evaluated for the preconcentration of NSAIDs from urine samples. The hybrid monolith was characterized by SEM and nitrogen intrusion porosimetry. A UV-polymerization strategy for the covalent functionalization of the housing polypropylene surface to achieve a robust attachment of monolith has been carried out, thus improving its mechanical stability, without the need of retaining frits, in comparison with non-anchored monoliths. To our knowledge, this is the first report that employs this modification protocol combined with organic monoliths confined within these microextraction supports. The high adsorption capacity and specific surface area has been attributed to the participation of o-SWNHs as monomer in the polymerization reaction due to their enhanced reactivity associated with their conical shape. The results obtained demonstrated that the hybrid monolithic spin columns can be successfully applied in bioanalysis owing to its unique characteristic including their cost-effective preparation, porosity and chemical stability in a wide pH range. Although the method requires high centrifugation rates in extraction procedure, it provides good analytical features in term of recoveries, excellent linearity and LODs compared to other nanomaterial-based extraction methods, and a high throughput sample preparation.
References
Svec F, Fréchet JM (1992) Continuous rods of macroporous polymer as high-performance liquid chromatography separation media. Anal Chem 64:820–822
Carrasco-Correa EJ, Vela-Soria F, Ballesteros O, Ramis-Ramos G, Herrero-Martínez JM (2015) Sensitive determination of parabens in human urine and serum using methacrylate monoliths and reversed-phase capillary liquid chromatography–mass spectrometry. J Chromatogr A 1379:65–73
Fresco-Cala B, Cárdenas S, Valcárcel M (2016) Improved microextraction of selected triazines using polymer monoliths modified with carboxylated multi-walled carbon nanotubes. Microchim Acta 183:465–474
Namera A, Nakamoto A, Nishida M, Saito T, Kishiyama I, Miyazaki S, Yahata M, Yashiki M, Nagao M (2008) Extraction of amphetamines and methylenedioxyamphetamines from urine using a monolithic silica disk-packed spin column and high-performance liquid chromatography–diode array detection. J Chromatogr A 1208:71–75
Yamada H, Kitagawa S, Ohtani H (2013) Simultaneous separation of water- and fat-soluble vitamins in isocratic pressure-assisted capillary electrochromatography using a methacrylate-based monolithic column. J Sep Sci 36:1980–1985
Xu L, Shi Z-G, Feng Y-Q (2011) Porous monoliths: sorbents for miniaturized extraction in biological analysis. Anal Bioanal Chem 399:3345–3357
Namera A, Saito T (2015) Spin column extraction as a new sample preparation method in bioanalysis. Bioanalysis 7:2171–2176
Güzel Y, Rainer M, Messner CB, Hussain S, Meischl F, Sasse M, Tessadri R, Bonn GK (2015) Development of erbium phosphate doped poly (glycidyl methacrylate/ethylene dimethacrylate) spin columns for selective enrichment of phosphopeptides. J Sep Sci 38:1334–1343
Tong S, Zhou X, Zhou C, Li Y, Li W, Zhou W, Jia Q (2013) A strategy to decorate porous polymer monoliths with graphene oxide and graphene nanosheets. Analyst 138:1549–1557
Chambers SD, Svec F, Fréchet JM (2011) Incorporation of carbon nanotubes in porous polymer monolithic capillary columns to enhance the chromatographic separation of small molecules. J Chromatogr A 1218:2546–2552
Wang X, Li X, Li Z, Zhang Y, Bai Y, Liu H (2014) Online coupling of in-tube solid-phase microextraction with direct analysis in real time mass spectrometry for rapid determination of triazine herbicides in water using carbon-nanotubes-incorporated polymer monolith. Anal Chem 86:4739–4747
Fresco-Cala B, Cárdenas S, Valcárcel M (2016) Preparation and evaluation of micro and meso porous silica monoliths with embedded carbon nanoparticles for the extraction of non-polar compounds from waters. J Chromatogr A 1468:55–63
Iijima S, Yudasaka M, Yamada R, Bandow S, Suenaga K, Kokai F, Takahashi K (1999) Nano-aggregates of single-walled graphitic carbon nano-horns. Chem Phys Lett 309:165–170
Murata K, Kaneko K, Kokai F, Takahashi K, Yudasaka M, Iijima S (2000) Pore structure of single-wall carbon nanohorn aggregates. Chem Phys Lett 331:14–20
Pagona G, Sandanayaka AS, Maigne A, Fan J, Papavassiliou GC, Petsalakis ID, Steele BR, Yudasaka M, Iijima S, Tagmatarchis N (2007) Photoinduced electron transfer on aqueous carbon nanohorn–pyrene–tetrathiafulvalene architectures. Chem Eur J 13:7600–7607
Jiménez-Soto JM, Cárdenas S, Valcárcel M (2012) Dispersive micro solid-phase extraction of triazines from waters using oxidized single-walled carbon nanohorns as sorbent. J Chromatogr A 1245:17–23
Zhu S, Niu W, Li H, Han S, Xu G (2009) Single-walled carbon nanohorn as new solid-phase extraction adsorbent for determination of 4-nitrophenol in water sample. Talanta 79:1441–1445
Fresco-Cala B, Jimenez-Soto JM, Cardenas S, Valcarcel M (2014) Single-walled carbon nanohorns immobilized on a microporous hollow polypropylene fiber as a sorbent for the extraction of volatile organic compounds from water samples. Microchim Acta 181:1117–1124
Jiménez-Soto JM, Cárdenas S, Valcárcel M (2012) Evaluation of single-walled carbon nanohorns as sorbent in dispersive micro solid-phase extraction. Anal Chim Acta 714:76–81
Bernabé-Zafón V, Beneito-Cambra M, Simó-Alfonso E, Herrero-Martínez J (2010) Comparison on photo-initiators for the preparation of methacrylate monolithic columns for capillary electrochromatography. J Chromatogr A 1217:3231–3237
Eeltink S, Svec F (2007) Recent advances in the control of morphology and surface chemistry of porous polymer-based monolithic stationary phases and their application in CEC. Electrophoresis 28:137–147
Huang NJ, Sundberg DC (1995) Fundamental studies of grafting reactions in free radical copolymerization. IV. Grafting of styrene, acrylate, and methacrylate monomers onto vinyl-polybutadiene using benzoyl peroxide and AIBN initiators in solution polymerization. J Polym Sci A Polym Chem 33:2587–2603
Karousis N, Suarez-Martinez I, Ewels CP, Tagmatarchis N (2016) Structure, properties, functionalization, and applications of carbon Nanohorns. Chem Rev 116:4850–4883
Suárez B, Simonet B, Cárdenas S, Valcárcel M (2007) Determination of non-steroidal anti-inflammatory drugs in urine by combining an immobilized carboxylated carbon nanotubes minicolumn for solid-phase extraction with capillary electrophoresis-mass spectrometry. J Chromatogr A 1159:203–207
Locatelli M, Ferrone V, Cifelli R, Barbacane RC, Carlucci G (2014) Microextraction by packed sorbent and high performance liquid chromatography determination of seven non-steroidal anti-inflammatory drugs in human plasma and urine. J Chromatogr A 1367:1–8
Kantor TG (1986) Ketoprofen: a review of its pharmacologic and clinical properties. Pharmacotherapy 6:93–102
Luo Z-Y, Li Z-Y, Liu H-Y, Tang M-Q, Shi Z-G (2015) Click chemistry-based synthesis of water-dispersible hydrophobic magnetic nanoparticles for use in solid phase extraction of non-steroidal anti-inflammatory drugs. Microchim Acta 182:2585–2591
Pourghazi K, Amoli-Diva M (2014) Magnetic nanoparticles solid phase extraction based on the formation of supramolecular mixed hemimicelle aggregates for the determination of naproxen in biological fluids using high-performance liquid chromatography-UV. Micro Nano Lett 9:577–581
Khoeini Sharifabadi M, Saber-Tehrani M, Waqif Husain S, Mehdinia A, Aberoomand-Azar P (2014) Determination of residual nonsteroidal anti-inflammatory drugs in aqueous sample using magnetic nanoparticles modified with cetyltrimethylammonium bromide by high performance liquid chromatography. Sci World J 2014:8 Article ID 127835
Hu C, He M, Chen B, Hu B (2015) Simultaneous determination of polar and apolar compounds in environmental samples by a polyaniline/hydroxyl multi-walled carbon nanotubes composite-coated stir bar sorptive extraction coupled with high performance liquid chromatography. J Chromatogr A 1394:36–45
Madrakian T, Ahmadi M, Afkhami A, Soleimani M (2013) Selective solid-phase extraction of naproxen drug from human urine samples using molecularly imprinted polymer-coated magnetic multi-walled carbon nanotubes prior to its spectrofluorometric determination. Analyst 138:4542–4549
Acknowledgements
Financial support from the Spanish Ministry of Science and Innovation (CTQ2014-52939-R and CTQ2014-52765-R) and PROMETEO/2016/145 (Conselleria de Educación, Investigación, Cultura y Deporte, Generalitat Valenciana, Spain) is gratefully acknowledged. B. Fresco-Cala expresses her gratitude for the predoctoral grant (ref FPU13/03896) from the Spanish Ministry of Education.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 793 kb)
Rights and permissions
About this article
Cite this article
Fresco-Cala, B., Cárdenas, S. & Herrero-Martínez, J.M. Preparation of porous methacrylate monoliths with oxidized single-walled carbon nanohorns for the extraction of nonsteroidal anti-inflammatory drugs from urine samples. Microchim Acta 184, 1863–1871 (2017). https://doi.org/10.1007/s00604-017-2203-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2203-6