Abstract
The high performance solid-phase microextraction (SPME) of polar phenols from aqueous matrices is a challenge due to the strong interaction between water molecules and polar phenols. The authors describe the fabrication of stainless steel fibers coated with multiple helix 3D metal-organic nanotubes via physical adhesion, and their application to the SPME of 2-chlorophenol, 2-nitrophenol, 2,4-dichlorophenol, 4-chloro-3-methylphenol and 2,4,6-trichlorophenol from water samples. GC/MS was used for sample quantification and detection. Box-Behnken design was adopted to optimize the experimental conditions through response surface methodology. The fibers display good thermal stability (~400 °C), high enrichment factors (418–1874), excellent repeatability (4.31%–8.37%), wide linear range (0.5–1000 ng⋅L−1) and low limits of detection (0.07–0.18 ng⋅L−1) for the phenols. This method was successfully applied to the analysis of phenols in environmental water samples.

Multiple helix 3D metal-organic nanotubes coated stainless steel fibers were fabricated. The fibers achieved good thermal stability (~400 °C), high enrichment factors (418–1874), excellent repeatability (4.31–8.37%), wide linear range (0.5–1000 ng L−1) and low limits of detection (0.07–0.18 ng L−1) for phenols.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenolic pollutants are toxic chemicals that are widely distributed in the environment [1]. Through the food chain, phenols can be accumulated in the human body which is harmful to organisms due to their carcinogenicity and high toxicity [2]. Many analytical techniques, such as gas/liquid chromatography coupled with mass spectrometry (GC-MS or LC-MS) [3, 4], high performance liquid chromatography (HPLC) [5], and capillary electrophoresis (CE) [6], have been used to monitor the phenol levels of different samples. However, direct determination of phenols is usually difficult because of the relatively low concentrations of phenols and the complexity of the sample matrices. Proper sample pretreatment techniques are usually required prior to instrumental analysis.
Many sample pretreatment techniques, such as dispersive liquid-liquid microextraction (DLLME) [7], solid-phase extraction (SPE) [8], dispersive micro-solid-phase extraction (d-μ-SPE) [9] and solid-phase microextraction (SPME) [10, 11], have been applied to the analysis of phenols in environmental samples. As a sample preparation technique that combines simplicity, rapidness, high enrichment factors and environmental friendly characteristics, SPME has attracted considerable interest [12]. The fiber coating plays an important role in SPME. The primary limit of a SPME technique is the number of commercially available SPME coatings. As a consequence, efforts in SPME research have been devoted to the design and implementation of coating materials that are able to select pollutants and offer high robustness [13].
Several commercial coatings, including polydimethylsiloxane (PDMS) [14], polyacrylate (PA) [15], and PDMS/divinylbenzene(PDMS/DVB) [16], and adsorbent coatings, such as polyaniline (PANI) [17], SBA-15 [18], polypyrrole (PPy) [19] and ionic liquids [20], have been used for the SPME of polar phenols. However, the efficient SPME of polar phenols from water samples is still challenging due to the strong interaction between the polar phenols and the aqueous matrices [21]. Metal-organic nanotubes (MONTs) are a class of hybrid materials that combine organic ligands and metal ions or metal-containing clusters [22]. As a bridge between organic and inorganic nanotubes, MONTs have the advantages of both carbon nanotubes and metal-organic frameworks, including open nanoporous structure, large specific surface area, and exceptional thermal and chemical stability [23]. These diverse properties make MONTs promising adsorbent candidates. Zhao et al. have successfully used several MONTs for the SPME and SPE of polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in environmental samples [22, 23]. Nevertheless, MONTs for the SPME of polar pollutants have rarely been studied to date.
The main goal of this work is to investigate the feasibility of using cobalt (II)-based metal-organic nanotubes (Co-MONTs) as a polar coating material for the SPME of phenols. Typical phenols in the environment, such as 2-chlorophenol, 2-nitrophenol, 2,4-dichlorophenol, 4-chloro-3-methylphenol and 2,4,6-trichlorophenol, were selected as target analytes. Box-Behnken design (BBD) was used to optimize experimental conditions. Finally, the method was used to analyze real environmental water samples for phenols.
Experimental
Reagents and chemicals
All chemicals and reagents used in this study were of at least analytical grade. Acetonitrile, N.N-dimethylformamide, sodium chloride, sodium hydroxide, and hydrochloric acid were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China, www.sinopharmholding.com). Cobalt(II) acetate tetrahydrate,4,4′-biphenyldicarboxylic acid (H2bpdc), and 3,3′,5,5′-tetramethyl-4,4′-bipyrazole (H2bpz) were purchased from Aladdin Reagent Co. Ltd. (Shanghai, China, www.aladdin-e.com). Phenols standard (500 μg⋅mL−1 for each phenol) in methanol was purchased from AccuStandard, Inc. (New Haven, Connecticut, USA, www.accustandard.com), including 2-Chlorophenol (2-CP), 2-nitrophenol (2-NP), 2,4-dichlorophenol (2,4-DCP), 4-chloro-3-methylphenol (PCMC) and 2,4,6-trichlorophenol (2,4,6-TCP). A 5 mL GC microsyringe (Shanghai Gaoge Industrial and Trade Co. Ltd., Shanghai, China, www.sh-gaoge.com) was used to construct the SPME device. A commercial SPME manual holder with 30 μm PDMS, 65 μm PDMS/DVB, 85 μm PA, 50/30 μm DVB/CAR/PDMS (Supelco, Bellefonte, PA, USA, www.sigmaaldrich.com/analytical-chromatography.html) coatings was used for comparison.
Instrumentation
A Bruker gas chromatography system (436GC, Bruker, USA) coupled with a triple quadrupole mass spectrometer (SCION TQ, Bruker, USA) (GC-MS/MS) was used during the experiment in multiple reaction monitoring (MRM) mode. A DB-35 MS fused silica capillary column (30 m × 0.25 mm × 0.25 μm) (Agilent Technologies, USA) was used for GC separation. The oven temperature was programmed as follows: initially held at 100 °C for 1 min, raised at a rate of 5 °C⋅min−1 to 120 °C, held for 5 min, and finally increased at a rate of 10 °C⋅min−1 to 220 °C and held for 1 min. The total analysis time was 21 min. Helium (99.999%) was used as the carrier gas, and the flow rate was set to 1.0 mL⋅min−1. High-purity nitrogen gas was used as the collision gas (1.5 mL⋅min−1). The mass spectrometer was operated in electron impact mode (EI) at 70 eV. Both the interface temperature and the ion source temperature were set to 200 °C. Such parameters as MRM transition (major parent ion → daughter ion) and collision energy are presented in Table S1.
Scanning electron microscopy (SEM) images were recorded on a SUPPA™ 55 (Zeiss, Germany). X-ray diffraction (XRD) spectra were recorded on a D/max-r8 diffractometer (Rigaku, Japan) using Cu Kα radiation (λ = 1.5418 Å) over an angular range of 5° to 40°. TGA experiments were performed on a STA 449F3-QMS403C system (Netzsch, Germany) from room temperature to 600 °C with a ramp rate of 10 °C⋅min−1 under a nitrogen atmosphere.
Preparation of [Co2(bpdc)1.5(Hbpz)]·DMF·CH3CN·H2O
[Co2(bpdc)1.5(Hbpz)]·DMF·CH3CN·H2O was synthesized using a hydrothermal growth method following Hou et al. [24]. Co(CH3COO)2·4H2O (0.05 g), H2bpdc (0.072 g, 0.30 mmol), H2bpz (0.038 g) in DMF (5 mL) and CH3CN (5 mL) were placed in a Teflon-lined stainless steel vessel (25 mL), which was heated at 160 °C for 72 h and later cooled to room temperature at a rate of 5 °C⋅h−1. Prism shaped purple crystals were obtained, isolated by washing with DMF, and dried in vacuo.
Fabrication of the Co-MONT-coated fibers
One end of a stainless steel wire (approximately 2 cm) was etched with 37% HCl solution for 30 min. The corroded portion was washed with methanol and ultrapure water and later dried in air. The etched end was vertically immersed in silicone glue for 30 s and removed quickly thereafter. The excess glue was scraped with a knife leaving a homogeneous glue film on the surface of the etched wire. The coated portion of the wire was inserted into a centrifuge tube full of Co-MONT powder for 60 s and later removed. Finally, the steel wire coated with Co-MONTs was installed by hand into a 5 μL GC syringe to fabricate a home-made SPME device. The coated fiber was conditioned in the GC injector at 300 °C under nitrogen until a stable GC baseline was obtained.
SPME procedure
All SPME experiments were performed in direct SPME mode. A 10 mL portion of standard or sample solution was added to a 25 mL glass vial that was capped with a butyl rubber stopper and wrapped with polytetrafluoroethylene sealing tape. The needle of the home-made SPME device was inserted into the stopper and the fabricated Co-MONT fiber was completely pushed into the sample solution. The fiber was retracted into the needle, removed from the vial, and later analyzed using GC-MS/MS. The fiber was aged at 300 °C for 5 min in the GC inlet before each use. Before the SPME procedure, the pH of the sample solution was adjusted to 6. And the ionic strength of the sample (10%) was controlled by the addition of NaCl. SPME is performed by immersing the fiber directly into the sample for 40 min with stirring rate 400 rpm. Then the fiber was removed from the sample and immediately inserted into the GC injector. The desorption time was 3 min and the desorption temperature was 270 °C. Between two consecutive SPMEs, the fiber was conditioned at 270 °C for 5 min.
Sample collection
River water, waste water and spring water were collected for use as real samples. River water was collected from the Yellow River (Jinan, China), waste water came from a sewage treatment of paper mill (Tianqiao District, Jinan, China), and spring water was collected from Heihu Spring (Jinan, China). All of the water samples were stored in amber glass bottles at 4 °C and filtered with 0.45-μm micropore membranes (polyether sulfone, Ameritech) prior to analysis.
Results and discussion
Characterization of the Co-MONT-coated fibers
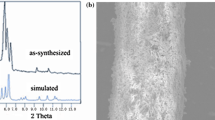
The Co-MONT-coated fibers have a homogenous coating on the surface of the stainless steel fiber which is presented in Fig. 1a. The wire shown in Fig. 1a is completely covered with the Co-MONTs. The diameter of the coated fiber is approximately 120 μm. A high-magnification SEM micrograph of the prepared [Co2(bpdc)1.5(Hbpz)]·DMF·CH3CN·H2O material is shown in Fig. 1b. The Co-MONTs exhibit an open nanoporous structure and a large specific surface area (593 m2⋅g−1). The nanotubes are square and have an opening of ca. 19.1 × 19.1 Å [24]. Fig. 1c illustrates that the XRD pattern of the synthesized material matches that of previous literature [24], which demonstrates the successful fabrication of [Co2(bpdc)1.5(Hbpz)]·DMF·CH3CN·H2O. TGA was used to evaluate the thermal stability of the epoxy resin, the Co-MONT and the Co-MONT-coated fibers (Fig. 1d). The decomposition temperature of the Co-MONT powder is approximately 300 °C. The adhesion of the epoxy resin increased the decomposition temperature of the Co-MONT coating to nearly 400 °C, which is beneficial for the thermal stability. To confirm that the Co-MONT-coated fibers have a higher extraction efficiency than the etched stainless steel or glue-coated stainless steel fibers, the fibers were used to enrich aqueous solutions of the five phenols (Fig. 2). Among the three fibers, only the Co-MONT-coated fiber showed clear enrichment efficiency. This observation shows that [Co2(bpdc)1.5(Hbpz)]·DMF·CH3CN·H2O may have an excellent adsorption capability.
Comparison of Co-MONT-coated fibers with commercial fibers
The Co-MONT-coated fibers were compared with four different commercial fibers: 30 μm PDMS, 65 μm PDMS/DVB, 85 μm PA, and 50/30 μm DVB/CAR/PDMS. The fibers were used to extract five phenols (1.0 μg⋅L−1) from spiked water samples under the same experimental conditions. All experiments were performed in triplicate (n = 3). In Fig. 3, the Co-MONT-coated fiber showed higher extraction efficiency than these commercial fibers. Compared with the best commercial coating (85 μm PA coating), the extraction efficiency of Co-MONT-coated fiber was improved by 28.84%, 12.94%, 20.70%, 24.91%, 61.84% for 2-CP, 2-NP, 2,4-DCP, PCMC and 2,4,6-TCP, respectively. Therefore, the Co-MONT-coated fibers possess excellent SPME performance and commercial value in the analysis for polar phenols.
Optimization of experimental parameters
A chemometric method was used to screen and optimize six factors important for increasing the extraction efficiency of any SPME method, including the extraction time (10 to 60 min), the pH (2 to 12), the agitation speed (100 to 900 rpm), the ionic strength (0 to 30%, m/v), the desorption temperature (260 to 300 °C) and the desorption time (1 to 5 min). Box-Behnken design (BBD) was used to identify the optimal levels for the experimental parameters through response surface methodology (RSM). The design matrix and factorial levels for optimization are presented in Table S2. The 3D response surfaces of the peak areas for the five phenols are illustrated in Fig. 4 and Fig. S1. A quadratic function perfectly describes the influences of these significant factors on the peak areas for the five phenols. The adjusted R-squared value for the degrees of freedom was 97.50%. This finding indicates that the model reliably estimated the optimal experimental parameters. An increase in the NaCl concentration would increase the ionic strength of the sample solution, causing salting-out and precompetitive effects [25]. An increase in ionic strength can decrease the solubility of numerous analytes and increase the distribution coefficient for aqueous solutions [26]. Phenols are polar species and can be ionized by changing the pH of an aqueous solution. They exist in an ionic form when the pH value of the solution is higher than their pKa value [27]. Therefore, choosing the proper pH can enhance the SPME selectivity and sensitivity. It is shown in the Fig. 4 and Fig. S1, the optimum experimental conditions were as follows: extraction time 40 min, ionic strength 10%, pH 6, agitation speed 400 rpm, desorption time 3 min, and desorption temperature 270 °C.
Possible mechanism for the extraction of phenols by Co-MONT-coated fibers
The enrichment factor (EF) was defined as the ratio of the target analyte sensitivity after extraction to the sensitivity observed by the direct injection of 1.0 μL of a standard solution using the chromatographic peak area for quantification [28]. The chemical structure, physical-chemical properties and EF values of the five phenols are listed in Table 1. From Table 1, It is obvious that phenols with larger log Kow values also had higher extraction efficiencies because a larger log Kow value means the phenol can be easily adsorbed onto the coating from the aqueous phase. Therefore, the hydrophobicity and the propensity for dissociation of the phenols affected the EF values of the five phenols. Secondly, the molecular structures of the phenols include hydrogen atoms that may form hydrogen bonds (X-H•••O) with the Co-MONTs because the Co-MONTs provide oxygen atoms. [29, 30] Additionally, the 2-NPs contain an intermolecular hydrogen bond. It can be observed in Table 1 that the adsorption efficiency for the 2-NP is sufficient enough indicating that the intramolecular hydrogen bond is stronger than the intermolecular hydrogen bond. Finally, the surface of the Co-MONTs has a unique porous network which is an advantage in the accommodation of the phenol molecules. Therefore, the Co-MONTs unique structural properties, ability to form hydrogen bonds and the natural hydrophobicity of the phenols contribute to the adsorption efficiency.
Analytical figures of merit
The analytical figures of merit of Co-MONT-coated fibers for phenol SPME under optimized conditions are summarized in Table 2. This method exhibits low limits of detection (LODs) (0.07–0.18 ng⋅L−1) (S/N = 3) and low limits of quantification (LOQs) (0.24–0.62 ng⋅L−1) (S/N = 10). The linearity of the phenols ranged from 0.5 to 1000 ng⋅L−1 and the correlation coefficient (r) ranged from 0.9946 to 0.9994. Table 3 showed that compared with other methods the method has various advantages such as high thermal stability and low LODs [10, 21, 31–34]. To determine the endurance and reusability of Co-MONT-coated fibers, a single Co-MONT-coated fiber device was used to complete 100 extractions of phenols from water samples (1.0 μg⋅L−1). The results show that the extraction efficiency of the coating decreased by 5.9%–18.3% after 100 extraction cycles. The relative standard deviations (RSDs) were in the range of 4.31% to 8.37% for the five phenols (1 μg⋅L−1, n = 5), using one Co-MONT-coated fiber. For three parallel Co-MONT-coated fibers, the fiber-to-fiber reproducibility (RSDs) ranged from 2.69% to 7.63%. These experimental results show that Co-MONT is suitable as a polar coating for the SPME of phenols.
Analysis of real environmental water samples
This method was applied to the analysis of environmental water samples for the presence of five phenols and the results are listed in Table 4. 2-NP, 2,4-DCP, and 2,4,6-TCP were detected in the waste water sample, and their concentrations were 68.2, 85.8 and 201.7 ng⋅L−1, respectively. Additionally, the spiked recoveries (10, 50 and 100 ng⋅L−1) of the three water samples were 72.5%–103.5%. Typical chromatograms of real water samples are shown in Fig. 5. The analytical method presented in this work can be applied to analyze environmental water samples for the presence of phenols.
Conclusions
In this work, we investigated the feasibility of using Co-MONT-coated fibers for the SPME of phenols from environmental water samples. The fibers showed excellent extraction performance for phenols. These findings demonstrate that Co-MONTs may be used as a polar coating material for SPME to monitor for other polar organic pollutants with benzene ring in environmental water samples. However, whether this coating has high extraction efficiency towards polar organic pollutants without benzene ring needs further more study.
References
Wang HL, Yan H, Wang CJ, Chen F, Ma MP, Wang WW (2012) Analysis of phenolic pollutants in human samples by high performance capillary electrophoresis based on pretreatment of ultrasound-assisted emulsification microextraction and solidification of floating organic droplet. J Chromatogr A 1253:16–21
Zhong C, He M, Liao HP, Chen BB, Wang C, Hu B (2016) Polydimethylsiloxane/covalent triazine frameworks coated stir bar sorptive extraction coupled with high performance liquid chromatography-ultraviolet detection for determination of phenols in environmental water samples. J Chromatogr A 1441:8–15
Fariña L, Boido E, Carrau F, Dellacassa E (2007) Determination of volatile phenols in red wines by dispersive liquid–liquid microextraction and gas chromatography–mass spectrometry detection. J Chromatogr A 1157:46–50
Seeram NP, Lee R, Scheuller HS, Heber D (2006) Identification of phenolic compounds in strawberries by liquid chromatography electrospray ionization mass spectroscopy. Food Chem 97:1–11
Shui GH, Leong LP (2002) Separation and determination of organic acids and phenolic compounds in fruit juices and drinks by high-performance liquid chromatography. J Chromatogr A 977:89–96
Puig D, Barceló D (1996) Determination of phenolic compounds in water and waste water. TrAC Trends Anal Chem 15:362–375
He H, Liu SH, Meng ZF, Hu SB (2014) Dispersive liquid–liquid microextraction for the determination of phenols by acetonitrile stacking coupled with sweeping-micellar electrokinetic chromatography with large-volume injection. J Chromatogr A 1361:291–298
Bagheri H, Mohammadi A, Salemi A (2004) On-line trace enrichment of phenolic compounds from water using a pyrrole-based polymer as the solid-phase extraction sorbent coupled with high-performance liquid chromatography. Anal Chim Acta 513:445–449
Zhao YG, Chen XH, Pan SD, Zhu H, Shen HY, Jin MC (2013) Simultaneous analysis of eight phenolic environmental estrogens in blood using dispersive micro-solid-phase extraction combined with ultra fast liquid chromatography–tandem mass spectrometry. Talanta 115:787–797
Gong SX, Wang X, Chen Y, Cheng CG, Wang ML, Zhao RS (2015) Carboxylated solid carbon spheres as a novel solid-phase microextraction coating for sensitive determination of phenols in environmental water samples. J Chromatogr A 1401:17–23
Mir MA, Sheyda P, Vahid Y (2016) A nanoporous anodized alumina wire with a nanosized hydroxyapatite coating for headspace solid-phase microextraction of phenol and chlorophenols. Microchim Acta 183:241–247
Arthur CL, Pawliszyn J (1990) Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal Chem 62:2145–2148
Xu J, Zheng J, Tian J, Zhu F, Zeng F, Su C, Ouyang GF (2013) New materials in solid phase microextraction. Trends Anal Chem 47:68–83
Huang SD, Cheng CP, Sung YH (1997) Determination of benzene derivatives in water by solid-phase microextraction. Anal Chim Acta 343:101–108
Buchholz KD, Pawliszyn J (1994) Optimization of solid-phase microextraction conditions for determination of phenols. Anal Chem 66:160–167
Campillo N, Penalver R, Hernández-Córdoba M (2006) Evaluation of solid-phase microextraction conditions for the determination of chlorophenols in honey samples using gas chromatography. J Chromatogr A 1125:31–37
Du W, Zhao FQ, Zeng BZ (2009) Novel multiwalled carbon nanotubes–polyaniline composite film coated platinum wire for headspace solid-phase microextraction and gas chromatographic determination of phenolic compounds. J Chromatogr A 1216:3751–3757
Zhu F, Liang YJ, Xia LY, Rong MZ, Su CY, Lai R, Li RY, Ouyang GF (2012) Preparation and characterization of vinyl-functionalized mesoporous organosilica-coated solid-phase microextraction fiber. J Chromatogr A 1247:42–48
Alizadeh N, Zarabadipour H, Mohammadi A (2007) Headspace solid-phase microextraction using an electrochemically deposited dodecylsulfate-doped polypyrrole film to determine of phenolic compounds in water. Anal Chim Acta 605:159–165
Meng YJ, Pinob V, Anderson JL (2011) Role of counteranions in polymeric ionic liquid-based solid-phase microextraction coatings for the selective extraction of polar compounds. Anal Chim Acta 687:141–149
Shang HB, Yang CX, Yan XP (2014) Metal–organic framework UiO-66 coated stainless steel fiber for solid-phase microextraction of phenols in water samples. J Chromatogr A 1357:165–171
Li QL, Wang X, Liu YL, Chen XF, Wang ML, Zhao RS (2014) Feasibility of metal–organic nanotubes [Cu3(μ3-O)(μ-OH)(triazolate)2]+-coated fibers for solid-phase microextraction of polychlorinated biphenyls in water samples. J Chromatogr A 1374:58–65
She XK, Wang X, Zhou JB, Zhao RS (2015) Porous lead (II)-based metal organic nanotubes as an adsorbent for dispersive solid-phase extraction of polybrominated diphenyl ethers from environmental water samples. J Chromatogr A 1423:31–38
Hou L, Jia LN, Shi WJ, Du LY, Li J, Wang YY, Shi QZ (2013) A 3D porous metal-organic framework containing nanotubes based on multiple helices. Dalton Trans 42:6306–6309
Ouyang GF, Vuckovic D, Pawliszyn J (2011) Nondestructive sampling of living systems using in vivo solid-phase microextraction. Chem Rev 111:2784–2814
Liu Y, Lee ML, Hageman KJ, Yang Y, Hawthorne SB (1997) Solid-phase microextraction of PAHs from aqueous samples using fibers coated with HPLC chemically bonded silica stationary phases. Anal Chem 69:5001–5005
Li SH, Wu DP, Yan XH, Guan YF (2015) Acetone-activated polyimide electrospun nanofiber membrane for thin-film microextraction and thermal desorption-gas chromatography–mass spectrometric analysis of phenols in environmental water. J Chromatogr A 1411:1–8
Wu YY, Yang CX, Yan XP (2014) Fabrication of metal–organic framework MIL-88B films on stainless steel fibers for solid-phase microextraction of polychlorinated biphenyls. J Chromatogr A 1334:1–8
Gautam RD (2005) C–H…O and other weak hydrogen bonds. From crystal engineering to virtual screening. Chem Commun 24:2995–3001
Zhang N, Ruan NH, Song YC, Liu Z, He GH (2016) Molecular dynamics simulation of the hydration structure and hydrogen bonding behavior of phenol in aqueous solution. J Mol Liq 221:942–948
Liu HM, Li JB, Jiang SX (2009) A novel multiwalled carbon nanotubes bonded fused-silica fiber for solid phase microextraction–gas chromatographic analysis of phenols in water samples. Talanta 78:929–935
Liu XY, Ji YS, Zhang YH, Zhang HX, Liu MC (2007) Oxidized multiwalled carbon nanotubes as a novel solid-phase microextraction fiber for determination of phenols in aqueous samples. J Chromatogr A 1165:10–17
Bagheri H, Mir A, Babanezhad E (2005) An electropolymerized aniline-based fiber coating for solid phase microextraction of phenols from water. Anal Chim Acta 532:89–95
López-Darias J, Pino V, Anderson JL, Graham CM, Afonso AM (2010) Determination of water pollutants by direct-immersion solid-phase microextraction using polymeric ionic liquid coatings. J Chromatogr A 1217:1236–1243
Acknowledgements
This study was supported by the National Natural Science Foundation of China (21477068 and 21407099), the Natural Science Foundation of Shandong Province (ZR2015YL003), and the Key Research and Development Program of Shandong Province (2015GSF117011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests. The experiments are compliance with ethical standards.
Electronic supplementary material
ESM 1
(DOC 337 kb)
Rights and permissions
About this article
Cite this article
Li, QL., Huang, F., Wang, XL. et al. Multiple-helix cobalt(II)-based metal-organic nanotubes on stainless steel fibers for solid-phase microextraction of chlorophenol and nitrophenols from water samples. Microchim Acta 184, 1817–1825 (2017). https://doi.org/10.1007/s00604-017-2167-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2167-6