Abstract
A nanocomposite consisting of polyaniline and multiwalled carbon nanotubes was tethered with a thiolated thrombin-specific aptamer and placed on a glassy carbon electrode (GCE) to obtain a biosensor for thrombin that has a limit of detection of 80 fM. Tethering was accomplished via a thiol-ene reaction between thiolated thrombin aptamer (TTA) and oxidized polyaniline (PANI) that was chemically synthesized in the presence of solution-dispersed multiwalled carbon nanotubes (MWCNTs). The modified GCE exhibits a pair of well-defined redox peaks (at 50/−25 mV) of self-doped PANI in neutral solution, and the tethered TTA-thrombin interaction gives a decreased electrochemical signal. Cyclic voltammetry, scanning electron microscopy and ultraviolet visible spectroscopy were used to characterize the film properties. This amperometric aptasensor is sensitive, selective and reproducible. It was applied to the determination of thrombin in spiked human serum (0.2 to 4 nM) and gave recoveries that ranged from 95 to 102%.

A nanocomposite consisting of polyaniline (PANI) and multiwalled carbon nanotubes (MWCNTs) was tethered with a thiolated thrombin aptamer (TTA) and placed on a glassy carbon electrode (GCE) to obtain a biosensor for thrombin that has a 80 f. detection limit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Detecting thrombin using aptasensors in a rapid, sensitive and cost-effective manner is a crucial issue in fundamental research and clinical applications [1]. Because of their fast response, high sensitivity, portability, inexpensiveness and simple instrumentation, the electrochemical methods have attracted substantial attention in the development of aptasensors [2–4]. In general, the electrochemical aptasensors were fabricated by employing label-free impedance strategy [5] or redox-labeled aptamer [2, 6]. However, it is not easy to obtain the redox-labeled aptamer, because the existing methods of covalent labeling of the aptamer require complicated and labor-intensive labeling procedures. Therefore, it is still an active and important research topic for developing sensitive, convenient, and cost-effective labeling protocols for aptasensing. Among various aptasensors that utilize differernt signal transduction techniques such as atomic force microscopy [7], optics [8, 9] and electrochemistry [10], electrochemical aptasensors have exhibited great promise in protein detection with high sensitivity, selectivity, and cost-effectiveness.
The conducting polymers (CPs), typically including polyaniline (PANI), polypyrrole (PPy) and polythiophene (PTH), can provide a suitable interface for immobilization of oligonucleotides probes and improve the analytical performance, various CP-modified electrodes has been widely applied for detection of oligonucleotides hybridization and damage [11, 12]. Interestingly, the CPs can be functionalized with thiol via thiol-ene reaction [13–18]. From the above description, we believe that the thiol-ene chemistry between CPs nanocomposite and thiolated aptamers should act as an aptamer-immobilization protocols for electrochemical aptasensing, however, to the best of our knowledge, such attempts have not been reported to date.
In addition, CPs nanocomposite such as poly(aminobenzenesulfonic acid)-modified single-walled carbon nanotubes (SWCNTs) [19] and PANI/gold nanoparticle multilayer film [20] exhibit a pair of well-defined redox peaks of self-doped PANI in neutral solution. In contrast, very few work have been reported for the incorporation of electroactive CPs nanocomposite into electrochemical aptasensors in neutral solution.
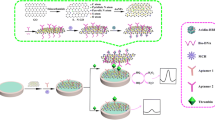
In this work, TTA-tethered PANI-MWCNTs are prepared for amperometric detection of thrombin. The thus-prepared PANI-MWCNTs exhibits a pair of well-defined redox peaks of self-doped PANI in neutral solution, and the tethered TTA-thrombin interaction can sensitively decrease the electrochemical signal. Herein, TTA-PANI-MWCNTs are used as electrode surface modifier providing thrombin recognition, and thiol-ene chemistry for the aptamer immobilization acts as an interesting method for the fabrication of amperometric thrombin biosensor. The prepared amperometric aptasensor was found to exhibit good analytical properties (sensitivity, low detection limit, and good selectivity and reproducibility) for thrombin detection.
Experimental
Chemicals and apparatus
Aniline was purchased from Shantou Xilong Chemical Engineering Factory (Guangdong, China, www.cn245785.21goods.net), which was purified via double distillation and stored in a refrigerator prior to use. Ammonium peroxydisulfate was purchased from Shanfu Chemical Engineering Inc., Ltd. (Huangshan, China, www.p-wholesale.com). Human α-thrombin and BSA were purchased from Sigma (USA, www.sigmaaldrich.com). Human lgG, glucose oxidase, lysozyme, fibrinogen, rabbit lgG and 39-Base aptamer (5′-SH-(CH2)6- T10AGTCCGTGGTAGGGCAGGTTGGGGTGACT-3′) were purchased from Sangon Co., Ltd. (Shanghai, China, www.sangon.com). Human blood sera were obtained from the Hospital of Hunan Agricultural University. MWCNTs, with an average diameter of ~20–40 nm were purchased from Shenzhen Nanotech Port Co., Ltd. (Shenzhen, China, www.nanotubes.com.cn). The MWCNTs were functionalized with -COOH groups by sonication in a 3:1 H2SO4/HNO3 mixture for 8 h [21]. Subsequently, the pretreated MWCNTs were washed with copious water to reach neutrality of pH 7.0, filtered, and dried. 10 mM pH 7.0 phosphate buffer (PB, 10 mM NaH2PO4–Na2HPO4) containing 100 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 1 mM CaCl2 was used for binding and rinse, and 0.10 M pH 7.4 PB (0.10 M KH2PO4–K2HPO4 + 0.10 M K2SO4) with a higher buffer capacity was used for pH-constant electrochemical experiments. All other chemicals were of analytical grade or better quality. Milli–Q ultrapure water (Millipore, >18 MΩ cm) was used throughout.
All electrochemical experiments were conducted on a CHI660E electrochemical workstation (CH Instrument Co., USA). A conventional three-electrode system consisted of a glassy carbon disk working electrode (GCE) of 3 mm diameter, a KCl-saturated calomel electrode (SCE) as the reference electrode and a platinum plate as the counter electrode. All potentials in this work are referenced to SCE. Scanning electron microscopy (SEM) pictures were collected on a JEM-6700F field emission scanning electron microscope. The PH-3B pH meter (Dazhong, Shanghai, China) was used for pH measurements. A Schimadzu UV-2450 ultraviolet-visible (UV-Vis) spectrophotometer was used to record UV-Vis spectra.
Procedures
To remove possible surface contamination, the GCE was polished with 1.0 and 0.05 μm alumina slurry sequentially and then washed ultrasonically in ethanol and water for 10 min, respectively. Then, the GCE was scanned between −0.2 and 1.0 V versus SCE in 0.2 mol L−1 HClO4 at 50 mV s−1 for a sufficient number of cycles to obtain reproducible cyclic voltammograms.
The preparation of the modified electrode is depicted in Scheme 1. The detailed preparation of the modified electrode, detection method and preparation of human sera samples are given in the Supplementary Material.
Results and discussion
Characterization of the amperometric aptasensor
CV tests of PANI-MWCNTs/GCE are shown in Fig. 1a. When potential cycling of the PANI-MWCNTs/GCE electrode at scan rates of 30, 50, 100, 200, 300, 400 and 500 mV s−1 in 0.1 M PB (pH 7.4), the modified GCE exhibits a pair of well-defined redox peaks (at −25 and 50 mV, scan rates: 50 mV s−1) of self-doped PANI in neutral solution [19, 20]. And we found that peak currents increase linearly with the scan rate, indicating a facile electrochemical and a surface-controlled [19, 20] redox process of self-doped PANI. These may imply that the PANI-MWCNTs film is highly conducting for a fast charge transfer across the film, because of excellent electronic conductivity of MWCNTs and self-doped PANI.
(a) CV responses of PANI-MWCNTs/GCE at scan rates of 30, 50, 100, 200, 300, 400 and 500 mV s−1 in 0.1 M PB (pH 7.4). Inset: anodic and cathodic peak currents as functions of scan rate. CV (b, scan rates: 50 mV s−1) and DPV responses (c) of PANI-MWCNTs/GCE (Dotted line), TTA-PANI/MWCNTs/GCE (Polyline), BSA-TTA-PANI-MWCNTs/GCE (Solid line) and thrombin-BSA-TTA-PANI-MWCNTs/GCE (Broken line, after addition of 2 nM thrombin onto BSA-TTA-PANI-MWCNTs/GCE and incubation for 2 h) in 0.1 M PB (pH 7.4)
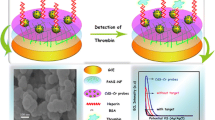
The morphologies of MWCNTs, PANI/MWCNTs and PANIe/MWCNTs were examined, as shown in Fig. S1. After the polymerization or electro-deposition on MWCNTs, the PANI layer of ca. 10 nm thickness was clearly seen on the MWCNTs, and its outer diameter was increased to ca. 50 nm. The nanocomposite was thus identified as a coaxial core-shell-shaped agglomerate, mainly as a result of the hydrophobic interaction between the PANI and MWCNTs.
Fig. S2 shows the UV–Vis spectra of PANI/MWCNTs and TTA-PANI/MWCNTs, collected successively in 10 mM PB (pH 7.0) aqueous solution. The absorption peak at 347 nm is assigned to the π → π* transition of benzenoid rings in PANI [22]. The absorption peak at 721 nm indicates the presence of bipolaronic species in PANI [23], which tends to disappear after the reaction with TTA. It is known that 100% leucoemeraldine exhibits only a π → π* transition at 347 nm [24]. The notable absorbance decrease at 721 nm should indicate that the thiol’s nucleophiles attack the more active diiminoquinoid rings and convert them into the more stable diaminobenzenoid rings [25]. A conversion of the less stable quinoid ring to the more stable benzenoid. Thus, the UV-Vis spectra should indicate the occurrence of the TTA–PANI interaction by ring–nucleophilic substitution of PANI with TTA.
Fig. 1b shows CV responses at PANI-MWCNTs/GCE, TTA-PANI-MWCNTs/GCE, BSA-TTA-PANI-MWCNTs/GCE and thrombin-BSA-TTA-PANI-MWCNTs/GCE in 0.1 M PB (pH 7.4). As is seen, the PANI-MWCNTs/GCE shows a pair of well-defined redox peaks in neutral solution. After the interaction between TTA and PANI-MWCNTs/GCE, the peak currents of PANI-MWCNTs/GCE decrease, indicating the occurrence of the TTA-PANI-MWCNTs interaction by thiol-ene chemistry of PANI and TTA. The treatment with BSA on the TTA-PANI-MWCNTs/GCE electrode leads to subdued peak currents. Then the TTA-PANI-MWCNTs/GCE electrode was incubated in 2 nM thrombin for 2 h, there was a large decrease in peak currents, the peak decrease of the TTA-PANI-MWCNTs/GCE electrode may originate from the bulky thrombin molecules blocking the electrode surface. The above findings also have been supported by DPV experiments, as shown in Fig. 1c.
Electrochemical probing of specific aptamer-thrombin interactions
The construction and assay principle of the amperometric aptasensor is schematically depicted in Scheme 1. The desired amount of PANI-MWCNTs nanocomposite were cast-coated on GCE, exhibiting a pair of well-defined redox peaks (at 50/−25 mV) of self-doped PANI in neutral solution, then the PANI-MWCNTs/GCE was activated for 60 s under 0.3 V in 0.1 M PBS (pH 7.4). TTA was tethered onto the activated PANI-MWCNTs/GCE via the thiol-ene chemistry to yield the TTA-PANI-MWCNTs/GCE. After BSA blocking, the tethered TTA-thrombin interaction gives a decreased electrochemical signal. Herein, TTA-tethered PANI-MWCNTs was used as electrode surface modifier providing thrombin recognition. And thiol-ene chemistry for the aptamer immobilization acts as a new method for the fabrication of amperometric thrombin biosensor.
Analytical properties of the amperometric aptasensor for thrombin detection
Fig. 2 shows the calibration curves corresponding to the DPV detection of thrombin based on changes of the DPV peak currents at thrombin-TTA-PANI-MWCNTs/GCE. The ΔI (ΔI = I 0-I s, where I 0 and I s are the DPV peak currents before and after thrombin addition) values of all electrodes increase with the increase of thrombin concentration. The amperometric aptasensor has a wide detection range from 0.1 pM to 4 nM, thrombin can be detected down to a concentration of 0.08 pM. In addition, we also used the electropolymerized PANI (PANIe) to prepare a TTA-PANIe/MWCNTs/GCE comparison, which are better than or comparable with those reported previously in most thrombin electrochemical aptasensor literatures, as listed in Table 1. This demonstrates that we have successfully developed a sensitive amperometric aptasensor for thrombin detection. And herein the thiol-ene chemistry serves as a convenient and effective aptamer-immobilization protocols for thrombin biosensing.
DPV curves (a) and the calibration curve (b) of the aptasensor for thrombin at various concentrations in 0.1 M PB (pH 7.4). Inset: an enlarged plot for the calibration curve. I 0 is the current with no added thrombin, and I s is the current with added thrombin. The optimal conditions were as follows: amplitude of 0.05 V; pulse width of 0.05 s; sampling width of 0.0167 s; pulse period of 0.2 s
In order to test the specificity of the aptasensor, the effect of other possible interferences were investigated. As demonstrated in Fig. 3, upon addition of each non-target molecules, such as glucose oxidase, human IgG, fabrinogen, lysozyme, and rabbit IgG, each of their DPV intensity near that of blank and addition of each.
The responses of our aptasensor to (a) 20.0 nM of glucose oxidase; (b) 20.0 nM of human lgG; (c) 20.0 nM of fibrinogen; (d) 20.0 nM of lysozyme; (e) 20.0 nM of rabbit lgG; (f):2.0 nM of thrombin; (g) mixture of 2.0 nM of thrombin and 20.0 nM of glucose oxidase; (h) mixture of 2.0 nM of thrombin and 20.0 nM of human lgG; (i) mixture of 2.0 nM of thrombin and 20.0 nM of fibrinogen; (j) mixture of 2.0 nM of thrombin and 20.0 nM of lysozyme; (k) mixture of 2.0 nM of thrombin and 20.0 nM of rabbit lgG. Every point was the mean of three measurements. Errorbar was the standard deviation interference had no effect on the intensity of thrombin. This observation indicates that the nonspecific adsorption of these foreign proteins is very minor and the aptasensor possesses high selectivity
The reproducibility of the amperometric aptasensor was investigated at the thrombin concentration of 2 nM, and the relative standard deviation for five times is 4.3%. In addition, five freshly prepared modified electrodes were used for the detection of 2 nM thrombin. All the five electrodes exhibit similar amperometric response behavior, and the relative standard deviation is 3.5%. These results demonstrate that the aptasensor for thrombin detection is highly reproducible.
The analytical application of the amperometric aptasensor was evaluated by the standard addition method. Samples were prepared by adding thrombin of different concentrations to human serum (from Hunan Agricultural University Hospital). Since no thrombin was found from the human sera [34], addition and recovery experiment was performed to estimate the application of the assay in complex sample [10, 34]. The analytical results for thrombin are listed in Table 2. The recovery ranges from 95% to 102%, and the relative standard deviation values were in the ranges of 6.7–8.2%. The acceptable recovery and RSD imply that the amperometric aptasensor has application potential in complex biological samples.
Conclusions
In summary, an amperometric thrombin aptasensor has been developed. The preparation of TTA-PANI-MWCNTs/GCE is simple, immobilization of thiolated aptamer onto PANI-MWCNTs/GCE via thiol-ene chemistry, and blocking with BSA. Importantly, the PANI-MWCNTs/GCE electrode exhibits a pair of well-defined redox peaks of self-doped PANI in neutral solution, and the tethered TTA-thrombin interaction can sensitively decrease the electrochemical signal. Under the optimum conditions, the peak current is linear with the thrombin concentration from 0.0001 to 4 nM, with a limit of detection of 0.08 pM. We also used the electropolymerized PANI (PANIe) to prepare a TTA-PANIe/MWCNTs/GCE for thrombin detection, the peak current is linear with the thrombin concentration from 0.001 to 10 nM, with a limit of detection of 0.5 pM. The method is simple and exhibits excellent selectivity for thrombin detection in the presence of other proteins. We successfully detected thrombin with the aptasensor in spiked human serum samples with acceptable recovery. The amperometric aptasensor based on thiolated aptamer, polymeric nanocomposite and thiol-ene chemistry may find wide application in the routine detection of proteins with high sensitivity and low detection limit.
References
Crivianu-Gaita V, Thompson M (2016) Aptamers, antibody scFv, and antibody Fab' fragments: an overview and comparison of three of the most versatile biosensor biorecognition elements. Biosens Bioelectron 85:32–45
Du Y, Li B, Wei H, Wang Y, Wang E (2008) Multifunctional label-free electrochemical biosensor based on an integrated aptamer. Anal Chem 80:5110–5117
Hansen JA, Wang J, Kawde AN, Xiang Y, Gothelf KV, Collins G (2006) Quantum-dot/aptamer-based ultrasensitive multi-Analyte electrochemical biosensor. J Am Chem Soc 128:2228–2229
Fu Y, Zou C, Bu L, Xie Q, Yao S (2013) Novel amperometric aptasensor based on Analyte-induced suppression of enzyme catalysis in polymeric Bionanocomposites. ACS Appl Mater Interfaces 5:934–939
Radi AE, Acero Sanchez JL, Baldrich E, O'Sullivan CK (2005) Reusable Impedimetric aptasensor. Anal Chem 77:6320–6323
Baker BR, Lai RY, Wood MS, Doctor EH, Heeger AJ, Plaxco KW (2006) An electronic, aptamer-based small-molecule sensor for the rapid, label-free detection of cocaine in adulterated samples and biological fluids. J Am Chem Soc 128:3138–3139
Basnar B, Elnathan R, Willner I (2006) Following aptamer−thrombin binding by force measurements. Anal Chem 78:3638–3642
Ma M, Zheng X (2015) Preparation of brightly fluorescent silica nanoparticles modified with lucigenin and chitosan, and their application to an aptamer-based sandwich assay for thrombin. Microchim Acta 182:2193–2199
Yue Q, Shen T, Wang L, Xu S, Li H, Xue Q, Zhang Y, Gu X, Zhang S, Liu J (2014) A convenient sandwich assay of thrombin in biological media using nanoparticle-enhanced fluorescence polarization. Biosens Bioelectron 56:231–236
Li J, Wang J, Guo X, Zheng Q, Peng J, Tang H, Yao S (2015) Carbon nanotubes labeled with aptamer and horseradish peroxidase as a probe for highly sensitive protein biosensing by postelectropolymerization of insoluble precipitates on electrodes. Anal Chem 87:7610–7617
Zhou Y, Yu B, Guiseppi EA, Sergeyev V, Levon K (2009) Potentiometric monitoring DNA hybridization. Biosens Bioelectron 24:3275–3280
Thompson LA, Kowalik J, Josowicz M, Janata J (2002) Label-free DNA hybridization probe based on a conducting polymer. J Am Chem Soc 125:324–325
Chen L, Li Z, Meng Y, Zhang P, Su Z, Liu Y, Huang Y, Zhou Y, Xie Q, Yao S (2014) Sensitive square wave anodic stripping voltammetric determination of Cd2+ and Pb2+ ions at Bi/Nafion/overoxidized 2-mercaptoethanesulfonate-tethered polypyrrole/glassy carbon electrode. Sensor Actua B 191:94–101
Su Z, Liu Y, Zhang Y, Xie Q, Chen L, Huang Y, Fu Y, Meng Y, Li X, Ma M (2013) Thiol-ene chemistry guided preparation of thiolated polymeric nanocomposite for anodic stripping voltammetric analysis of Cd2+ and Pb2+. Analyst 138: 1180–1186
Liu Y, Su Z, Zhang Y, Chen L, Gu T, Huang S, Liu Y, Sun L, Xie Q, Yao S (2013) Amperometric determination of ascorbic acid using multiwalled carbon nanotube-thiolated polyaniline composite modified glassy carbon electrode. J Electroanal Chem 709:19–25
Su Z, Liu Y, Xie Q, Chen L, Zhang Y, Meng Y, Li Y, Fu Y, Ma M, Yao S (2012) Preparation of thiolated polymeric nanocomposite for sensitive electroanalysis of dopamine. Biosens Bioelectron 36:154–160
Chen L, Su Z, He X, Liu Y, Qin C, Zhou Y (2012) Square wave anodic stripping voltammetric determination of Cd and Pb ions at a Bi/Nafion/thiolated polyaniline/glassy carbon electrode. Electrochem Commun 15:34–37
Hoyle CE, Lowe AB, Bowman CN (2010) Thiol-click chemistry: a multifaceted toolbox for small molecule and polymer synthesis. Chem Soc Rev 39:1355–1387
Liu J, Tian S, Knoll W (2005) Properties of polyaniline/carbon nanotube multilayer films in neutral solution and their application for stable low-potential detection of reduced β-nicotinamide adenine dinucleotide. Langmuir 21:5596–5599
Tian S, Liu J, Zhu T, Knoll W (2004) Polyaniline/gold nanoparticle multilayer films: assembly, properties, and biological applications. Chem Mater 16:4103–4108
Wang Z, Liu J, Liang Q, Wang Y, Luo G (2002) Carbon nanotube-modified electrodes for the simultaneous determination of dopamine and ascorbic acid. Analyst 127:653–658
Shreepathi S, Holze R (2005) Spectroelectrochemical investigations of soluble polyaniline synthesized via new inverse emulsion pathway. Chem Mater 17:4078–4085
Elwahed A, Holze R (2002) Ion size and size memory effects with electropolymerized polyaniline. Synth Met 131:61–70
Masters JG, Ginder JM, MacDiarmid AG, Epstein AJ (1992) Thermochromism in the insulating forms of polyaniline: role of ring-torsional conformation. J Chem Phys 96:4768–4778
Zhou Y, Yu B, Levon K (2004) The role of cysteine residues in electrochemistry of cytochrome c at a polyaniline modified electrode. Synth Met 142:137–141
Lin Z, Pan D, Hu T, Liu Z, Su X (2015) A near-infrared fluorescent bioassay for thrombin using aptamer-modified CuInS2 quantum dots. Microchim Acta 182:1933–1939
Wang GL, Hu XL, Wu XM, Dong YM, Li ZJ (2016) Fluorescent aptamer-based assay for thrombin with large signal amplification using peroxidase mimetics. Microchim Acta 183:765–771
Xu Z, Huang X, Dong C, Ren J (2014) Fluorescence correlation spectroscopy of gold nanoparticles, and its application to an aptamer-based homogeneous thrombin assay. Microchim Acta 181:723–730
Zhang H, Shuang S, Sun L, Chen A, Qin Y (2014) Label-free aptasensor for thrombin using a glassy carbon electrode modified with a graphene-porphyrin composite. Microchim Acta 181:189–196
Shangguan L, Zhu W, Xue Y, Liu S (2015) Construction of photoelectrochemical thrombin aptasensor via assembling multilayer of graphene-CdS nanocomposites. Biosens Bioelectron 64:611–617
Zhang L, Li L (2016) Colorimetric thrombin assay using aptamer-functionalized gold nanoparticles acting as a peroxidase mimetic. Microchim Acta 183:485–490
Sinha B, Ramulu TS, Kim KW, Venu R, Lee JJ, Kim CG (2014) Planar hall magnetoresistive aptasensor for thrombin detection. Biosens Bioelectron 59:140–144
Cunningham JC, Brenes NJ, Crooks RM (2014) Paper electrochemical device for detection of DNA and thrombin by target-induced conformational switching. Anal Chem 86:6166–6170
Li Y, Ling L (2015) Aptamer-based fluorescent solid-phase thrombin assay using a silver-coated glass substrate and signal amplification by glucose oxidase. Microchim Acta 182:1849–1854
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21305039, 21475041, 21175042 and 21075036), the Foundation of Hunan Province (14JJ3097), the Program for Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province, the Foundation of Hunan Provincial Education Department for Young Scholar, and the Foundation of Hunan Agricultural University (12YJ05). Thank Dr. Rui Tan (Postdoctoral associate of Department of Chemistry, Brown University) for checking the language.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 781 kb)
Rights and permissions
About this article
Cite this article
Su, Z., Xu, X., Xu, H. et al. Amperometric thrombin aptasensor using a glassy carbon electrode modified with polyaniline and multiwalled carbon nanotubes tethered with a thiolated aptamer. Microchim Acta 184, 1677–1682 (2017). https://doi.org/10.1007/s00604-017-2164-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2164-9