Abstract
The authors describe a composite material prepared from carbon nanohorns and poly(2-aminopyridine) that was obtained by electrochemical polymerization of 2-aminopyridine on carbon nanohorns. The material was used to modify a glassy carbon electrode (GCE) to obtain a sensor for non-enzymatic determination of hydrogen peroxide. The modified GCE was characterized by cyclic voltammetry, electrochemical impedance spectroscopy and chronoamperometry. The modified electrode is shown to display excellent electrical conductivity and catalytic activity towards hydrogen peroxide, mainly due to the large specific surface area of carbon nanohorns, the good electron charge transfer properties resulting from the use of poly(2-aminopyridine), and their synergistic effect. The response of the modified GCE (best operated at a working potential of −0.45 V vs. SCE) to H2O2 is linear in the 0.05 to 8 mM concentration range. The limits of detection (LOD) and quantitation (LOQ) are 3.6 μM and 12.4 μM, respectively. The electrode is selective, stable and reproducible, this making it a promising tool for non-enzymatic determination of hydrogen peroxide.

A glassy carbon electrode was modified with carbon nanohorns and poly(2-aminopyridine) to obtain a sensor for H2O2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrogen peroxide (H2O2) is of great importance in many areas [1]. The rapid, accurate and reliable determination of H2O2 is of significance to scientific research and practical applications [2]. Some methods have been applied for the detection of H2O2, mainly including optical [3] and electrochemical methods [4–6]. Electrochemical methods have attracted special attention because of its fast detection, low cost, easy operation and efficiency. Electrochemical sensors of H2O2 are roughly divided into two types: enzymatic and non-enzymatic determination. Non-enzymatic sensors have several advantages, such as high stability, favorable selectivity and easy handing. Therefore, great efforts have been given to develop desirable non-enzymatic electrochemical sensors [7].
With the development of nanotechnology, nanomaterials, especially nanostructured carbon materials, play an important role in improving sensor performance [8, 9]. Nanostructured carbon materials mainly include graphene, carbon nanotubes and carbon nanohorns (CNH), etc. CNH have horn-shaped sheaths composed of graphene sheets and a conical structure with a particularly sharp apical angle, possessing excellent electrical conductivity, high specific surface area and internal spaces [10]. Due to these striking features, CNH have attracted great interest with respect to potential applications, such as adsorption [11], drug delivery [12], fuel cells [13] and supercapacitors [14]. Dai et al. built CNH and carboxylic ionic liquid composite and achieved fast determination of 4-aminophenylarsonic acid [15].
As good electrically conductive materials, conducting polymers modified glassy carbon electrode (GCE) can be easily fabricated via electropolymerization for the development of sensors [16]. Hassan et al. fabricated sensor by electropolymerization of 1,5-diaminonaphthalene on modified electrode for determination of dihydronicotinamide adenine dinucleotide [17]. 2-aminopyridine (2-AP) was an excellent kind of electrochemical polymerization reagent. It would be interesting to utilize poly(2-aminopyridine) (PAP) on modified electrode for the fabricate of sensor.
We fabricated a non-enzymatic electrochemical sensor of H2O2 by utilizing CNH as matrix for electropolymerization of 2-AP on GCE. With the advantages of CNH and PAP, CNH/PAP electrode exhibited excellent catalytic performance towards H2O2 reduction with low detection limit, wide linear range. CNH/PAP electrode showed prominent selectivity, sensitivity and reproducibility, demonstrating the potential application in electrochemical sensing devices.
Experimental
Reagents and materials
CNH were purchased from Qingda Jiguang Scientific Development Co. (www.qingdajiguang.com). N,N-dimethylformamide (DMF), 2-aminopyridine (2-AP), H2O2 (30 wt%), NaNO3, H3PO4, H3BO3 and acetic acid were analytical grade and purchased from Chengdu Kelong Chemical Reagent Factory (www.cdkelong.com). Glucose, acetaminophen (AC), ascorbic acid (AA) and uric acid (UA) were obtained from Sigma-Aldrich (www.sigmaaldrich.com). Britton-Robinson (BR) buffer was prepared by mixing stock solutions of 0.040 M H3PO4, H3BO3 and acetic acid, and then adjusted to pH of 5.7 with 0.20 M NaOH solution. The different concentrations of H2O2 solution were prepared just before electrochemical measurements. Doubly distilled water was used throughout the whole experiment. High purity nitrogen (99.999 %) was used for deaeration in the electrochemical detection.
Electrodes preparation and characterization
Prior to use, GCE (Φ = 3.0 mm, S = 0.071 cm2) was polished with 500 and 50 nm aluminum oxide powders to a mirror-like, respectively, and then washed successively with double-distilled water and ethanol for several times. A homogeneous mixture was formed by adding 1.0 mg CNH into 1.0 mL DMF, and the mixture was sonicated for at least 30 min. The preparation procedures of CNH/PAP/GCE were as follows: 5.0 μL the mixture was dropped on the surface of GCE and allowed to dry in ambient air. And then electropolymerization of 2-AP on CNH/GCE was carried out by cyclic voltammetry between 1.60 V ~ −0.80 V at scan rate of 50 mV s−1 for 10 cycles in the solution containing 0.050 M 2-AP and 0.10 M NaNO3. CNH/PAP electrode was thoroughly washed with distilled water and dried in air. For comparison, the preparation procedures of PAP/GCE and CNH/GCE were similar to that of CNH/PAP/GCE just without dropping or electropolymerization. Before the electrochemical measurements, the electrolytes were purged with N2 for 1 h to remove oxygen completely.
The morphology of CNH was characterized by transmission electron microscopy (TEM, Libra 200FE, Zeiss Corporation, Germany). The data for cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS) and chronoamperometry (CA) were obtained with a PARSTAT 2273 electrochemical workstation (Princeton Applied Research, USA) with a three-electrode test system using a platinum electrode as counter electrode, a bare or modified GCE as working electrode referred to as saturated calomel electrode.
Results and discussion
Choice of materials
Nanostructured carbon materials (graphene, carbon quantum dots and carbon nanotubes, etc.) and conductive polymer (polythiophene, polypyrrole and polyaniline, etc.) have potential application in electrochemical sensor. As mentioned in Introduction, CNH possessed excellent electrical conductivity and PAP had an excellent electrocatalytic response. Therefore, taking full advantages of CNH and PAP excellent performance, we fabricated CNH/PAP composite by electrochemical polymerization of 2-AP on CNH and obtained a sensor for non-enzymatic determination of hydrogen peroxide.
Modification of electrode surface
TEM images of CNH are shown in Fig. 1. The individual CNH structural unit is shown clearly in the images. CNH formed dahlia-like assemblies with a diameter about 70 nm. The unique nanohorn structure of CNH might have plenty of edge plane-like defects. On the other hand, the excellent electrocatalytic ability of CNH can be ascribed to edge plane-like defects on the surface of CNH, providing many favorable sites for electron transfer to molecule [18].
Figure 2 shows CVs for electropolymerization of 2-AP on CNH/GCE between 1.60 V ~ −0.80 V at a scan rate of 50 mV s−1 for 10 cycles in 0.10 M NaNO3 solution containing 0.050 M 2-AP. The peak current density decreased with successive scanning from 1 to 4 cycles, which was due to the increased resistance of the formed conducting polymeric film PAP. After 4 cycles of potential scanning, it was observed that the peak current densities were almost constant, indicating the complete formation of conducting polymeric film PAP on CNH/GCE electrode surface [19].
EIS is utilized to study the electron charge transfer property between the electrolyte and the electrode surface [20]. Figure 3 shows the Nyquist plots of 1.0 mM K3Fe(CN)6 + 0.10 M KCl solution on GCE (a), PAP/GCE (b), CNH/GCE (c) and CNH/PAP/GCE (d).
As indicated in Fig. 3, the bare GCE (curve a) exhibited an almost straight line at low frequency region which corresponded to the diffusion process. The electron charge transfer resistance was estimated from the diameter of the semicircle. The semicircle-like shape with larger diameter was observed on PAP/GCE (curve b), indicating that PAP was successfully assembled on electrode surface and a larger increase in resistance was achieved due to the hindrance to electron-transfer kinetics caused by PAP film. Lower resistance on CNH/GCE (curve c) was observed compared with PAP/GCE, showing conducting effect by CNH in facilitating the electron transfer process on electrode interface. However, an obvious decrease of the charge transfer resistance was observed when modified with CNH/PAP (curve d). Such complex charge transfer reduced the ion intercalation distances and facilitated better charge transfers and lower resistances, which confirmed the cyclic voltammetric results. Besides, it also further proved that CNH/PAP composite was more suitable as electrode materials for detection of H2O2.
Electrochemical detection of H2O2 on CNH/PAP/GCE
The electrocatalytic reduction of H2O2 on the surface of modified electrodes was investigated by CV in the potential range of 0.0 to −0.80 V. To delineate the main impact of different components of modified electrode on the observed responses in electrochemical reduction of H2O2, a series of experiments was designed.
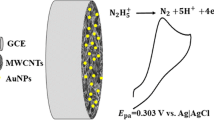
Figure 4 shows CVs of 0.2 mM H2O2 on modified electrodes in BR buffer (pH 5.7). No obvious response appears in curve a of Fig. 4, indicating that it was difficult to determine H2O2 on bare GCE. However, on the surface of PAP/GCE (b) and CNH/GCE (c), reduction peaks respectively appeared at −0.50 V or −0.55 V owning to reduction of H2O2, and the cathodic peak current densities increased on CNH/GCE compared to that obtained on PAP/GCE. This indicates that the presence of PAP or CNH species is necessary for H2O2 reduction. CNH/PAP/GCE (d) showed a clear cathodic peak at −0.45 V and the largest cathodic peak current density for H2O2 reduction, which was more positive than that observed on PAP/GCE or CNH/GCE. The above results indicated that CNH/PAP/GCE possess remarkable catalytic ability for H2O2 reduction.
The electrocatalytic mechanism of CNH/PAP/GCE electrode for H2O2 reduction might be that the electrons were obtained on surface of H2O2 molecules, and anions were formed in BR buffer. Nitrogen atoms of 2-AP molecule which have lone pairs of electrons combined with the H+, making polymer film with high density of positive charge. Hydrogen bonds interaction between -NH2 of polymer and molecules of H2O2 also may occur. Based on electrostatic attraction and hydrogen-bonds interaction between polymer film and H2O2, as well as conducting effect by CNH, CNH/PAP modified electrode exhibited catalytic performance towards H2O2 [21].
Figure S1 shows CVs of 0.2 mM H2O2 on CNH/PAP/GCE at different scan rates (10, 20, 50, 80, 100, 120, 150, 180, 200 mV s−1) in BR buffer (pH 5.7). The inset shows the dependence of cathodic peak currents on the square root of scan rate (υ1/2). Clearly the peak current increases gradually with increasing the square root of scan rate and a linear relationship exists between them. This was because that H+ attacking was accompanied with redox reaction of 2-AP to the surface of electrode. And it diffused from the surface of solution to electrode surface then crossed the adsorption layer, which suggested that a diffusion controlled process probably occurred in the reduction process [22].
Amperometric detection of H2O2 on CNH/PAP/GCE
Figure 5 shows the amperometric response of H2O2 on CNH/PAP/GCE obtained by successively adding H2O2 to a continuously stirred electrochemical cell. The inset shows the linear calibration plot of peak current densities versus concentrations.
Figure 5 showed that the reduction current density of H2O2 varies linearly with concentrations. The linear regression equation was calculated as j pc / (μA cm−2) = (−42.91 ± 1.22) + (2.11 ± 0.01) × c / (μM) in the range of 0.05 mM to 8 mM (R = 0.992, n = 5). Based on the signal-to-noise ratio (S/N), the limits of detection (LOD) and quantization (LOQ) were calculated to be 3.6 μM (S/N = 3) and 12.4 μM (S/N = 10), respectively. CNH/PAP/GCE showed a wide linear range and a low detection limit, and the comparison results with other sensors are shown in Table 1 [4, 5, 23–28].
Effect of interferences on analytical response
One of the important analytical factors for an amperometric sensor was its ability to discriminate the interfering species having electroactivities similar to the target analyte [29]. To investigate the selectivity of sensor toward H2O2 reduction, the effect of some electroactive interferences such as glucose, AC, AA, and UA, which commonly present in physiological samples were studied by amperometric detection at −0.45 V.
Figure 6 shows the current density responses of CNH/PAP/GCE upon the addition of 1.0 mM H2O2, 5.0 mM glucose, 0.10 mM AC, 0.150 mM AA, 0.50 mM UA, 1.0 mM H2O2, 1.0 mM H2O2 and 1.0 mM H2O2 in succession. The concentration of interferences was selected based on their levels of endogenous in the blood samples [30]. Observed in Fig. 6 were the measured effects of different interferents along with H2O2 at −0.45 V. For all interfering species, CNH/PAP/GCE showed no significant responses, indicating that this sensor had high selectivity, which was owing to relative low applied potential of sensor.
Stability and reproducibility
The stability of CNH/PAP/GCE was evaluated by measuring the decrease of current response to 0.2 mM H2O2 over 30 min. The electrochemical sensor exhibited no obvious decrease in current response and maintained about 93 % of its initial response. Ten newly-prepared electrodes for 0.2 mM H2O2 were investigated and intra-day relative standard deviation was 2.8 %, confirming that CNH/PAP/GCE had high reproducibility. The results above showed that the CNH/PAP/GCE presented excellent stability and satisfactory reproducibility for the determination of H2O2.
Conclusions
CNH/PAP composite film modified electrode was successfully developed via electropolymerization process and used for non-enzymatic determination of H2O2. CNH/PAP composite combined the merits of CNH and PAP and enhanced electrochemical properties toward the reduction of H2O2. CNH/PAP-based H2O2 sensor exhibited excellent stability and satisfactory reproducibility, making it one of the promising candidates for efficient and sensitive determination of H2O2.
References
Chen W, Cai S, Ren QQ, Wen W, Zhao YD (2012) Recent advances in electrochemical sensing for hydrogen peroxide: a review. Analyst 137:49–58
Yang ZY, Qi CC, Zheng XH, Zheng JB (2016) Sensing hydrogen peroxide with a glassy carbon electrode modified with silver nanoparticles, AlOOH and reduced grapheme oxide. Microchim Acta 183:1131–1136
Schäferling M, Grögel DBM, Schreml S (2011) Luminescent probes for detection and imaging of hydrogen peroxide. Microchim Acta 174:1–18
Song HY, Ma CH, You LY, Cheng ZY, Zhang XH, Yin BS, Ni YN, Zhang KQ (2015) Electrochemical hydrogen peroxide sensor based on a glassy carbon electrode modified with nanosheets of copper-doped copper (II) oxide. Microchim Acta 182:1543–1549
Wang N, Sun JC, Chen LJ, Fan H, Ai SY (2015) A Cu2(OH)3Cl-CeO2 nanocomposite with peroxidase-like activity, and its application to the determination of hydrogen peroxide, glucose and cholesterol. Microchim Acta 182:1733–1738
Cui X, Wu SN, Li YX, Wan G (2015) Sensing hydrogen peroxide using a glassy carbon electrode modified with in-situ electrodeposited platinum-gold bimetallic nanoclusters on a graphene surface. Microchim Acta 182:265–272
Wu Q, Sheng QL, Zheng JB (2016) Nonenzymatic amperometric sensing of hydrogen peroxide using a glassy carbon electrode modified with a sandwich-structured nanocomposite consisting of silver nanoparticles, Co3O4 and reduced graphene oxide. Microchim Acta 183:1943–1951
Chen XJ, Wang YZ, Zhang YY, Chen ZH, Liu Y, Li ZL, Li JH (2014) Sensitive electrochemical aptamer biosensor for dynamic cell surface n-glycan evaluation featuring multivalent recognition and signal amplification on a dendrimer-graphene electrode interface. Anal Chem 86:4278–4286
Zhu XH, Zhao TB, Nie Z, Liu Y, Yao SZ (2015) Non-redox modulated fluorescence strategy for sensitive and selective ascorbic acid detection with highly photoluminescent nitrogen-doped carbon nanoparticles via solid-state synthesis. Anal Chem 87:8524–8530
Xu JX, Shingaya Y, Tomimoto H, Kubo O, Nakayama T (2011) Irreversible and reversible structural deformation and electromechanical behavior of carbon nanohorns probed by conductive AFM. Small 9:1169–1174
Vaiva K, Ziegler CA, Banjara SR, Masako Y, Iijima S, Migone AD (2013) Neon and CO2 adsorption on open carbon nanohorns. Langmuir 29:9388–9397
Li NN, Zhao Q, Shu C, Ma XN, Li RX, Shen HJ, Zhong WY (2015) Targeted killing of cancer cells in vivo and in vitro with IGF-IR antibody-directed carbon nanohorns based drug delivery. Int J Pharm 478:644–654
Unni SM, Ramadas S, Illathvalappil R, Bhange SN, Kurungot S (2015) Surface-modified single wall carbon nanohorn as an effective electrocatalyst for platinum-free fuel cell cathodes. J Mater Chem A 3:4361–4367
Yang CM, Kim YJ, Miyawaki J, Kim YA, Yudasaka M, Iijima S, Kaneko K (2015) Effect of the size and position of ion-accessible nanoholes on the specific capacitance of single-walled carbon nanohorns for supercapacitor applications. J Phys Chem C 119:2935–2940
Dai H, Gong LS, Lu SY, Zhang QR, Li YL, Zhang SP, Xu GF, Li XH, Lin YY, Chen GN (2015) Determination of 4-aminophenylarsonic acid using a glassy carbon electrode modified with an ionic liquid and carbon nanohorns. Microchim Acta 182:1247–1254
Hosseini H, Rezaei SJT, Rahmani P, Sharifi R, Nabid MR, Bagheri A (2014) Nonenzymatic glucose and hydrogen peroxide sensors based on catalytic properties of palladium nanoparticles/poly(3,4-ethylenedioxythiophene) nanofibers. Sensors Actuators B Chem 195:85–91
Hassan KM, Hathoot AA, Ashour WFD, Abdel-Azzem M (2015) Electrochemical and analytical applications for NADH detection at glassy carbon electrode modified with nickel nanoparticles dispersed on poly 1,5-diaminonaphthalene. J Solid State Electrochem 19:1063–1072
Zhu SY, Xu GB (2010) Single-walled carbon nanohorns and their applications. Nanoscale 2:2538–2549
Kan JQ, Li X, Li YF (2002) Synthesis and properties of poly-2-aminopyridine. Acta Phys -Chim Sin 18:106–111
Zhang SS, He P, Lei W, Zhang GL (2014) Novel attapulgite/polyaniline/phosphomolybdic acid-based modified electrode for the electrochemical determination of iodate. J Electroanal Chem 724:29–35
Wu J, Liu GD, Huang SS, Yu RQ (2001) Electrochemical behavior of 2-pyridinamine modified glassy carbon electrode and its application to the determination of ascorbic acid. Chin J Anal Chem 29:1140–1143
Gao P, Gong YX, Mellott NP, Liu DW (2015) Non-enzymatic amperometric detection of hydrogen peroxide using grass-like copper oxide nanostructures calcined in nitrogen atmosphere. Electrochim Acta 173:31–39
Janyasupab M, Liu CW, Zhang Y, Wang KW, Liu CC (2013) Bimetallic Pt-M (M = Cu, Ni, Pd, and Rh) nanoporous for H2O2 based amperometric biosensors. Sensors Actuators B Chem 179:209–214
Liu M, Liu R, Chen W (2013) Graphene wrapped Cu2O nanocubes: non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens Bioelectron 45:206–212
Frontera P, Malara A, Stelitano S, Leonardi SG, Bonavita A, Fazio E, Antonucci P, Neri G, Neri F, Santangelo S (2016) Characterization and H2O2 sensing properties of TiO2-CNTs/Pt electro-catalysts. Mater Chem Phys 170:129–137
Woo S, Kim YR, Chung TD, Piao Y, Kim H (2012) Synthesis of a grapheme-carbon nanotube composite and its electrochemical sensing of hydrogen peroxide. Electrochim Acta 59:509–514
Xie AJ, Liu QX, Ge HL, Kong Y (2015) Novel H2O2 electrochemical sensor based on grapheme-polyacrylamide composites. Mater Technol 30:50–53
Kumar SA, Chen SM (2007) Electrocatalytic reduction of oxygen and hydrogen peroxide at poly (p-aminobenzene sulfonic acid)-modified glassy carbon electrodes. J Mol Catal A Chem 278:244–250
Sophia J, Muralidharan G (2015) Polyvinylpyrrolidone stabilized palladium nanospheres as simple and novel electrochemical sensor for amperometric hydrogen peroxide detection. J Electroanal Chem 739:115–121
Liu Q, Lu XB, Li J, Yao X, Li JH (2007) Direct electrochemistry of glucose oxidase and electrochemical biosensing of glucose on quantum dots/carbon nanotubes electrodes. Biosens Bioelectron 22:3203–3209
Acknowledgments
This work was supported by the Open Project of State Key Laboratory Cultivation Base for Nonmetal Composites and Functional Materials (11zxfk26). Also we are grateful for the help of Analytical and Testing Center of Southwest University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 103 kb)
Rights and permissions
About this article
Cite this article
Chen, J., He, P., Bai, H. et al. A glassy carbon electrode modified with a nanocomposite consisting of carbon nanohorns and poly(2-aminopyridine) for non-enzymatic amperometric determination of hydrogen peroxide. Microchim Acta 183, 3237–3242 (2016). https://doi.org/10.1007/s00604-016-1975-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1975-4