Abstract
We are introducing functionalized carbon dots (C-dots) coated with a shell of molecularly imprinted sol-gel as a new tool in molecular imprint-based detection. Specifically, an imprint recognizing nicotinic acid (NA) was prepared in two steps. The first involves pyrolytic decomposition of citric acid in the presence of aminopropyltriethoxysilane to yield triethoxysilyl-modified C-dots with a typical size of 2.8 ± 1.1 nm. These are then polycondensed in the presence of tetraethoxysilane and NA at room temperature to give spherical silica nanoparticles (SiNPs) with a typical size of ~300 nm and containing C-dots and NA in the silica matrix. NA was then removed by extraction. The resulting SiNPs are well permeable to NA, photostable, display strong blue luminescence and can bind NA fairly selectively. The fluorometric detection scheme is based on the finding that increasing concentrations of NA quench the fluorescence of the C-dots in the SiNPs. NA can be determined by this method in the 0.5 to 10.5 μM concentration range, with a 12.6 nM detection limit. The composite was successfully utilized as a fluorescent probe for the determination of NA in spiked human urine samples. The method is believed to have a wider scope in being applicable to other analytes that are capable of quenching the fluorescence of C-dots.

A new type of nicotinic acid sensitive molecularly imprinted polymers functionalized carbon dots (SiNPs) were fabricated by the sol-gel polymerization. The composite was utilized as a fluorescent probe for the determination of nicotinic acid in human urine samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Molecular imprinting technique refers to taking a particular target molecule as a template, and preparing a polymer which is specific for the molecule by simulating antigen-antibody interaction. Based on this theory, bulk molecularly imprinted polymers (MIPs) have been commonly prepared by self-assembly approach and applied for a wide scope [1], for instance, in separation processes, catalysis, antibody mimics and artificial enzymes. Because most binding sites are in the inner part of bulk MIPs, it is hard for the large molecules, especially proteins, to go into and out of the polymers [2]. The approach by using small molecule as template to prepare MIPs can effectively improve the adsorption capacity and specific identification of prepared polymers. A further improvement of their efficiency can be achieved by placing the active site inside soluble nanoparticles [3]. Many approaches have been developed for the preparation of MIPs. Among them, sol-gel process has many advantages, such as mild processing conditions, eco-friendly reaction solvent, etc. As we known, many kinds of MIPs with great sensitivity and selectivity necessary for sensing applications have been prepared by sol-gel approach [4].
C-dots are a class of nano-structured materials, which are strongly fluorescent, emission-color-tuning and non-blinking. Owing to low cytotoxicity and impressive photostability of C-dots, they have garnered considerable interest since their discovery. Among the physicochemical characteristics of C-dots, their optical properties, especially their fluorescence emissions, have attracted considerable attention [5]. However, the main problem, lacking molecular recognition properties, has limited their application. With the increasing complexity and diversity of samples for drug analysis, the separation and detection of trace components have become a prominent issue.
As the increasing demand of complicated sample analysis, reports about the functional composite materials have increased greatly [6]. MIPs not only were synthesized on the surface of carbon nanotubes [7], but also have been combined with many other kinds of materials to prepare composite materials for a wider scope application. For instance, Lin et al. [8] synthesized MIPs thin films on the surface of CdSe/ZnS core-shell. Similarly, Lin et al. [9] used poly(ethylene-co-ethylene alcohol) as cross-linker to incorporate quantum dots into MIPs. Wang et al. [10] anchored the MIP layer on the surface of quantum dots via a surface molecular imprinting process using tetraethoxysilane as cross-linker. Some researchers have coated fluorescence C-dots with molecularly imprinted silica film by sol-gel polymerization approach [11]. Therefore, combining the molecular imprinting technique with fluorescence technique, functional composite materials can be prepared, which will not only have molecular specific identifying characteristics, but also show high optical sensitivity. These materials can be used as a fluorescent probe to detect trace substances in complex samples.
Nicotinic acid (NA) belongs to the vitamin B group. It participates in the oxidizing process of cells, lowers plasma levels of cholesterol, and maintains the normal functions of skin. NA has been widely used in medicine and food additives. Detection and quantification of NA are important in diagnoses and treatments of several disorders [12]. Different analytical methods have been developed for NA determination, including high-performance liquid chromatography (HPLC) [13], capillary electrophoresis [14], enzymic method [15] and supercritical fluid chromatography coupled to tandem mass spectrometry [16]. Enzymic method was not sensitive enough in some case. HPLC and capillary electrophoresis were susceptible to ambient temperature. Supercritical fluid chromatography coupled to tandem mass spectrometry has detection limit as low as 1 ng mL−1, but this method is complex, expensive, and the sample has to be diluted with organic solvent. Therefore, low cost, sensitive and selective sensor systems have become increasingly needed for NA determinations.
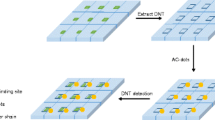
To the best of our knowledge, there are no previous reports on NA fluorescent optosensing based on molecular imprinting technology. We describe the preparation of a fluorescent probe for NA detection. First of all, using anhydrous citric acid as precursor, we prepared silane groups coated C-dots [17]. Secondly, we used NA as template molecules, which were mixed with silane groups coated C-dots, functional monomer and cross-linker in ethanol, SiNPs were fabricated by sol-gel method. Once more, upon removal of the templates, the recognition specificity of SiNPs and the effect of NA on the fluorescence intensity were studied. Moreover, linear regression analysis was performed to assess the relationship between fluorescence response and NA concentration. Eventually, SiNPs were utilized as a fluorescent probe for the detection of NA in biological samples.
Experiment
Apparatus
Solution pH values were determined by use of a pHS-25 pH meter (Shanghai Albert Instrument Factory, China). UV–vis absorption was characterized by a UV1800 UV–vis spectrophotometer (Shimadzu Corporation, Japan). Photoluminescence emission measurements were performed using a RF-5301PC fluorescence spectrophotometer (Shimadzu Corporation, Japan). FT-IR spectrum was performed on a Shimadzu IR-prespige-21 FT-IR spectrometer (Kyoto, Japan) in the range of 400–4000 cm−1. The morphology of the nanoparticles was studied using a JEM-2100 (HR) transmission electron microscope (TEM) (JEOL, Japan). The X-ray photoelectron spectra (XPS) of C-dots were obtained with an X-ray photoelectron spectrometer (K-Alpha, Thermo Fisher). Time-resolved fluorescence spectra were carried out in a time-correlated single-photon counting (TCSPC) system from an Edinburgh FLS920 spectrometer (Edinburgh Instruments, UK) excited at the wavelength of 370 nm. Dynamic light scattering studies and zeta potential measurements were recorded on a Malvern Instruments (Malvern, UK) Zetasizer Nano ZS90.
Materials and reagents
Ascorbic acid (AA) and isonicotinic acid (INA) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China; www.reagent.com.cn), anhydrous citric acid was purchased from Shanghai Ziyi Reagent Factory (www.ziyi-reagent.com), 3-aminopropyl triethoxysilane (APTES), tetraethoxysilane (TEOS), ammonia solution (25 % in water) and NA were obtained from Aladdin (Shanghai, China; http://www.aladdin-e.com). Doubly deionized water was used throughout.
Fabrication of C-dots
The overall synthetic procedure is illustrated in Scheme. 1. C-dots were prepared from anhydrous citric acid according to the previously described method with some minor modifications [17]. 10 mL APTES was added into a 100 mL three-necked flask, purged with nitrogen, and was heated to 210 °C, then 0.5 g anhydrous citric acid was quickly added with vigorous stirring. After keeping the temperature at 210 °C for 10 min, the mixture was naturally cooled down to room temperature. The final product was separated by precipitation with petroleum ether, and was purified with dichloromethane to remove the unreacted organic moieties, then centrifuged at 10,000 rpm. The obtained nanoparticles were dispersed in ethanol for the next process.
Synthesis of silica nanoparticles (SiNPs)
Nicotinic acid (NA; 30 mg) acting as the template was mixed with 18 mL of a solution of C-dots in ethanol (containing 1824 mg of C-dots) in a 50 mL flask, sonicated to dissolve NA. To the resulting mixture, 155 μL APTES (functional monomer) and 0.7 mL TEOS (cross-linker) were added under magnetic stirring. Finally, 430 μL water and 60 μL ammonia solution were added, the mixture was magnetically stirred for 24 h at room temperature.
After the reaction was finished, the solution became turbid, and a thick white emulsion was obtained. Then it was centrifuged at 3000 rpm, the white precipitate was washed with ethanol to discard the unreacted monomers. Templates in the composite were extracted with the mixture of methanol and acetic acid (9/1, v/v) several times, until the template molecules in the eluent were not detected with UV spectrometer at 263 nm. The obtained SiNPs were washed with water three times, dried at 60 °C in a vacuum drying oven overnight.
Following the same procedure, non-imprinted polymer nanoparticles were prepared as a control, except that no template was added.
Fluorescence properties of SiNPs
The obtained SiNPs were added into deionized water (pH 7.0), and 20 μg mL−1 mixed solution was prepared. 10 μL NA aqueous solution of certain concentration was diluted to produce 5 mL by adding the SiNPs solution, mixed thoroughly. After reaction for 8 min at room temperature, the fluorescence emission spectra were obtained by scanning the emission from 300 to 700 nm on the spectrofluorimeter. The measurement of non-imprinted polymer nanoparticles as control was the same as SiNPs.
Analytical application
Human urine samples were collected from healthy volunteers. 1 mL of urine sample was diluted with deionized water to 100 mL. 20 μg mL−1 SiNPs mixed solution was prepared using the diluted urine sample. 10 μL NA aqueous solution of certain concentration was diluted to produce 5 mL by adding the mixed solution, the resulting urine solutions were detected by the spectrofluorimeter. The amount of NA was determined according to the calibration curves.
Results and discussion
Selection of materials
Even though many composite materials have been prepared using quantum dots and organic cross-linkers, synthesis of conventional semiconductor quantum dots usually required laborious processes. Meanwhile, most of the metal-based quantum dots in use are toxic, resulting in environmental and biological hazards [5]. MIPs prepared using organic cross-linkers such as poly(ethylene-co-vinylalcohol) [9] were hydrophobic, so they are poorly water-soluble [18]. Furthermore, when quantum dots were wrapped in a shell of MIPs, their fluorescence intensity decreased and emission peak red-shifted, fluorescence property would change [8]. To solve the above-mentioned questions, C-dots have inspired extensive studies. C-dots have unique attributes, such as benign chemical composition, high aqueous solubility, low toxicity, good biocompatibility, facile functionalization, non-photobleaching, low cost and easy of synthesis [19]. What’s more, C-dots with high quantum yields have been fabricated by hydrothermal method [20]. Silica matrix is a kind of inert and transparent material [21], composite materials using TEOS as cross-linker can keep the photoluminescence properties of C-dots. Because of many hydrophilic hydroxyl groups on the surface of SiNPs, they are water-soluble and their imprinting efficiency can be improved. So C-dots and organic silanes are applicable as initial materials to synthesize SiNPs.
Characterization of C-dots
Highly luminescent C-dots functionalized with triethoxysilane groups were prepared via a pyrolytic process. The functionalized C-dots were characterized with FTIR spectroscopy, TEM and fluorescent spectroscopy.
The TEM image (Fig. 1a) showed that the C-dots with microspherical shape were uniform, mono-dispersed. A particle diameter of 2.8 ± 1.1 nm was estimated from TEM image by investigating 87 particles, which was consistent with the dynamic light scattering result (Fig. S1, Electronic Supplementary Material, ESM). The zeta potential of the C-dots in water at pH 7.0 was measured to be 3.51 mV. In the process of C-dots synthesis, anhydrous citric acid was decomposited and carbonized under high temperature conditions. Surface passivation was achieved using the reaction of the amine group in APTES with the carboxyl groups on the surface of pyrolyzed organics, thereby achieving bright luminescence and surface functionalization of C-dots simultaneously [17].
FT-IR spectra comparison between APTES and C-dots was shown in Fig. 2a. Spectra of APTES and C-dots displayed some common characteristic absorption bands. The FTIR peak at 3456 cm−1 was ascribed to N-H stretching vibration. The peak at 1078 cm−1 corresponded to O-Si-O asymmetric stretching vibration. The peak at 1554 cm−1 was attributed to secondary amine R-NH-R bending vibration, 1639 cm−1 was attributed to the stretching vibration of secondary amide carbonyl (C = O). The two typical signals of C-dots (1554, 1639 cm−1) demonstrated that acylation reaction had occurred between C-dots and APTES, and that silane groups had been connected on the surface of C-dots via amido linkage. The surface elemental analysis for the resultant nanoparticles was determined by XPS. The full range XPS analysis (Fig. 2b) of the resultant C-dots clearly showed four peaks at 284.08, 399.08, 531.08 and 101.58 eV, which were attributed to C 1 s, N 1 s, O 1 s and Si 2p, respectively. Functionalization of C-dots was further confirmed by the XPS result.
The fluorescent emission spectra were detected by scanning the emission from 300 to 700 nm on the spectrofluorimeter (with 5 and 5 nm slit width for excitation and emission, respectively). Figure 2c depicted the UV–vis absorption and photoluminescence spectra of C-dots in ethanol. The maximum UV absorption peak was observed at 360 nm. When excited at the wavelength from 320 to 450 nm, the C-dots showed blue-green photoluminescence. The fluorescent intensity gradually increased with increasing excitation wavelength from 320 to 380 nm, the photoluminescence spectra showed the highest fluorescence intensity when C-dots were excited at 380 nm, however, the fluorescent intensity gradually decreased as the excitation wavelength continued increasing. The emission peak position shifted from 448 nm to 507 nm with increasing excitation wavelength from 320 to 450 nm. Their emission spectra were highly dependent on the excitation wavelength.
Characterization of SiNPs
Catalyzed by aqueous ammonia, polymerization took place on the surface of C-dots through the hydrolysis and condensation reaction of APTES and TEOS, MIPs were successfully anchored on the surface of C-dots. The morphology of the SiNPs was characterized by TEM. As shown in Fig. 1b, the resulting particles showed spherical nanostructure with ca. 300 nm diameter, and they were in relatively narrow size distribution.
The fluorescent emission spectra of SiNPs in ethanol were detected by scanning the emission from 350 to 700 nm on the spectrofluorimeter. As shown in Fig. 3, with the decrease of the incident light wavelength, the ultraviolet absorption of SiNPs (unlabelled curve) gradually increased, but no UV absorption peak was observed. When excited from 320 nm to 360 nm, the fluorescence intensity of SiNPs gradually increased, the maximum fluorescence emission peak at 452 nm was obtained when excited at 360 nm. From 360 nm to 390 nm, with the excitation wavelength increasing, the fluorescence intensity gradually decreased. These results demonstrated that the silica coating had little influence on the fluorescence intensity of C-dots [22]. Different from C-dots, the emission peak position of SiNPs had no shift as the excitation wavelength increasing from 320 to 390 nm. This phenomenon might be caused by fluorescence resonance energy transfer [23]. The C-dots acted as donors, MIPs served as acceptors, this donor-acceptor system lead to intra-energy transfer [24], so there were no wavelength shifts.
The fluorescence intensity of an aqueous solution of SiNPs (20 μg mL−1) was recorded every 5 min under 360 nm excitation. As shown in Fig. S2 (ESM), the fluorescence intensity of SiNPs in water was relatively strong and stable.
Recognition capability of SiNPs
Figure 4 compared the FTIR spectra between non-imprinted polymer nanoparticles and SiNPs. All spectra exhibited strong N-H stretching vibration at 3438 cm−1. The presence of the carbonyl group in the spectrum of SiNPs before template molecules extraction was confirmed by the peak at 1668 cm−1, corresponding to the C = O stretching mode. The characteristic peak at 1486 cm−1 was attributed to pyridine ring skeletal vibration of NA molecule. The presence of pyridine ring and carbonyl group peaks in the spectrum of SiNPs before template molecules extraction, combined with the absence of these characteristic peaks after solvent elution, demonstrated the successful imprinting of NA into the silica matrix. The broad and strong peak around 1078 cm−1 was assigned to the O-Si-O asymmetric stretching vibration, and the peaks at 458 cm−1 and 795 cm−1 were assigned to the Si-O stretching vibration. The peak at 1638 cm−1 was attributed to the stretching vibration of secondary amide carbonyl (C = O). All these bonds demonstrate that molecular imprint incorporating C-dots have successfully been prepared by sol-gel condensation approach.
As shown in Fig. 5a, b and c, after template extraction, the fluorescence intensity of SiNPs increased greatly, while quenched a lot when interacted with NA solution. These spectra confirmed that the extraction and rebinding of NA were a reversible process.
The selectivity adsorption of the SiNPs was investigated with INA and AA as the reference compound. As can be seen from Fig. 5b, the fluorescence responses of SiNPs towards NA were much larger than its structure analogues AA and INA, which indicated SiNPs had better adsorption and binding capacity for the template molecules. With increasing concentration of NA, fluorescence quenching degree increased. Because of nonspecific adsorption, there were some fluorescence responses towards AA and INA, but much lower than that of NA. After the removal of template molecules, imprinted cavities and specific binding sites in a predetermined orientation were formed. The shapes of the cavities were fit for the unique molecular structure of NA, but the imprinted cavities were not suitable for other structure analogues. Other analogues were not bound tightly in the imprinted cavities. This was attributed to the imprinting effect, different molecular interactions and their specific structures [25], so the SiNPs had highly specific recognition for the template molecules.
Template measurement
The fluorescence intensity of the SiNPs at 452 nm (exCitation 360 nm) gradually decreased with increment of NA concentration, revealing that this method was sensitive to NA concentration in aqueous solution. The photoluminescence quenching data followed the Stern-Volmer equation:
F and F0 are the fluorescent intensities of the SiNPs in the presence and absence of NA respectively. [CNA] is the concentration of NA, KSV is the quenching constant of the quencher (NA). The fluorescence quenching efficiency of SiNPs was 2.8 times as large as that of non-imprinted polymer nanoparticles, which confirmed the efficient imprinting effect of SiNPs. Owing to better steric matching of recognition sites with NA in the SiNPs, more template molecules were adsorbed in the imprinted silica matrix via hydrogen bonds and van der Wall forces [26], leading to C-dots fluorescence quenching. To get further insight into the fluorescence quenching mechanism, the time-resolved fluorescence of SiNPs in the presence and absence of NA was performed under the excitation of 370 nm (Fig. S3, ESM). After adding NA solution, the decay time of SiNPs changed little, indicating that a static mechanism dominated for the quenching [27, 28]. Calibration for NA was constructed from the fluorescence spectra results with concentration increment of NA, and a linear regression equation was obtained as follows:
A good linear relationship was observed up to NA concentration ranging from 0.5 to 10.5 μM with a correlation coefficient of 0.9981. KSV was found to be 5.85 × 104 L mol−1. The detection limit, calculated according to the 3σ IUPAC criteria, was 12.6 nM.
Applications of SiNPs
Urine of healthy adults was diluted 100-fold with deionized water, 20 μg mL−1 SiNPs mixed solution was prepared using the diluted urine. Determinations were carried out for a set of measurements of 10, 50 and 100 × 10−7 M of NA in diluted urine, respectively. Table 1 showed that the quantification results of NA were in good agreement with the added values. The recovery was calculated as the ratio of the responses of NA obtained from the spiked urine against those of the standard solutions at the same levels (n = 6, average of six replicate measurements), and the RSD were 4.76 %, 4.21 % and 3.45 %, respectively. These values indicated that this method was accurate and precise.
Table 2 summarized the detection limit, linear range and comments using different methods for the determination of NA. Our method was comparable with micellar liquid chromatography [30], micellar electrokinetic chromatography with ultraviolet detector [31] and reverse phase high-performance liquid chromatography [33]. The results demonstrate that this method offers a facile and selective approach for the detection of NA in urine solution.
Conclusions
A facile method for the highly selective recognition of NA was developed based on the combination of the fluorescence and molecularly imprinting techniques. C-dots were prepared at relatively low temperature in APTES. Using NA as template molecules, SiNPs were fabricated by sol-gel polymerization at room temperature. The fabrication process was facile without any complex or post-treatment procedures. The starting materials are low cost and easily available. The fabricated SiNPs showed some advantages such as strong fluorescence, stability and monodispersity. They were used as optical sensor, which selectively bound to the template molecules. A good linear relationship between fluorescence intensity ratio of the system and concentration of NA in aqueous solution was observed in a wide response range. Furthermore, the method was successfully developed for the determination of trace NA in human urine samples. Combining their low cost, stability, selectivity and convenient synthesis, we expect the method will play an important role in probing other analytes which can quench the fluorescence of C-dots.
References
Vlatakis G, Andersson LI, Muller R, Mosbach K (1993) Drug assay using antibody mimics made by molecular imprinting. Nature 361:645–647. doi:10.1038/361645a0
Ge Y, Turner AP (2008) Too large to fit? Recent developments in macromolecular imprinting. Trends Biotechnol 26(4):218–224. doi:10.1016/j.tibtech.2008.01.001
Wulff G (2013) Fourty years of molecular imprinting in synthetic polymers: origin, features and perspectives. Microchim Acta 180(15–16):1359–1370. doi:10.1007/s00604-013-0992-9
Gupta R, Kumar A (2008) Molecular imprinting in sol-gel matrix. Biotechnol Adv 26(6):533–547. doi:10.1016/j.biotechadv.2008.07.002
Lim SY, Shen W, Gao ZQ (2015) Carbon quantum dots and their applications. Chem Soc Rev 44:362–381. doi:10.1039/C4CS00269E
Shughart EL, Ahsan K, Detty MR, Bright FV (2006) Site selectively templated and tagged xerogels for chemical sensors. Anal Chem 78(9):3165–3170. doi:10.1021/ac060113m
Dai H, Xiao DL, He H, Li H, Yuan DH, Zhang C (2015) Synthesis and analytical applications of molecularly imprinted polymers on the surface of carbon nanotubes: a review. Microchim Acta. doi:10.1007/s00604-014-1376-5
Lin CI, Joseph AK, Chang CK, Lee YD (2004) Synthesis and photoluminescence study of molecularly imprinted polymers appended onto CdSe/ZnS core-shells. Biosens Bioelectron 20(1):127–131. doi:10.1016/j.bios.2003.10.017
Lin HY, Ho MS, Lee MH (2009) Instant formation of molecularly imprinted poly(ethylene-co-vinyl alcohol)/quantum dot composite nanoparticles and their use in one-pot urinalysis. Biosens Bioelectron 25(3):579–586. doi:10.1016/j.bios.2009.03.039
Wang HF, He Y, Ji TR, Yan XP (2009) Surface molecular imprinting on Mn-doped ZnS quantum dots for room-temperature phosphorescence optosensing of pentachlorophenol in water. Anal Chem 81(4):1615–1621. doi:10.1021/ac802375a
Mao Y, Bao Y, Han DX, Li FH, Niu L (2012) Efficient one-pot synthesis of molecularly imprinted silica nanospheres embedded carbon dots for fluorescent dopamine optosensing. Biosens Bioelectron 38(1):55–60. doi:10.1016/j.bios.2012.04.043
Carlson LA (2005) Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J Intern Med 258(2):94–114. doi:10.1111/j.1365-2796.2005.01528.x
Pfuhl P, Karcher U, Haring N, Baumeister A, Tawab MA, Schubert-Zsilavecz M (2005) Simultaneous determination of niacin, niacinamide and nicotinuric acid in human plasma. J Pharm Biomed Anal 36(5):1045–1052. doi:10.1016/j.jpba.2004.08.033
Iwaki M, Murakami E, Kikuchi M, Wada A, Ogiso T, Oda Y, Kubo K, Kakehi K (1998) Simultaneous determination of nicotinic acid and its metabolites in rat urine by micellar electrokinetic chromatography with photodiode array detection. J Chromatogr B Biomed Sci Appl 716(1–2):335–342. doi:10.1016/S0378-4347(98)00327-2
HamanoT MY, Kojima N, Aoki N, Semma M, Ito Y, Oji Y (1995) Enzymic method for the amperometric determination of nicotinic acid in meat products. Analyst 120:135–138. doi:10.1039/AN9952000135
Taquchi K, Fukusaki E, Bamba T (2014) Determination of niacin and its metabolites using supercritical fluid chromatography coupled to tandem mass spectrometry. Mass Spectrom (Tokyo) 3(1):A0029. doi:10.5702/massspectrometry.A0029
Wang F, Xie Z, Zhang H, Liu CY, Zhang YG (2011) Highly luminescent organosilane-functionalized carbon dots. Adv Funct Mater 21(6):1027–1031. doi:10.1002/adfm.201002279
Chen L, Xu S, Li J (2011) Recent advances in molecular imprinting technology: current status, challenges and highlighted applications. Chem Soc Rev 40:2922–2942. doi:10.1039/C0CS00084A
Ding CQ, Zhu AW, Tian Y (2014) Functional surface engineering of C-dots for fluorescent biosensing and in vivo bioimaging. Acc Chem Res 47(1):20–30. doi:10.1021/ar400023s
Zhu SJ, Meng QN, Wang L, Zhang JH, Song YB, Jin H, Zhang K, Sun HC, Wang HY, Yang B (2013) Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew Chem 125(14):4045–4049. doi:10.1002/ange.201300519
Yang Y, Gao MY (2005) Preparation of fluorescent SiO2 particles with single CdTe nanocrystal cores by the reverse microemulsion method. Adv Mater 17(19):2354–2357. doi:10.1002/adma.200500403
Sun J, Zhuang JQ, Guan SW, Yang WS (2008) Synthesis of robust water-soluble ZnS:Mn/SiO2 core/shell nanoparticles. J Nanoparticle Res 10(4):653–658. doi:10.1007/s11051-007-9296-5
Dong YQ, Wang RX, Li GL, Chen CQ, Chi YW, Chen GN (2012) Polyamine-functionalized carbon quantum dots as fluorescent probes for selective and sensitive detection of copper ions. Anal Chem 84(14):6220–6224. doi:10.1021/ac3012126
Lunz M, Bradley AL, Chen WY, Gerard VA, Byrne SJ, Gun’ko YK, Lesnyak V, Gaponik N (2010) Influence of quantum dot concentration on förster resonant energy transfer in monodispersed nanocrystal quantum dot monolayers. Phys Rev B 81:201356. doi:10.1103/PhysRevB.81.205316
Wang X, Fang Q, Liu S, Chen L (2012) Preparation of a magnetic molecularly imprinted polymer with pseudo template for rapid simultaneous determination of cyromazine and melamine in bio-matrix samples. Anal Bioanal Chem 404(5):1555–1564. doi:10.1007/s00216-012-6200-7
Li HB, Li YL, Cheng J (2010) Molecularly imprinted silica nanospheres embedded CdSe quantum dots for highly selective and sensitive optosensing of pyrethroids. Chem Mater 22(8):2451–2457. doi:10.1021/cm902856y
Lakowicz J (2006) Principles of fluorescence spectroscopy. Springer Science Business Media, New York
Backhus DA, Golini C, Castellanos E (2003) Evaluation of fluorescence quenching for assessing the importance of interactions between nonpolar organic pollutants and dissolved organic matter. Environ Sci Technol 37(20):4717–4723. doi:10.1021/es026388a
Yao LD, Tang YW, Huang ZF (2007) Nicotinic acid voltammetric sensor based on molecularly imprinted polymer membrane-modified electrode. Anal Lett 40(4):677–688. doi:10.1080/00032710601017755
Capella-Peiró ME, Carda-Broch S, Monferrer-Pons L, Esteve-Romero J (2004) Micellar liquid chromatographic determination of nicotinic acid and nicotinamide after precolumn könig reaction derivatization. Anal Chim Acta 517(1–2):81–87. doi:10.1016/j.aca.2004.05.014
Zhang J, Chakraborty U, Foley JP (2009) Determination of residual cell culture media components by MEKC. Electrophoresis 30(22):3971–3977. doi:10.1002/elps.200900169
Saccani G, Tanzi E, Mallozzi S, Cavalli S (2005) Determination of niacin in fresh and dry cured pork products by ion chromatography: experimental design approach for the optimisation of nicotinic acid separation. Food Chem 92(2):373–379. doi:10.1016/j.foodchem.2004.10.007
Ciulu M, Solinas S, Floris I, Panzanelli A, Pilo MI, Piu PC, Spano N, Sanna G (2011) RP-HPLC determination of water-soluble vitamins in honey. Talanta 83(3):924–929. doi:10.1016/j.talanta.2010.10.059
Liu M, Zhang D, Wang XL, Zhang LN, Han J, Yang M, Xiao X, Zhang YN, Liu HC (2012) Simultaneous quantification of niacin and its three main metabolites in human plasma by LC–MS/MS. J Chromatogr B 904:107–114. doi:10.1016/j.jchromb.2012.07.030
Graeff R, Lee HC (2002) A novel cycling assay for nicotinic acid–adenine dinucleotide phosphate with nanomolar sensitivity. Biochem J 367:163–168. doi:10.1042/BJ20020644
Acknowledgments
This work was supported by Graduate Students Innovative Projects of Jiangsu Province (No.CXZZ12_0308). We thank the editors and co-workers for help and constructive suggestions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 15855 kb)
Rights and permissions
About this article
Cite this article
Zuo, P., Gao, J., Peng, J. et al. A sol-gel based molecular imprint incorporating carbon dots for fluorometric determination of nicotinic acid. Microchim Acta 183, 329–336 (2016). https://doi.org/10.1007/s00604-015-1630-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1630-5